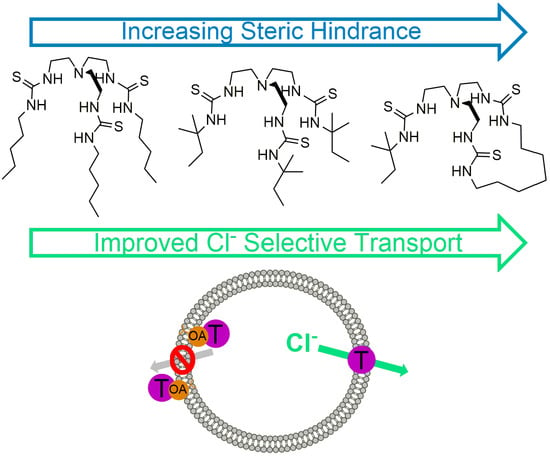
Investigating the Influence of Steric Hindrance on Selective Anion Transport
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Anion Binding Studies
2.3. Anion Transport Studies
2.3.1. Transport Mechanism
2.3.2. Chloride Transport Selectivity
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gale, P.A.; Davis, J.T.; Quesada, R. Anion transport and supramolecular medicinal chemistry. Chem. Soc. Rev. 2017, 46, 2497–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentzsch, A.V.; Emery, D.; Mareda, J.; Nayak, S.K.; Metrangolo, P.; Resnati, G.; Sakai, N.; Matile, S. Transmembrane anion transport mediated by halogen-bond donors. Nat. Commun. 2012, 3, 905. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Valkenier, H.; Judd, L.W.; Brotherhood, P.R.; Hussain, S.; Cooper, J.A.; Jurček, O.; Sparkes, H.A.; Sheppard, D.N.; Davis, A.P. Efficient, non-toxic anion transport by synthetic carriers in cells and epithelia. Nat. Chem. 2016, 8, 24–32. [Google Scholar] [CrossRef]
- Hernando, E.; Capurro, V.; Cossu, C.; Fiore, M.; García-Valverde, M.; Soto-Cerrato, V.; Pérez-Tomás, R.; Moran, O.; Zegarra-Moran, O.; Quesada, R. Small molecule anionophores promote transmembrane anion permeation matching CFTR activity. Sci. Rep. 2018, 8, 2608. [Google Scholar] [CrossRef] [PubMed]
- Valkenier, H.; Akrawi, O.; Jurček, P.; Sleziaková, K.; Lízal, T.; Bartik, K.; Šindelář, V. Fluorinated Bambusurils as Highly Effective and Selective Transmembrane Cl−/HCO3− Antiporters. Chem 2019, 5, 429–444. [Google Scholar] [CrossRef]
- Lee, L.M.; Tsemperouli, M.; Poblador-Bahamonde, A.I.; Benz, S.; Sakai, N.; Sugihara, K.; Matile, S. Anion Transport with Pnictogen Bonds in Direct Comparison with Chalcogen and Halogen Bonds. J. Am. Chem. Soc. 2019, 141, 810–814. [Google Scholar] [CrossRef]
- Fiore, M.; Cossu, C.; Capurro, V.; Picco, C.; Ludovico, A.; Mielczarek, M.; Carreira-Barral, I.; Caci, E.; Baroni, D.; Quesada, R.; et al. Small molecule-facilitated anion transporters in cells for a novel cystic fibrosis therapeutic approach. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Ashcroft, F.M. Ion Channels and Disease; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Vankeerberghen, A.; Cuppens, H.; Cassiman, J.-J. The cystic fibrosis transmembrane conductance regulator: An intriguing protein with pleiotropic functions. J. Cyst. Fibros. 2002, 1, 13–29. [Google Scholar] [CrossRef]
- Vergani, P.; Lockless, S.W.; Nairn, A.C.; Gadsby, D.C. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature 2005, 433, 876–880. [Google Scholar] [CrossRef] [Green Version]
- Gadsby, D.C.; Vergani, P.; Csanády, L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 2006, 440, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Judd, L.W.; Howe, E.N.W.; Withecombe, A.M.; Soto-Cerrato, V.; Li, H.; Busschaert, N.; Valkenier, H.; Pérez-Tomás, R.; Sheppard, D.N.; et al. Nonprotonophoric Electrogenic Cl− Transport Mediated by Valinomycin-like Carriers. Chem 2016, 1, 127–146. [Google Scholar] [CrossRef]
- Soto-Cerrato, V.; Llagostera, E.; Montaner, B.; Scheffer, G.L.; Pérez-Tomás, R. Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem. Pharmacol. 2004, 68, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Tsukimoto, M.; Harada, H.; Ikari, A.; Takagi, K. Involvement of Chloride in Apoptotic Cell Death Induced by Activation of ATP-sensitive P2X7 Purinoceptor. J. Biol. Chem. 2005, 280, 2653–2658. [Google Scholar] [CrossRef]
- Ko, S.K.; Kim, S.K.; Share, A.; Lynch, V.M.; Park, J.; Namkung, W.; Van Rossom, W.; Busschaert, N.; Gale, P.A.; Sessler, J.L.; et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nat. Chem. 2014, 6, 885–892. [Google Scholar] [CrossRef]
- Hosogi, S.; Kusuzaki, K.; Inui, T.; Wang, X.; Marunaka, Y. Cytosolic chloride ion is a key factor in lysosomal acidification and function of autophagy in human gastric cancer cell. J. Cell. Mol. Med. 2014, 18, 1124–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busschaert, N.; Park, S.-H.; Baek, K.-H.; Choi, Y.P.; Park, J.; Howe, E.N.W.; Hiscock, J.R.; Karagiannidis, L.E.; Marques, I.; Félix, V.; et al. A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations. Nat. Chem. 2017, 9, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Gale, P.A. Small-Molecule Uncoupling Protein Mimics: Synthetic Anion Receptors as Fatty Acid-Activated Proton Transporters. J. Am. Chem. Soc. 2016, 138, 16508–16514. [Google Scholar] [CrossRef]
- Clarke, H.J.; Howe, E.N.W.; Wu, X.; Sommer, F.; Yano, M.; Light, M.E.; Kubik, S.; Gale, P.A. Transmembrane Fluoride Transport: Direct Measurement and Selectivity Studies. J. Am. Chem. Soc. 2016, 138, 16515–16522. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. The Chelate, Cryptate and Macrocyclic Effects. Comments Inorg. Chem. 1988, 6, 237–284. [Google Scholar] [CrossRef]
- Supramolecular.org—Online Tools for Supramolecular Chemistry Research and Analysis. Available online: http://supramolecular.org (accessed on 14 February 2019).
- Howe, E.N.W.; Busschaert, N.; Wu, X.; Berry, S.N.; Ho, J.; Light, M.E.; Czech, D.D.; Klein, H.A.; Kitchen, J.A.; Gale, P.A. pH-Regulated Nonelectrogenic Anion Transport by Phenylthiosemicarbazones. J. Am. Chem. Soc. 2016, 138, 8301–8308. [Google Scholar] [CrossRef]
- Jowett, L.A.; Howe, E.N.W.; Soto-Cerrato, V.; Van Rossom, W.; Pérez-Tomás, R.; Gale, P.A. Indole-based perenosins as highly potent HCl transporters and potential anti-cancer agents. Sci. Rep. 2017, 7, 9397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jowett, L.A.; Howe, E.N.W.; Wu, X.; Busschaert, N.; Gale, P.A. New Insights into the Anion Transport Selectivity and Mechanism of Tren-based Tris-(thio)ureas. Chem. Eur. J. 2018, 24, 10475–10487. [Google Scholar] [CrossRef]
- Pressman, B.C. Biological Applications of Ionophores. Annu. Rev. Biochem. 1976, 45, 501–530. [Google Scholar] [CrossRef]
- Mollenhauer, H.H.; James Morré, D.; Rowe, L.D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta, Rev. Biomembr. 1990, 1031, 225–246. [Google Scholar] [CrossRef]
- Dawson, R.E.; Hennig, A.; Weimann, D.P.; Emery, D.; Ravikumar, V.; Montenegro, J.; Takeuchi, T.; Gabutti, S.; Mayor, M.; Mareda, J.; et al. Experimental evidence for the functional relevance of anion–π interactions. Nat. Chem. 2010, 2, 533–538. [Google Scholar] [CrossRef]
- Hill, A.V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem. J. 1913, 7, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






| Receptor | Ka (M−1) for 1:1 Receptor:Anion Complex | ||
|---|---|---|---|
| Ka Cl− a | Ka HCO3− b | Ka H2PO4− b | |
| 1 | 346 | 5010 c | n.d. d |
| 2 | 770 e | 5560 f | n.d. g |
| 3 | 1080 e | 2460 f | n.d. g |
| 4 | 1530 | 1500 | 7460 |
| 5 | 1110 | 2670 | 2600 |
| 6 | 2170 b, e | 4930 h | n.d. g |
| Receptor | EC50 (mol%) a | FGra e | FOA f | FOA/Gra g | ||
|---|---|---|---|---|---|---|
| (a) BSA b | (b) Gra c | (c) OA d | ||||
| 1 | 0.236 | 0.0113 | 0.0214 | 21 | 11 | 1.9 |
| 2 | 0.218 | 0.00748 | 0.0195 | 29 | 11 | 2.6 |
| 3 | 0.175 | 0.00420 | 0.00834 | 42 | 21 | 1.9 |
| 4 | 0.164 | 0.00223 | 0.00970 | 74 | 17 | 4.3 |
| 5 | 0.295 | 0.00483 | 0.0121 | 61 | 24 | 2.5 |
| 6 | 1.900 | 0.0238 | 0.126 | 80 | 15 | 5.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jowett, L.A.; Ricci, A.; Wu, X.; Howe, E.N.W.; Gale, P.A. Investigating the Influence of Steric Hindrance on Selective Anion Transport. Molecules 2019, 24, 1278. https://doi.org/10.3390/molecules24071278
Jowett LA, Ricci A, Wu X, Howe ENW, Gale PA. Investigating the Influence of Steric Hindrance on Selective Anion Transport. Molecules. 2019; 24(7):1278. https://doi.org/10.3390/molecules24071278
Chicago/Turabian StyleJowett, Laura A., Angela Ricci, Xin Wu, Ethan N. W. Howe, and Philip A. Gale. 2019. "Investigating the Influence of Steric Hindrance on Selective Anion Transport" Molecules 24, no. 7: 1278. https://doi.org/10.3390/molecules24071278