2-Deoxyglucose-Modified Folate Derivative: Self-Assembling Nanoparticle Able to Load Cisplatin
Abstract
1. Introduction
2. Results
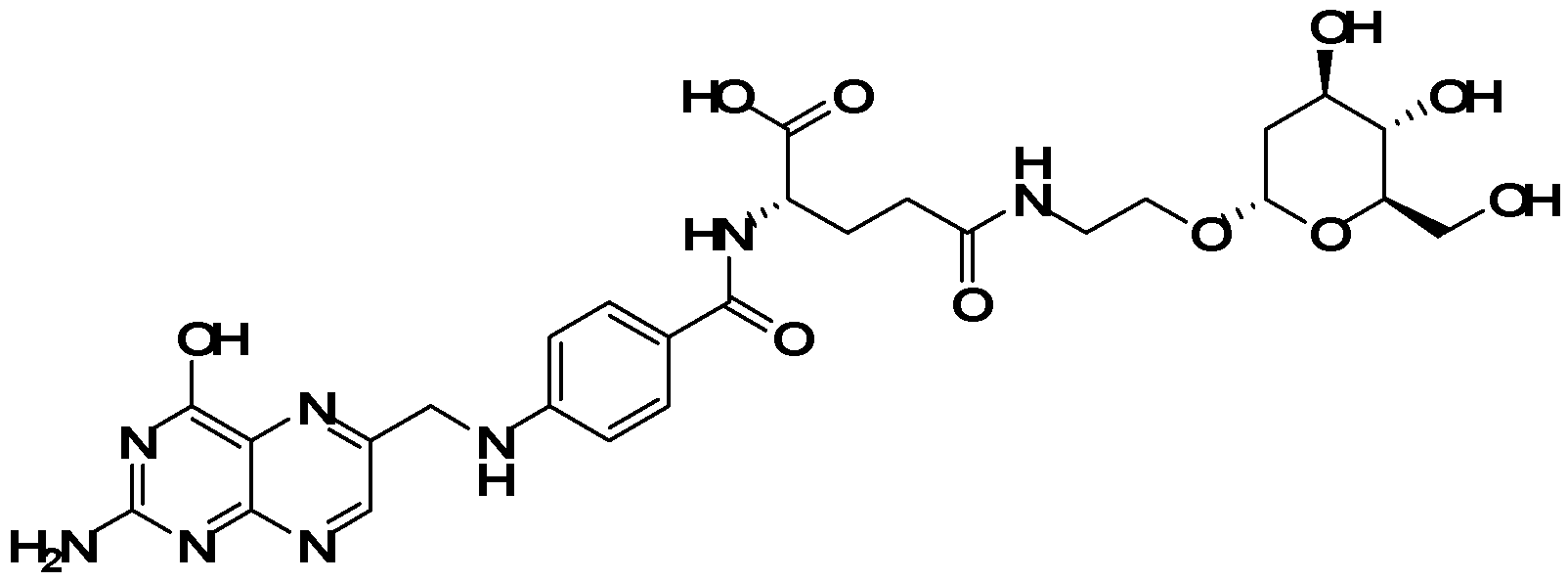
2.1. Water Solubility of FA-2-DG
2.2. Intermolecular Hydrogen Bonding
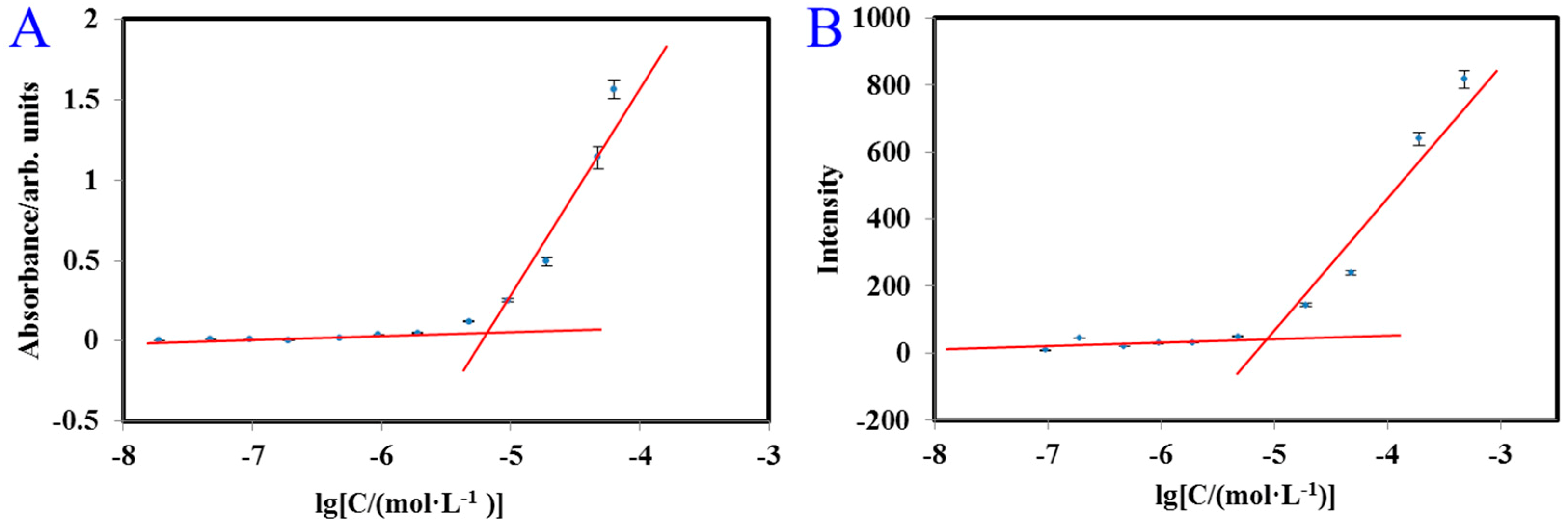
2.3. Critical Micelle Concentration (CMC) of FA-2-DG
2.4. Dynamic Light Scattering (DLS) Measurement of FA-2-DG
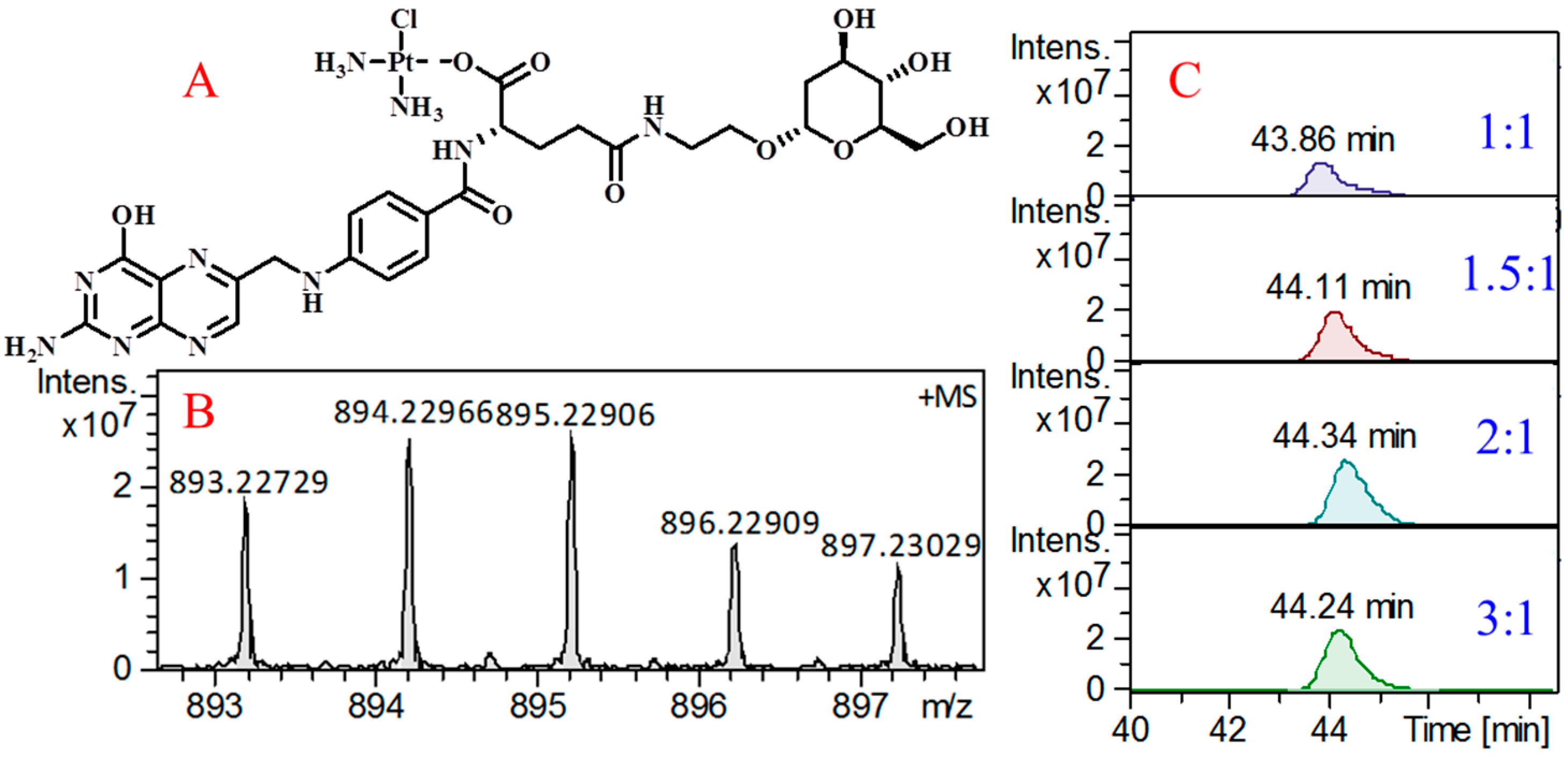
2.5. Ability of FA-2-DG to Load Cisplatin
3. Materials and Methods
3.1. Preparation of FA-2-DG
3.2. Optical Characterization Method
3.3. DLS Characterization
3.4. Cisplatin Loading Method and Evaluation of Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. New Eng. J. Med. 1999, 341, 1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, X.; Demirtas, H.; Li, J.; Mao, G.; Huo, Y.; Sun, N.; Liu, L.; Xu, X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar] [CrossRef]
- Wibowo, A.S.; Mirage, S.; Reeder, K.M.; Carter, J.J.; Kovach, A.R.; Wuyi, M.; Manohar, R.; Faming, Z.; Dann, C.E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 15180–15188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Diopbove, N.; Visentin, M.; Goldman, I.D. Mechanisms of membrane transport offolates into cells and across epithelia. Annu. Rev. Nutr. 2011, 31, 177. [Google Scholar] [CrossRef]
- Shi, H.; Guo, J.; Li, C.; Wang, Z. A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des. Dev. Ther. 2015, 9, 4989–4996. [Google Scholar]
- Tai, X.; Wang, Y.; Zhang, L.; Yang, Y.; Shi, K.; Ruan, S.; Liu, Y.; Gao, H.; Zhang, Z.; He, Q. Cabazitaxel and indocyanine green co-delivery tumor-targeting nanoparticle for improved antitumor efficacy and minimized drug toxicity. J. Drug Target. 2017, 25, 9. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Kingston, D.G.I.; Tamarkin, L. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2010, 67, 47–54. [Google Scholar] [CrossRef]
- Maxwell, T.; Banu, T.; Price, E.; Tharkur, J.; Campos, M.G.; Gesquiere, A.; Santra, S. Non-cytotoxic quantum dot-chitosan nanogel biosensing probe for potential cancer targeting agent. Nanomaterials (Basel) 2015, 5, 2359–2379. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Savitha, S.; Wu, C.J.; Lin, F.H.; Stobiński, L. Carbon encapsulated iron oxide nanoparticles surface engineered with polyethylene glycol-folic acid to induce selective hyperthermia in folate over expressed cancer cells. Int. J. Pharm. 2015, 480, 8–14. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Patra, C.R.; Earl, A.; Wang, S.; Katarya, A.; Lu, L.; Kizhakkedathu, J.N.; Yaszemski, M.J.; Greipp, P.R.; Mukhopadhyay, D. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 224–238. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Luo, L.; Wang, Y.; Zhong, Y.; Dai, H.B.; Sun, D.; Luo, M.L.; Wu, W.; Wang, G.X. Dual tumor-targeted poly(lactic-co-glycolic acid)-polyethylene glycol-folic acid nanoparticles: a novel biodegradable nanocarrier for secure and efficient antitumor drug delivery. Int. J. Nanomed. 2017, 12, 5745–5760. [Google Scholar] [CrossRef] [PubMed]
- Suriamoorthy, P.; Zhang, X.; Hao, G.; Joly, A.G.; Singh, S.; Hossu, M.; Sun, X.; Chen, W. Folic acid-CdTe quantum dot conjugates and their applications for cancer cell targeting. Cancer Nanotechnol. 2010, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Morosini, V.; Bastogne, T.; Frochot, C.; Schneider, R.; Franã Ois, A.; Guillemin, F.; Barberi-Heyob, M. Quantum dot-folic acid conjugates as potential photosensitizers in photodynamic therapy of cancer. Photochem. Photobiol. 2011, 10, 842–851. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Y.; Li, G.; Yang, S.; Xiong, F.; Chu, F. Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin. Nanomaterials 2019, 9, 188. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Alitheen, N.B.; Hussein, M.Z.; Abu, N.; Mohammed, N.E.; Nordin, N.; Zamberi, N.R. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J. Colloid Interface Sci. 2016, 480, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gao, M.; Song, M.; Shi, C.; Zhang, P.; Xu, D.; You, L.; Zhuang, R.; Su, X.; Liu, T.; Du, J.; Zhang, X. Synthesis and Evaluation of (99m)Tc-Labeled dimeric folic acid for FR-targeting. Molecules 2016, 21, 817. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, X.; Su, X.; Li, J.; Tian, Y.; Xue, H.; Xu, H. 99mTc-labeled oligomeric nanoparticles as potential agents for folate receptor-positive tumor targeting. J. Label. Compd. Radiopharm. 2018, 61, 54–60. [Google Scholar] [CrossRef]
- Kettenbach, K.; Reffert, L.M.; Schieferstein, H.; Pektor, S.; Eckert, R.; Miederer, M.; Rösch, F.; Ross, T.L. Comparison study of two differently clicked 18F-folates-lipophilicity plays a key role. Pharmaceuticals 2018, 11, 30. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, X.; Hou, M.; Xue, P.; Gao, Y.E.; Liu, S.; Kang, Y. Disassembly of amphiphilic small molecular prodrug with fluorescence switch induced by pH and folic acid receptors for targeted delivery and controlled release. Colloids Surf. B 2017, 150, 50–58. [Google Scholar] [CrossRef]
- Zhen, W.; Li, X.; Hou, C.; Yu, Q. Solubility of folic acid in water at pH values between 0 and 7 at temperatures (298.15, 303.15, and 313.15) K. J. Chem. Eng. Data 2010, 55, 3958–3961. [Google Scholar]
- Xie, Y.; Li, S.S.; Jiang, X.; Xiang, T.; Wang, R.; Zhao, C.S. Zwitterionic glycosyl modified polyethersulfone membranes with enhanced anti-fouling property and blood compatibility. J. Colloid Interface Sci. 2015, 443, 36–44. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, H.; Ma, X.; Tai, X.; Wang, W.; Du, Z.; Wang, G. Synthesis, characterization and physicochemical properties of glycosyl-modified polysiloxane. J. Mol. Liquids 2018, 266, 90–98. [Google Scholar] [CrossRef]
- Dan, L.; Wu, J.; Yang, S.; Zhang, W.; Niu, X.; Chen, Y.; Ran, F. Hydrophilicity and anti-fouling performance of polyethersulfone membrane modified by grafting block glycosyl copolymers via surface initiated electrochemically mediated atom transfer radical polymerization. New J. Chem. 2018, 42, 2692–2701. [Google Scholar]
- O’Byrne, K.J.; Baird, A.; Kilmartin, L.; Leonard, J.; Sacevich, C.; Gray, S.G. Epigenetic regulation of glucose transporters in non-small cell lung cancer. Cancers 2011, 3, 1550–1565. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, H.; Lleonart, M.E.; Gil, J.; Wang, J.; Degan, P.; Peters, G.; Martinez, D.; Carnero, A.; Beach, D. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005, 65, 177–185. [Google Scholar]
- Sottnik, J.L.; Lori, J.C.; Rose, B.J.; Thamm, D.H. Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic phenotype in vitro and in vivo. Clin. Exp. Metastasis 2011, 28, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Wang, F.; Hu, J.; Wang, S.; Sun, Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014, 355, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, J.; Han, X. Lidocaine Sensitizes the Cytotoxicity of Cisplatin in Breast Cancer Cells via Up-Regulation of RARβ2 and RASSF1A Demethylation. Int. J. Mol. Sci. 2014, 15, 23519–23536. [Google Scholar] [CrossRef]
- Pan, H.; Kim, E.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Theaflavin-3,3′-Digallate Enhances the Inhibitory Effect of Cisplatin by Regulating the Copper Transporter 1 and Glutathione in Human Ovarian Cancer Cells. Int. J. Mol. Sci. 2018, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Song, F.; Liu, Z.; Li, J.; Wu, B.; Fu, Z.; Jiang, J.; Chen, Z. MLKL-PITPα signaling-mediated necroptosis contributes to cisplatin-triggered cell death in lung cancer A549 cells. Cancer Lett. 2018, 414, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Toro-Cordova, A.; Flores-Cruz, M.; Santoyo-Salazar, J.; Carrillo-Nava, E.; Jurado, R.; Figueroa-Rodriguez, P.A.; Lopez-Sanchez, P.; Medina, L.A.; Garcia-Lopez, P. Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy. Molecules 2018, 23, 2272. [Google Scholar] [CrossRef]
- Sun, M.; Shi, Y.; Dang, U.J.; Di Pasqua, A.J. Phenethyl Isothiocyanate and Cisplatin Co-Encapsulated in a Liposomal Nanoparticle for Treatment of Non-Small Cell Lung Cancer. Molecules 2019, 24, 801. [Google Scholar] [CrossRef]
- Durazzo, A.; Kiefer, J.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Aguzzi, A.; Gambelli, L.; Lisciani, S.; Marletta, L. Qualitative Analysis of Traditional Italian dishes: FTIR approach. Sustainability 2018, 10, 4112. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Y.; Chen, W.; Ren, J.; Zhang, L.; Lu, L.; Luo, G.; Huang, H. Preparation, characterization and evaluation of α-Tocopherol succinate-modified dextran micelles as potential drug carriers. Materials 2015, 8, 6685–6696. [Google Scholar] [CrossRef]
- Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Dorkoosh, F.A.; Handali, S. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur. J. Pharm. Sci. 2018, 114, 166–174. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, N.N.T.; Tran, N.T.N.; Le, P.N.; Nguyen, T.B.T.; Nguyen, N.H.; Bach, L.G.; Doan, V.N.; Tran, H.L.B.; Le, V.T.; Tran, N.Q. Synergic activity against MCF-7 breast cancer cell growth of nanocurcumin-encapsulated and cisplatin-complexed nanogels. Molecules 2018, 23, 3347. [Google Scholar] [CrossRef]
- Badea, M.A.; Prodana, M.; Dinischiotu, A.; Crihana, C.; Ionita, D.; Balas, M. Cisplatin loaded multiwalled carbon nanotubes induce resistance in triple negative breast cancer cells. Pharmaceutics 2018, 10, 228. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, H.; Zhan, J.; Lin, M.; Dai, L.; Ren, C.; Shi, Y.; Liu, J.; Gao, J.; Yang, Z. Supramolecular “Trojan Horse” for nuclear delivery of dual anticancer drugs. J. Am. Chem. Soc. 2017, 139, 2876–2879. [Google Scholar] [CrossRef]
- Jin, S.; Du, Z.; Guo, H.; Zhang, H.; Ren, F.; Wang, P. Novel targeted anti-tumor nanoparticles developed from folic acid-modified 2- Deoxyglucose. Int. J. Mol. Sci. 2019, 20, 697. [Google Scholar] [CrossRef]
- Stopková, L.; Gališinová, J.; Šuchtová, Z.; Čižmárik, J.; Andriamainty, F. Determination of critical micellar concentration of homologous 2-alkoxyphenylcarbamoyloxyethyl-morpholinium chlorides. Molecules 2018, 23, 1064. [Google Scholar] [CrossRef] [PubMed]
- Quanten, T.; Shestakova, P.; Kondinski, A.; Parac-Vogt, T.N. Effect of [Zr(α-PW11O39)2]10− polyoxometalate on the self-assembly of surfactant molecules in water studied by fluorescence and DOSY NMR Spectroscopy. Inorganics 2018, 6, 112. [Google Scholar] [CrossRef]
- Kuen, C.Y.; Fakurazi, S.; Othman, S.S.; Masarudin, M.J. Increased loading, efficacy and sustained release of silibinin, a poorly soluble drug using hydrophobically-modified chitosan nanoparticles for enhanced delivery of anticancer drug delivery systems. Nanomaterials 2017, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, C.; Chen, C.; Ren, F.; Li, Y.; Mu, Z.; Wang, P. The use of trisodium citrate to improve the textural properties of acid-Induced, transglutaminase-treated micellar casein gels. Molecules 2018, 23, 1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schrader, W. Selective analysis of sulfur-containing species in a heavy crude oil by deuterium labeling reactions and ultrahigh resolution mass spectrometry. Int. J. Mol. Sci. 2015, 16, 30133–30143. [Google Scholar] [CrossRef]
Sample Availability: Samples of the FA-2-DG is available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Du, Z.; Wang, P.; Guo, H.; Zhang, H.; Lei, X.; Ren, F. 2-Deoxyglucose-Modified Folate Derivative: Self-Assembling Nanoparticle Able to Load Cisplatin. Molecules 2019, 24, 1084. https://doi.org/10.3390/molecules24061084
Jin S, Du Z, Wang P, Guo H, Zhang H, Lei X, Ren F. 2-Deoxyglucose-Modified Folate Derivative: Self-Assembling Nanoparticle Able to Load Cisplatin. Molecules. 2019; 24(6):1084. https://doi.org/10.3390/molecules24061084
Chicago/Turabian StyleJin, Shaoming, Zhongyao Du, Pengjie Wang, Huiyuan Guo, Hao Zhang, Xingen Lei, and Fazheng Ren. 2019. "2-Deoxyglucose-Modified Folate Derivative: Self-Assembling Nanoparticle Able to Load Cisplatin" Molecules 24, no. 6: 1084. https://doi.org/10.3390/molecules24061084
APA StyleJin, S., Du, Z., Wang, P., Guo, H., Zhang, H., Lei, X., & Ren, F. (2019). 2-Deoxyglucose-Modified Folate Derivative: Self-Assembling Nanoparticle Able to Load Cisplatin. Molecules, 24(6), 1084. https://doi.org/10.3390/molecules24061084





