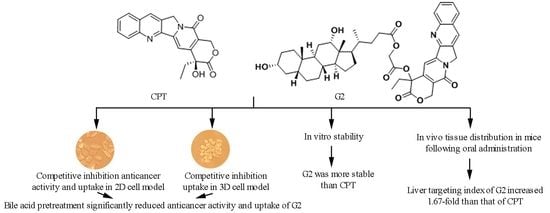
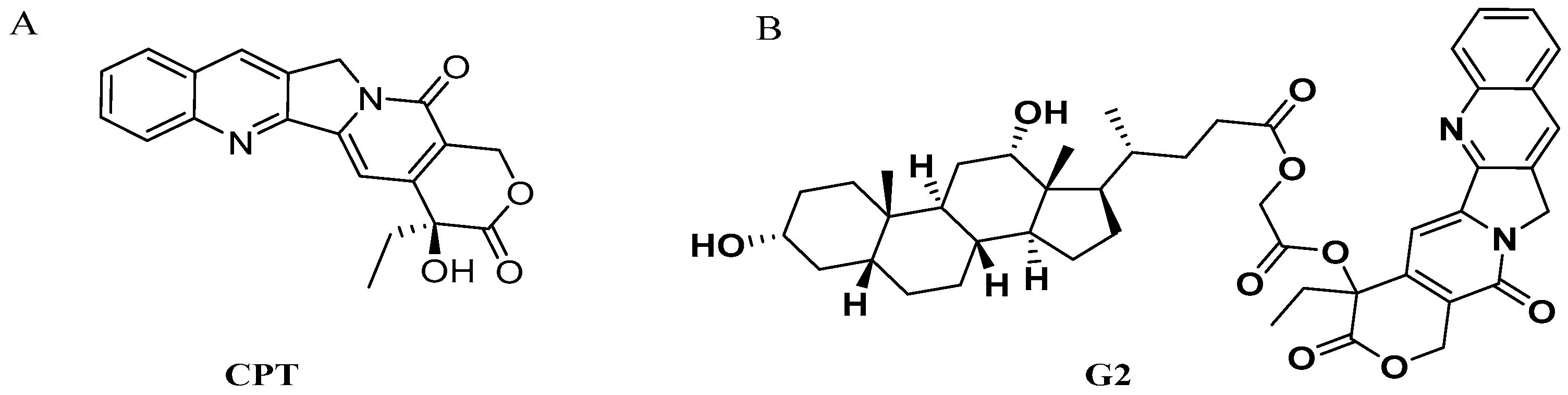
Enhanced Liver Targeting of Camptothecin via Conjugation with Deoxycholic Acid
Abstract
:1. Introduction
2. Results and Discussion
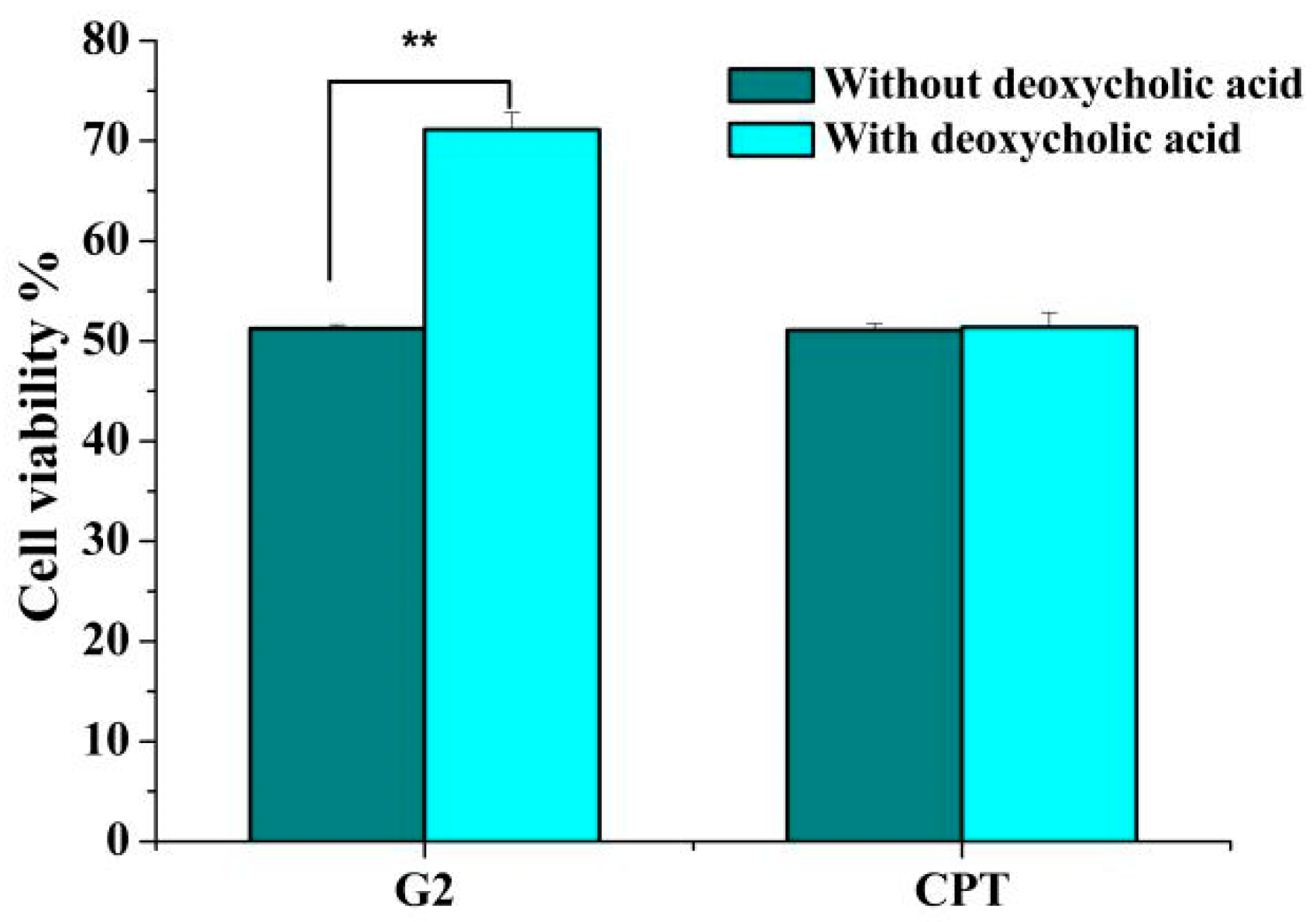
2.1. Effect of Deoxycholic Acid on Cytotoxicity of G2 and CPT
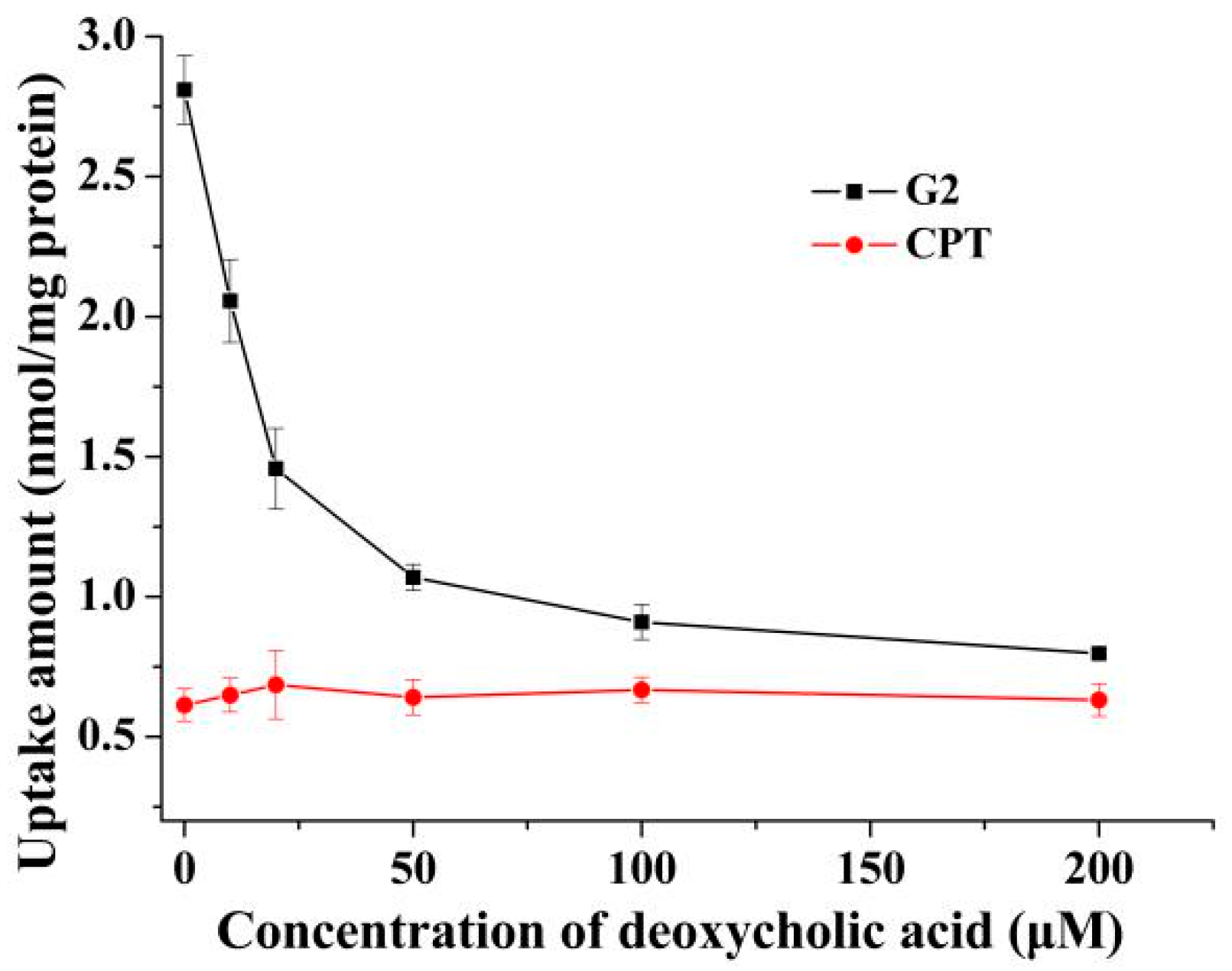
2.2. Effects of Deoxycholic Acid on Uptake of G2 and CPT in 2D Cell Model
2.3. Effects of Deoxycholic Acid on Uptake of G2 and CPT in 3D Cell Model
2.4. Stability of G2 and CPT
2.5. Method Validation
2.6. Body Distribution
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. 3D Multicellular Tumor Spheroids Culture
3.4. Competitive Inhibition Assays
3.5. Uptake of G2 into 2D Cell Model
3.6. Uptake of G2 into 3D Cell Model
3.7. Stability of G2
3.8. In Vivo Distribution of G2 in Mice
3.9. Calibration Curves and Quality Control Samples Preparation
3.10. Plasma and Tissue Homogenate Samples Processing
3.11. HPLC Analysis
3.12. Method Validation
3.13. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wani, M.C.; Wall, M.E.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant antitumor agents. I. The isolation and structure of camptothecin a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar]
- Hertzberg, R.P.; Caranfa, M.J.; Hecht, S.M. On the mechanism of topoisomerase I inhibition by camptothecin: Evidence for binding to an enzyme-DNA complex. Biochemistry 1989, 28, 4629–4638. [Google Scholar] [CrossRef]
- Kehrer, D.F.; Soepenberg, O.; Loos, W.J.; Verweij, J.; Sparreboom, A. Modulation of camptothecin analogs in the treatment of cancer: A review. Anti-Cancer Drug 2001, 12, 89–105. [Google Scholar] [CrossRef]
- Park, E.S.; Kang, S.; Yoo, K.; Lee, M.Y.; Yoo, H.S.; Hong, J.T.; Shin, H.S.; Kim, B.; Yun, Y.P. Camptothecin inhibits platelet-derived growth factor-BB-induced proliferation of rat aortic vascular smooth muscle cells through inhibition of PI3K/Akt signaling pathway. Exp. Cell Res. 2013, 319, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Li, W.Q.; Morris-Natschke, S.L.; Qian, K.; Yang, L.; Zhu, G.X.; Wu, X.B.; Chen, A.L.; Zhang, S.Y.; Nan, X.; et al. Perspectives on biologically active camptothecin derivatives. Med. Res. Rev. 2015, 35, 753–789. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Ho, Y.F.; Li, T.K.; Chen, C.S.; Hsu, L.C.; Guh, J.H. Rottlerin potentiates camptothecin-induced cytotoxicity in human hormone refractory prostate cancers through increased formation and stabilization of topoisomerase I-DNA cleavage complexes in a PKCδ-independent pathway. Biochem. Pharmacol. 2012, 84, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Yeo, C.D.; Lee, S.H.; Kim, J.S.; Kim, S.J.; Kim, S.C.; Kim, Y.K.; Kang, H.H.; Yoon, H.K.; Song, J.S.; Moon, H.S.; et al. A multicenter phase II study of belotecan, a new camptothecin analogue, in elderly patients with previously untreated, extensive-stage small cell lung cancer. Cancer Chemoth. Pharm. 2013, 72, 809–814. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zu, Y.G.; Shi, R.Z.; Yao, L.P. Review camptothecin: Current perspectives. Curr. Med. Chem. 2006, 13, 2021–2039. [Google Scholar] [CrossRef] [PubMed]
- He, X.G.; Lu, W.; Jiang, X.Q.; Cai, J.C.; Zhang, X.W.; Ding, J. Synthesis and biological evaluation of bis and monocarbonate prodrugs of 10-hydroxycamptothecins. Bioorgan. Med. Chem. 2004, 12, 4003–4008. [Google Scholar] [CrossRef]
- Oledzka, E.; Horeglad, P.; Gruszczynska, Z.; Plichta, A.; Nalecz-Jawecki, G.; Sobczak, M. Polylactide conjugates of camptothecin with different drug release abilities. Molecules 2014, 19, 19460–19470. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zhu, Q.C.; Zhang, X.K.; Hua, C.L.; Deng, X.Q.; Zhao, T.F.; Sun, B.H. A liquid chromatography-tandem mass spectrometry method for pharmacokinetics and tissue distribution of a camptothecin quaternary derivative in rats. Fitoterapia 2013, 90, 57–64. [Google Scholar] [CrossRef]
- Du, H.Z.; Huang, Y.; Hou, X.Y.; Quan, X.P.; Jiang, J.W.; Wei, X.H.; Liu, Y.; Li, H.Y.; Wang, P.H.; Zhan, M.X.; et al. Two novel camptothecin derivatives inhibit colorectal cancer proliferation via induction of cell cycle arrest and apoptosis in vitro and in vivo. Eur. J. Pharm. Sci. 2018, 123, 546–559. [Google Scholar] [CrossRef]
- Guo, Z.P.; Zhou, X.Z.; Xu, M.Z.; Tian, H.Y.; Chen, X.S.; Chen, M.W. Dimeric camptothecin-loaded RGD-modified targeted cationic polypeptide-based micelles with high drug loading capacity and redox-responsive drug release capability. Biomater. Sci. 2017, 5, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, H.X.; Liu, Z.J.; Yao, P. Effective enhancement of hypoglycemic effect of insulin by liver-targeted nanoparticles containing cholic acid-modified chitosan derivative. Mol. Pharm. 2016, 13, 2433–2442. [Google Scholar] [CrossRef]
- Chen, D.Q.; Wang, X.; Chen, L.; He, J.X.; Miao, Z.H.; Shen, J.K. Novel liver-specific cholic acid-cytarabine conjugates with potent antitumor activities: Synthesis and biological characterization. Acta Pharmacol. Sin. 2011, 32, 664–672. [Google Scholar] [CrossRef]
- Dong, Z.Q.; Li, Q.; Guo, D.; Shu, Y.; Polli, J.E. Synthesis and evaluation of bile acid-ribavirin conjugates as prodrugs to target the liver. J. Pharm. Sci.-US 2015, 104, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.Y. Enhanced hepatic-targeted delivery via oral administration using nanoliposomes functionalized with a novel DSPE-PEG-cholic acid conjugate. RSC Adv. 2016, 6, 28110–28120. [Google Scholar] [CrossRef]
- Zhang, D.; Li, D.P.; Shang, L.; He, Z.G.; Sun, J. Transporter-targeted cholic acid-cytarabine conjugates for improved oral absorption. Int. J. Pharmaceut. 2016, 511, 161–169. [Google Scholar] [CrossRef]
- Vivian, D.; Polli, J.E. Synthesis and in vitro evaluation of bile acid prodrugs of floxuridine to target the liver. Int. J. Pharmaceut. 2014, 475, 597–604. [Google Scholar] [CrossRef]
- Halilbasic, E.; Claudel, T.; Trauner, M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 2013, 58, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Dawson, P.A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharmacol. 2011, 201, 169–203. [Google Scholar]
- Li, Q.Y.; Gao, Y.; Qiu, W.; Zu, Y.G.; Su, L.; He, W.N.; Deng, X.Q. Synthesis and anti-tumour activity of novel camptothecin-bile acid analogues. Lett. Drug Des. Discov. 2011, 8, 698–703. [Google Scholar] [CrossRef]
- Li, X.N.; Zhao, T.F.; Cheng, D.P.; Chu, C.; Tong, S.Q.; Yan, J.Z.; Li, Q.Y. Synthesis and biological activity of some bile acid-based camptothecin analogues. Molecules 2014, 19, 3761–3776. [Google Scholar] [CrossRef]
- Jarockyte, G.; Dapkute, D.; Karabanovas, V.; Daugmaudis, J.V.; Ivanauskas, F.; Rotomskis, R. 3D cellular spheroids as tools for understanding carboxylated quantum dot behavior in tumors. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 914–923. [Google Scholar] [CrossRef]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Van den Brand, D.; Veelken, C.; Massuger, L.; Brock, R. Penetration in 3D tumor spheroids and explants: Adding a further dimension to the structure-activity relationship of cell-penetrating peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 1342–1349. [Google Scholar] [CrossRef]
- Li, D.Z.; Li, Y.; Chen, X.G.; Zhu, C.G.; Yang, J.; Liu, H.Y.; Pan, X.D. Synthesis and antitumor activity of heterocyclic acid ester derivatives of 20S-camptothecins. Chin. Chem. Lett. 2007, 18, 1335–1338. [Google Scholar] [CrossRef]
- Chu, C.; Xu, J.L.; Cheng, D.P.; Li, X.N.; Tong, S.Q.; Yan, J.Z.; Li, Q.Y. Anti-proliferative and apoptosis-inducing effects of camptothecin-20(s)-O-(2-pyrazolyl-1) acetic ester in human breast tumor MCF-7 cells. Molecules 2014, 19, 4941–4955. [Google Scholar] [CrossRef]
- Zhao, J.C.; Lu, H.X.; Yao, Y.; Ganda, S.; Stenzel, M.H. Length vs. stiffness: Which plays a dominant role in the cellular uptake of fructose-based rod-like micelles by breast cancer cells in 2D and 3D cell culture models? J. Mater. Chem. B 2018, 6, 4223–4231. [Google Scholar] [CrossRef]
- Zeng, Z.; Shen, Z.L.; Zhai, S.; Xu, J.L.; Liang, H.; Li, Q.Y. Transport of curcumin derivatives in Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2017, 117, 123–131. [Google Scholar] [CrossRef]
- Yang, T.C.; Chao, H.F.; Shi, L.S.; Chang, T.C.; Lin, H.C.; Chang, W.L. Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes. Fitoterapia 2014, 93, 239–244. [Google Scholar] [CrossRef]
- He, C.B.; Yin, L.C.; Tang, C.; Yin, C.H. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef]
- Yin, L.C.; Ding, J.Y.; He, C.B.; Cui, L.M.; Tang, C.; Yin, C.H. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Analyte | Organ | Tmax (h) | Cmax (nmol/mL) | AUC0–t (nmol h/mL) | AUC0–∞ (nmol h/mL) | MRT0–∞ (h) |
|---|---|---|---|---|---|---|
| G2 | Plasma | 0.600 ± 0.200 | 0.441 ± 0.084 | 1.987 ± 0.319 | 2.303 ± 0.500 | 4.355 ± 0.645 |
| Heart | 0.600 ± 0.200 | 0.899 ± 0.165 | 3.362 ± 1.065 | 4.589 ± 1.937 | 7.799 ± 3.102 | |
| Liver | 1.000 ± 0.000 | 2.425 ± 0.279 | 16.043 ± 2.783 | 20.093 ± 6.046 | 7.194 ± 2.298 | |
| Spleen | 1.600 ± 1.200 | 1.526 ± 0.140 | 10.371 ± 1.382 | 12.112 ± 2.056 | 6.379 ± 1.352 | |
| Lung | 1.600 ± 1.200 | 1.386 ± 0.269 | 10.180 ± 2.318 | 12.889 ± 4.211 | 7.446 ± 1.748 | |
| Kidney | 1.800 ± 1.166 | 1.096 ± 0.193 | 6.055 ± 1.197 | 6.627 ± 1.740 | 5.049 ± 1.003 | |
| CPT | Plasma | 0.500 ± 0.000 | 0.465 ± 0.084 | 1.556 ± 0.260 | 1.677 ± 0.252 | 3.224 ± 0.578 |
| Heart | 0.600 ± 0.200 | 0.954 ± 0.207 | 2.860 ± 0.983 | 3.639 ± 1.615 | 6.350 ± 2.712 | |
| Liver | 1.000 ± 0.000 | 2.079 ± 0.243 | 6.954 ± 1.319 | 7.459 ± 1.539 | 4.261 ± 0.380 | |
| Spleen | 0.900 ± 0.200 | 1.526 ± 0.195 | 7.562 ± 1.718 | 9.292 ± 2.527 | 6.958 ± 2.948 | |
| Lung | 1.200 ± 0.400 | 1.641 ± 0.325 | 9.798 ± 2.594 | 12.431 ± 4.429 | 7.248 ± 1.775 | |
| Kidney | 0.800 ± 0.245 | 1.328 ± 0.218 | 4.827 ± 1.045 | 5.448 ± 1.193 | 5.322 ± 0.460 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Yu, E.; Yue, H.; Li, Q. Enhanced Liver Targeting of Camptothecin via Conjugation with Deoxycholic Acid. Molecules 2019, 24, 1179. https://doi.org/10.3390/molecules24061179
Xiao L, Yu E, Yue H, Li Q. Enhanced Liver Targeting of Camptothecin via Conjugation with Deoxycholic Acid. Molecules. 2019; 24(6):1179. https://doi.org/10.3390/molecules24061179
Chicago/Turabian StyleXiao, Linxia, Endian Yu, Hanlin Yue, and Qingyong Li. 2019. "Enhanced Liver Targeting of Camptothecin via Conjugation with Deoxycholic Acid" Molecules 24, no. 6: 1179. https://doi.org/10.3390/molecules24061179