Zinc Porphyrin-Functionalized Fullerenes for the Sensitization of Titania as a Visible-Light Active Photocatalyst: River Waters and Wastewaters Remediation
Abstract
:1. Introduction
2. Results and Discussion
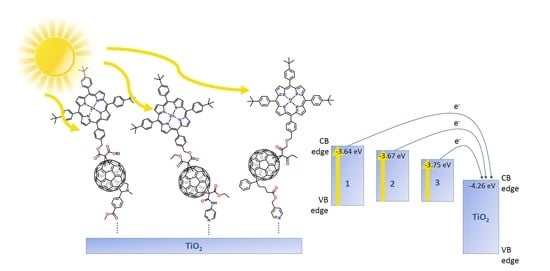
2.1. Photoelectronic Properties of the Fullerene Derivatives
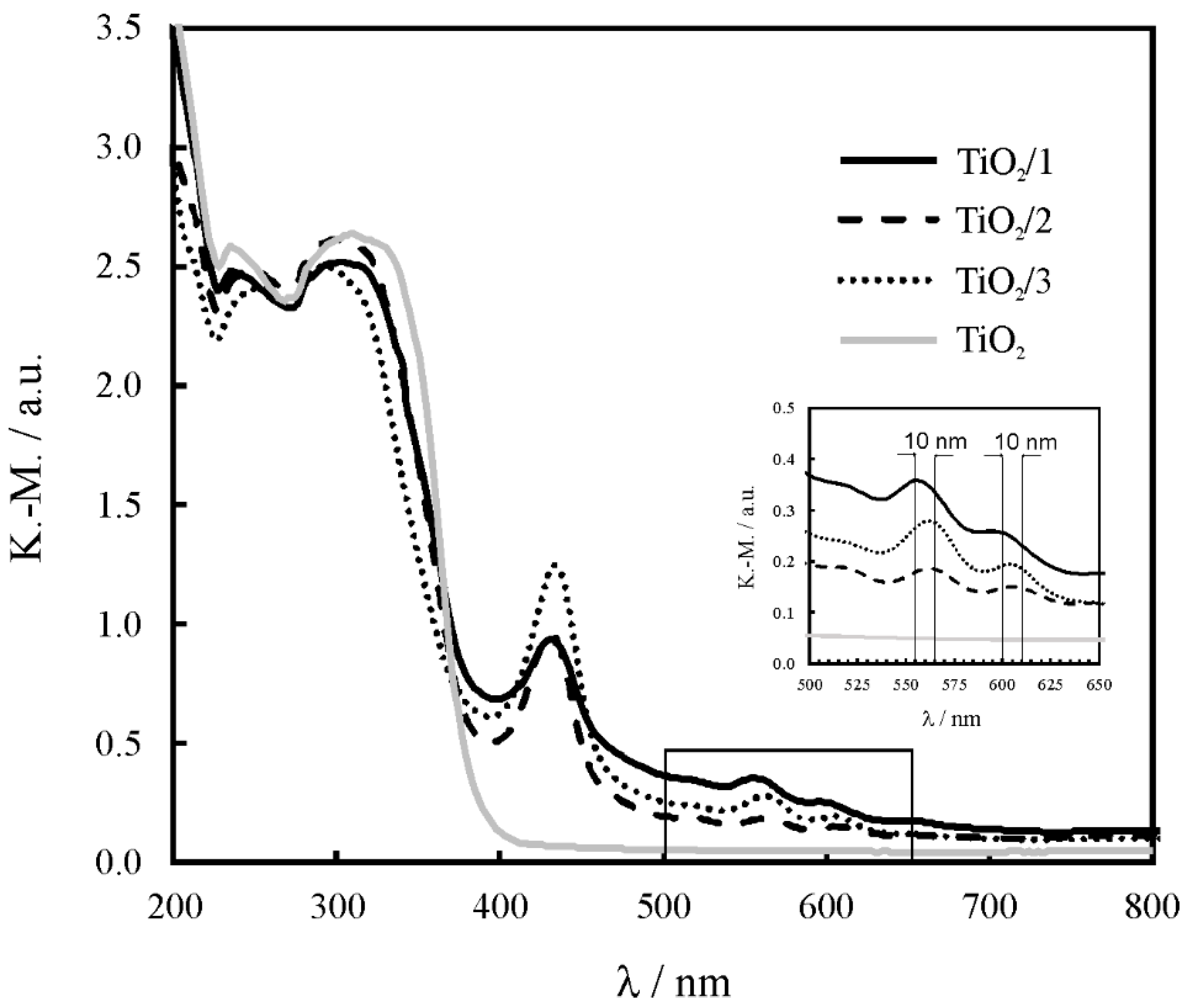
2.2. Characterization of the Titania
2.3. Characterization of the Composites
2.4. Photocatalytic Performance
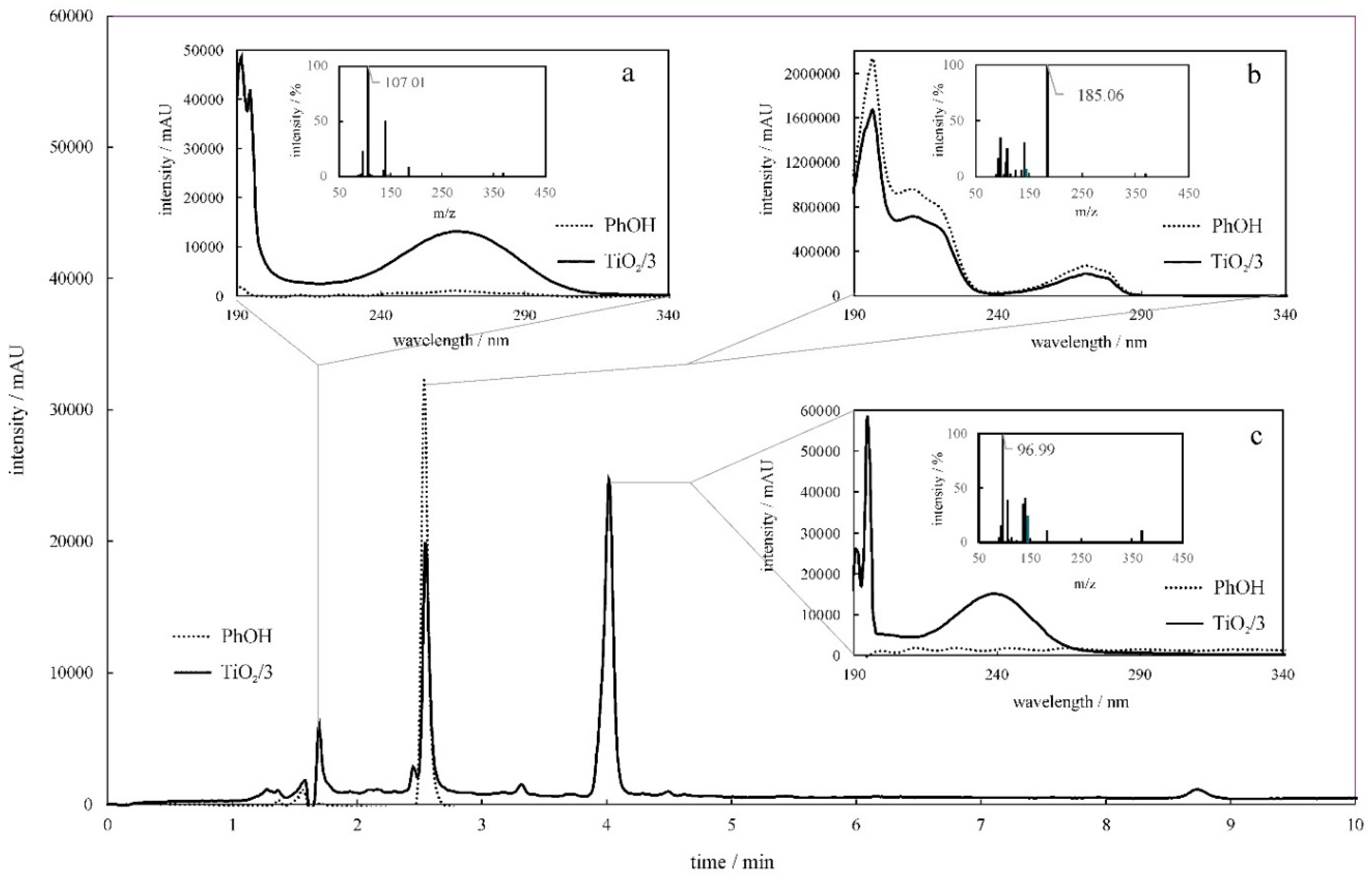
2.5. UV–Vis Absorbance Spectra of the Photodegradation Products
2.6. Photocatalytic Performance Using Natural Samples
3. Experimental
3.1. Methods
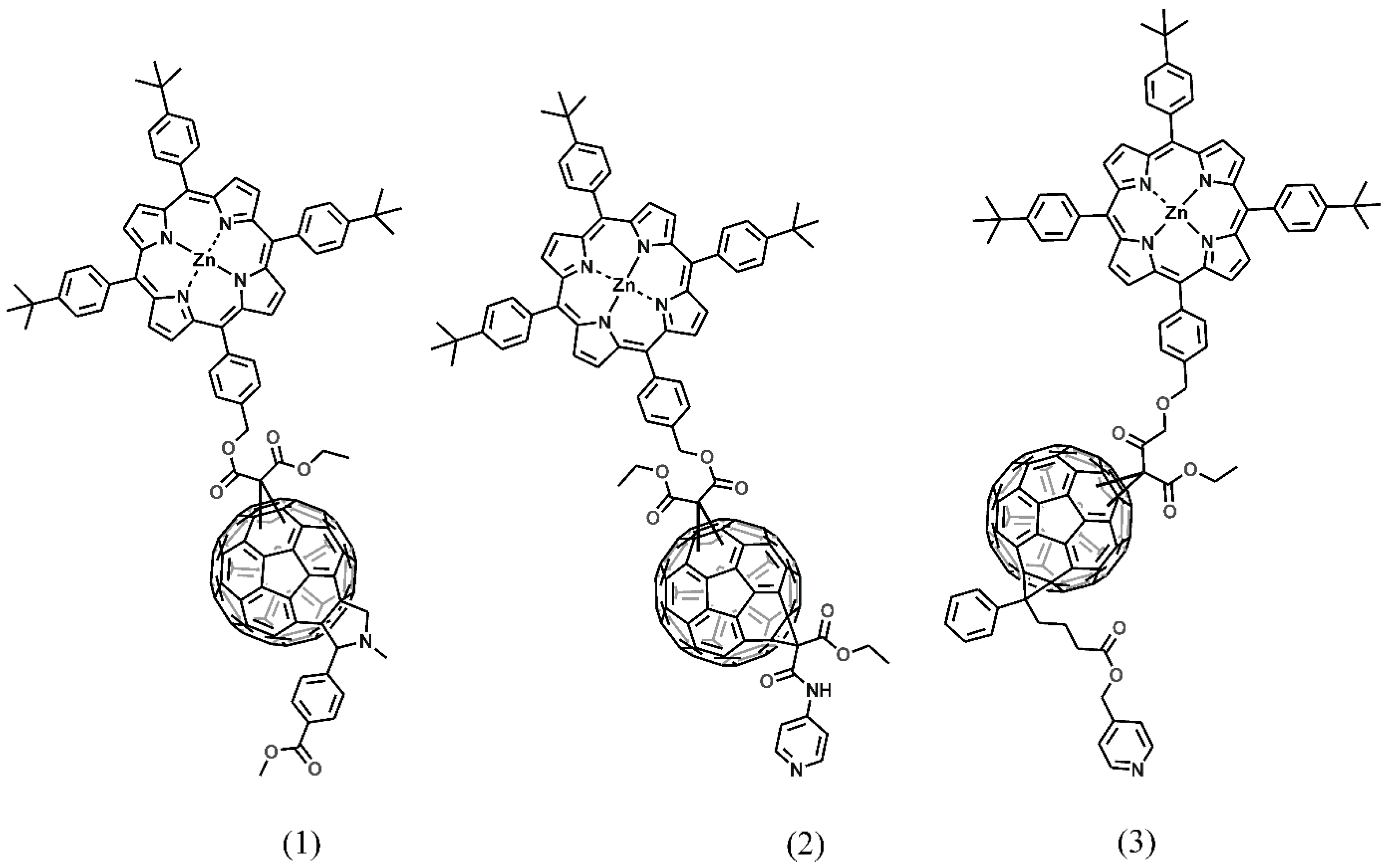
3.2. Synthesis of Fullerene Derivatives
3.3. Synthesis of Titania
3.4. Synthesis of Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Fujishima, A.; Honda, K. Photolysis-decomposition of water at the surface of an irradiated semiconductor. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Savinkina, E.; Obolenskaya, L.; Kuzmicheva, G. Efficiency of sensitizing nano-titania with organic dyes and peroxo complexes. Appl. Nanosci. 2015, 5, 125–133. [Google Scholar] [CrossRef]
- Guarisco, C.; Palmisano, G.; Calogero, G.; Ciriminna, R.; di Marco, G.; Loddo, V.; Pagliaro, M.; Parrino, F. Visible-light driven oxidation of gaseous aliphatic alcohols to the corresponding carbonyls via TiO2 sensitized by a perylene derivative. Environ. Sci. Pollut. Res. 2014, 21, 11135–11141. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.G.; Dhital, B.; He, Y.; Lu, H.P. Single-Molecule Interfacial Electron Transfer Dynamics of Porphyrin on TiO2 Nanoparticles: Dissecting the Complex Electronic Coupling Dependent Dynamics. J. Phys. Chem. C 2014, 118, 20209–20221. [Google Scholar] [CrossRef]
- Machado, A.E.H.; França, M.D.; Velani, V.; Magnino, G.A.; Velani, H.M.M.; Freitas, F.S.; Müller, P.S.; Sattler, C.; Schmücker, M. Characterization and Evaluation of the Efficiency of TiO2/Zinc Phthalocyanine Nanocomposites as Photocatalysts for Wastewater Treatment Using Solar Irradiation. Int. J. Photoenergy 2008, 2008, 482373. [Google Scholar] [CrossRef]
- Ardo, S.; Achey, D.; Morris, A.J.; Abrahamsson, M.; Meyer, G.J. Non-Nernstian Two-Electron Transfer Photocatalysis at Metalloporphyrin–TiO2 Interfaces. J. Am. Chem. Soc. 2011, 133, 16572–16580. [Google Scholar] [CrossRef]
- Ren, X.-B.; Chen, M.; Qian, D.-J. Pd(II)-Mediated Triad Multilayers with Zinc Tetrapyridylporphyrin and Pyridine-Functionalized Nano-TiO2 as Linkers: Assembly, Characterization, and Photocatalytic Properties. Langmuir 2012, 28, 7711–7719. [Google Scholar] [CrossRef]
- Duan, M.; Li, J.; Mele, G.; Wang, C.; Lü, X.; Vasapollo, G.; Zhang, F. Photocatalytic Activity of Novel Tin Porphyrin/TiO2 Based Composites. J. Phys. Chem. C 2010, 114, 7857–7862. [Google Scholar] [CrossRef]
- Li, D.; Dong, W.; Sun, S.; Shi, Z.; Feng, S. Photocatalytic Degradation of Acid Chrome Blue K with Porphyrin-Sensitized TiO2 under Visible Light. J. Phys. Chem. C 2008, 112, 14878–14882. [Google Scholar] [CrossRef]
- Mele, G.; del Sole, R.; Vasapollo, G.; Marcì, G.; Garcìa-Lòpez, E.; Palmisano, L.; Coronado, J.M.; Hernández-Alonso, M.D.; Malitesta, C.; Guascito, M.R. TRMC, XPS, and EPR Characterizations of Polycrystalline TiO2 Porphyrin Impregnated Powders and Their Catalytic Activity for 4-Nitrophenol Photodegradation in Aqueous Suspension. J. Phys. Chem. B 2005, 109, 12347–12352. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Zhu, L.; Choi, J.-G.; Chen, M.-L.; Oh, W.-C. Effect of Pt treated fullerene/TiO2 on the photocatalytic degradation of MO under visible light. J. Mater. Chem. 2011, 21, 7596. [Google Scholar] [CrossRef]
- Krishna, V.; Noguchi, N.; Koopman, B.; Moudgil, B. Enhancement of titanium dioxide photocatalysis by water-soluble fullerenes. J. Colloid Interface Sci. 2006, 304, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Yanes, D.; Imaram, W.; Angerhofer, A.; Koopman, B.; Moudgil, B. Mechanism of enhanced photocatalysis with polyhydroxy fullerenes. Appl. Catal. B Environ. 2008, 79, 376–381. [Google Scholar] [CrossRef]
- Lin, J.; Zong, R.; Zhou, M.; Zhu, Y. Photoelectric catalytic degradation of methylene blue by C60-modified TiO2 nanotube array. Appl. Catal. B Environ. 2009, 89, 425–431. [Google Scholar] [CrossRef]
- Apostolopoulou, V.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Preparation and characterization of [60] fullerene nanoparticles supported on titania used as a photocatalyst. Colloids Surf. Physicochem. Eng. Asp. 2009, 349, 189–194. [Google Scholar] [CrossRef]
- Oh, W.-C.; Jung, A.-R.; Ko, W.-B. Preparation of Fullerene/TiO2 Composite and Its Photocatalytic Effect. J. Ind. Eng. Chem. 2007, 13, 1208–1214. [Google Scholar]
- Katsumata, K.; Matsushita, N.; Okada, K. Preparation of TiO2-Fullerene Composites and Their Photocatalytic Activity under Visible Light. Int. J. Photoenergy 2012, 2012, 256096. [Google Scholar] [CrossRef]
- Oh, W.-C.; Zhang, F.-J.; Meng, Z.-D.; Zhang, K. Relative Photonic Properties of Fe/TiO2-Nanocarbon Catalysts for Degradation of MB Solution under Visible Light. Bull. Korean Chem. Soc. 2010, 31, 1128–1134. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Synthesis of Fullerene–, Carbon Nanotube–, and Graphene–TiO2 Nanocomposite Photocatalysts for Selective Oxidation: A Comparative Study. ACS Appl. Mater. Interfaces 2013, 5, 1156–1164. [Google Scholar] [CrossRef]
- Zhao, Z.; Ozoemena, K.I.; Maree, D.M.; Nyokong, T. Synthesis and electrochemical studies of a covalently linked cobalt(II) phthalocyanine-cobalt(II) porphyrin conjugate. Dalton Trans. 2005, 7, 1241–1248. [Google Scholar] [CrossRef]
- Kadish, K.M.; van Caemelbecke, E. Electrochemistry of porphyrins and related macrocycles. J. Solid State Electrochem. 2003, 7, 254–258. [Google Scholar] [CrossRef]
- Fernández-García, M.; Wang, X.; Belver, C.; Hanson, J.C.; Rodriguez, J.A. Anatase-TiO2 Nanomaterials: Morphological/Size Dependence of the Crystallization and Phase Behavior Phenomena. J. Phys. Chem. C 2007, 111, 674–682. [Google Scholar] [CrossRef]
- Burgos, M.; Langlet, M. The sol-gel transformation of TIPT coatings: A FTIR study. Thin Solid Films 1999, 349, 19–23. [Google Scholar] [CrossRef]
- Zhao, D.; Peng, T.; Liu, M.; Lu, L.; Cai, P. Fabrication, characterization and photocatalytic activity of Gd3+-doped titania nanoparticles with mesostructured. Microporous Mesoporous Mater. 2008, 114, 166–174. [Google Scholar] [CrossRef]
- Regulska, E.; Karpinska, J. Investigation of Photocatalytic Activity of C60/TiO2 Nanocomposites Produced by Evaporation Drying Method. Pol. J. Environ. Stud. 2014, 23, 2175–2182. [Google Scholar]
- Regulska, E.; Karpinska, J. Investigation of novel material for effective photodegradation of bezafibrate in aqueous samples. Environ. Sci. Pollut. Res. 2014, 21, 5242–5248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol–gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Bahnemann, D.; Henglein, A.; Spanhel, L. Detection of the intermediates of colloidal TiO2-catalysed photoreactions. Faraday Discuss. Chem. Soc. 1984, 78, 151–163. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Dz. U 2014 poz. 1482—Regulation of the Minister of the Environment on the Method of Classifying the Status of Uniform Bodies of Surface Water and Environmental Quality Standards for Priority Substances (Journal of Laws, Item 1482, 2014); 2014.
- Dz. U 2014 poz. 1800—Regulation of the Minister of the Environment on the Conditions to Be Met When Discharging Sewage to Waters or to the Soil and on Substances of Particular Adverse Impact on the Water Environment (Journal of Laws, Item 1800, 2014); 2014.
- Mariquit, E.G.; Salim, C.; Hinode, H. The Role of Calcium Ions in the Photocatalytic Oxidation of Humic Acid at Neutral pH. Ann. N. Y. Acad. Sci. 2008, 1140, 389–393. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Lu, Z.; Huo, P.; Xing, W.; He, M.; Li, J.; Yan, Y. Influence of Inorganic Ions and pH on the Photodegradation of 1-Methylimidazole-2-thiol with TiO2 Photocatalyst Based on Magnetic Multi-walled Carbon Nanotubes. Bull. Korean Chem. Soc. 2014, 35, 76–82. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Martínez-Zapata, M.; Aristizábal, C.; Peñuela, G. Photodegradation of the endocrine-disrupting chemicals 4n-nonylphenol and triclosan by simulated solar UV irradiation in aqueous solutions with Fe(III) and in the absence/presence of humic acids. J. Photochem. Photobiol. Chem. 2013, 251, 41–49. [Google Scholar] [CrossRef]
- Canonica, S. Oxidation of Aquatic Organic Contaminants Induced by Excited Triplet States. Chim. Int. J. Chem. 2007, 61, 641–644. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from E.R. |









| Catalyst | TiO2 | TiO2/1 | TiO2/2 | TiO2/3 |
|---|---|---|---|---|
| BET surface area/m2∙g−1 | 269 | 178 | 151 | 158 |
| Diameter/μm | 3.24 | 1.07 | 0.86 | 0.48 |
| Parametr | Goldapa River | Sapina River | Suprasl River | The Range of Acceptable Values for Rivers [30] | Norms |
|---|---|---|---|---|---|
| pH | 7.8 | 7.8 | 7.5 | 6.5-8.5 | PN-90/C-04540/01 |
| Acidity/mg CaCO3 ∙ L−1 | 111.6 | 99.2 | 80.6 | nd | PN-90/C-04540/03 |
| Alkalinity/mg CaCO3 ∙ L−1 | 240.2 | 260.2 | 180.2 | ≤150 (I) *; ≤250 (II) ** | PN-90/C-04540/03 |
| COD ***/mg O2 ∙ L−1 | 1.9 | 2.3 | 2.3 | ≤6 (I); ≤12 (II) | PN-85/C-04578/02 |
| Conductivity/S ∙ cm−1 | 245 | 270 | 360 | ≤1000 (I); ≤1500 (II) | |
| Chlorine (Cl−)/mg Cl− ∙ L−1 | 156.0 | 113.4 | 170.2 | ≤200 (I); ≤300 (II) | PN-75/C-04617/02 |
| Calcium (Ca2+)/ mg Ca2+ ∙ L−1 | 56.1 | 74.1 | 104.2 | ≤100 (I); ≤200 (II) | PN-91/C-04551/01 |
| Magnesium (Mg2+)/ mg Mg2+ ∙ L−1 | 27.3 | 16.0 | 35.3 | ≤50 (I); ≤100 (II) | PN-71/C-04554/10 |
| Hardness/mg CaCO3 ∙ L−1 | 207.6 | 223.6 | 347.3 | ≤300 (I); ≤500 (II) | PN-71/C-04554/02 |
| Dissolved oxygen/mg O2 ∙ L−1 | 0.9 | 2.2 | 1.0 | ≥7 (I); ≥5 (II) | ISO 5813:1983 |
| Nitrate (V)/mg ∙ L−1 | 3.7 | 3.4 | 69.3 | ≤2.2 (I); ≤5.0 (II) | PN-82/C-04576/08 |
| Phosphate/mg ∙ L−1 | 11.5 | 7.3 | nd | ≤0.20 (I); 0.31 (II) | PN-88/C-04537/04 |
| Parameter | Municipal Wastewater | Wastewater Effluents | The range of Acceptable Values for Effluents [31] | Norms |
|---|---|---|---|---|
| pH | 10.8 | 10.7 | 6.5-9.0 | PN-90/C-04540/01 |
| Acidity/mg CaCO3 ∙ L−1 | 2.3 | 1.1 | nd | PN-90/C-04540/03 |
| Alkalinity/mg CaCO3 ∙ L−1 | 580.0 | 285.0 | 150-350 | PN-90/C-04540/03 |
| COD/mg O2 ∙ L−1 | 89.8 | 36.7 | <125 | PN-85/C-04578/02 |
| Conductivity/S ∙ cm−1 | 8500 | 7000 | nd | |
| Chlorine (Cl−)/mg Cl− ∙ L−1 | 368.7 | 326.1 | <1000 | PN-75/C-04617/02 |
| Calcium (Ca2+)/mg Ca2+ ∙ L−1 | 78.6 | 86.7 | nd | PN-91/C-04551/01 |
| Magnesium (Mg2+)/mg Mg2+ ∙ L−1 | 41.7 | 17.6 | >300 | PN-71/C-04554/10 |
| Hardness/mg CaCO3 ∙ L−1 | 300.2 | 260.2 | nd | PN-71/C-04554/02 |
| Dissolved oxygen/mg O2 ∙ L−1 | nd | 1.4 | nd | ISO 5813:1983 |
| Nitrate (V)/mg ∙ L−1 | 1226.3 | 45.0 | <30 | PN-82/C-04576/08 |
| Phosphate/mg ∙ L−1 | 3418.5 | 18.1 | nd | PN-88/C-04537/04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regulska, E.; Rivera-Nazario, D.M.; Karpinska, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Zinc Porphyrin-Functionalized Fullerenes for the Sensitization of Titania as a Visible-Light Active Photocatalyst: River Waters and Wastewaters Remediation. Molecules 2019, 24, 1118. https://doi.org/10.3390/molecules24061118
Regulska E, Rivera-Nazario DM, Karpinska J, Plonska-Brzezinska ME, Echegoyen L. Zinc Porphyrin-Functionalized Fullerenes for the Sensitization of Titania as a Visible-Light Active Photocatalyst: River Waters and Wastewaters Remediation. Molecules. 2019; 24(6):1118. https://doi.org/10.3390/molecules24061118
Chicago/Turabian StyleRegulska, Elzbieta, Danisha Maria Rivera-Nazario, Joanna Karpinska, Marta Eliza Plonska-Brzezinska, and Luis Echegoyen. 2019. "Zinc Porphyrin-Functionalized Fullerenes for the Sensitization of Titania as a Visible-Light Active Photocatalyst: River Waters and Wastewaters Remediation" Molecules 24, no. 6: 1118. https://doi.org/10.3390/molecules24061118