Excited State Frequencies of Chlorophyll f and Chlorophyll a and Evaluation of Displacement through Franck-Condon Progression Calculations
Abstract
:1. Introduction
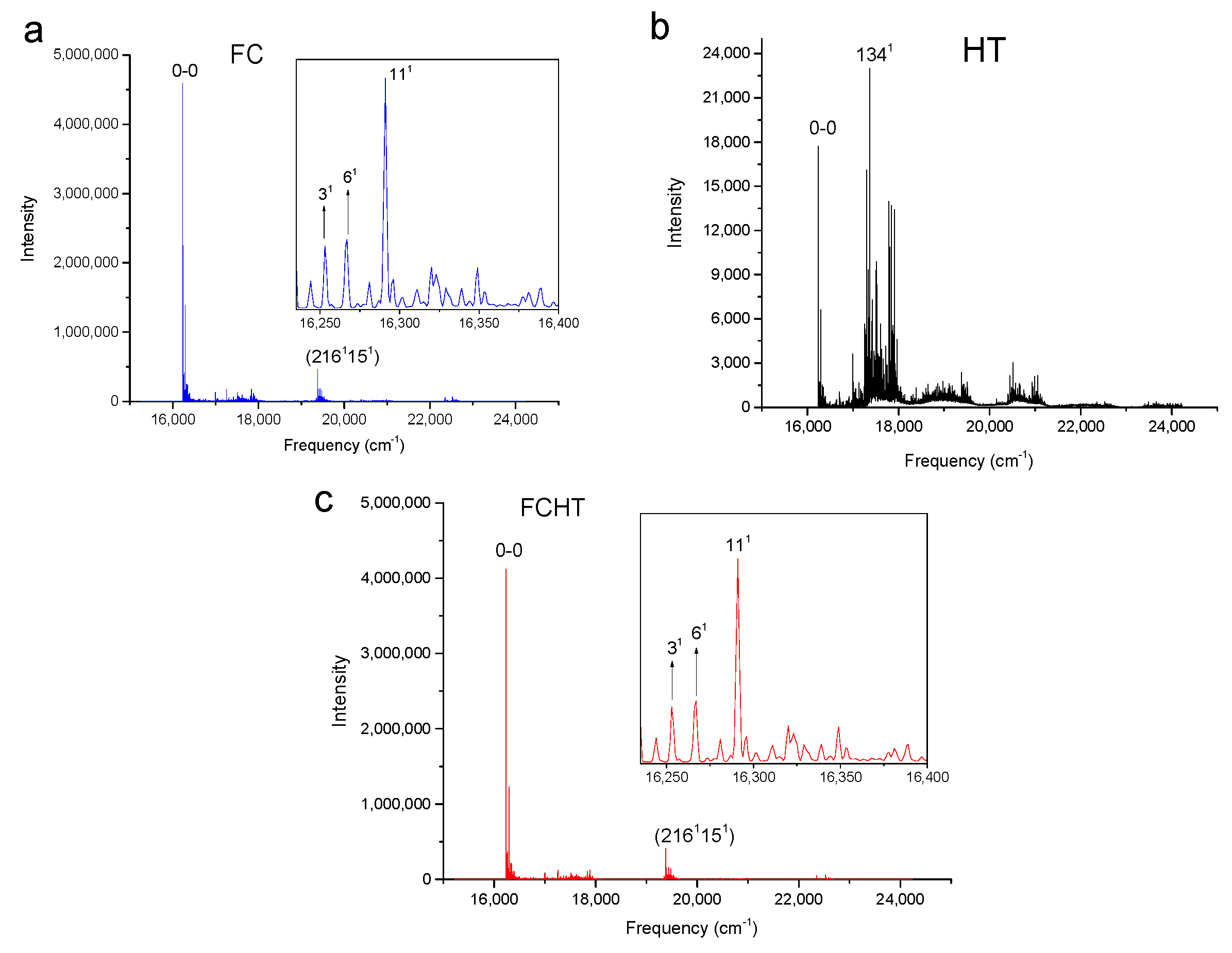
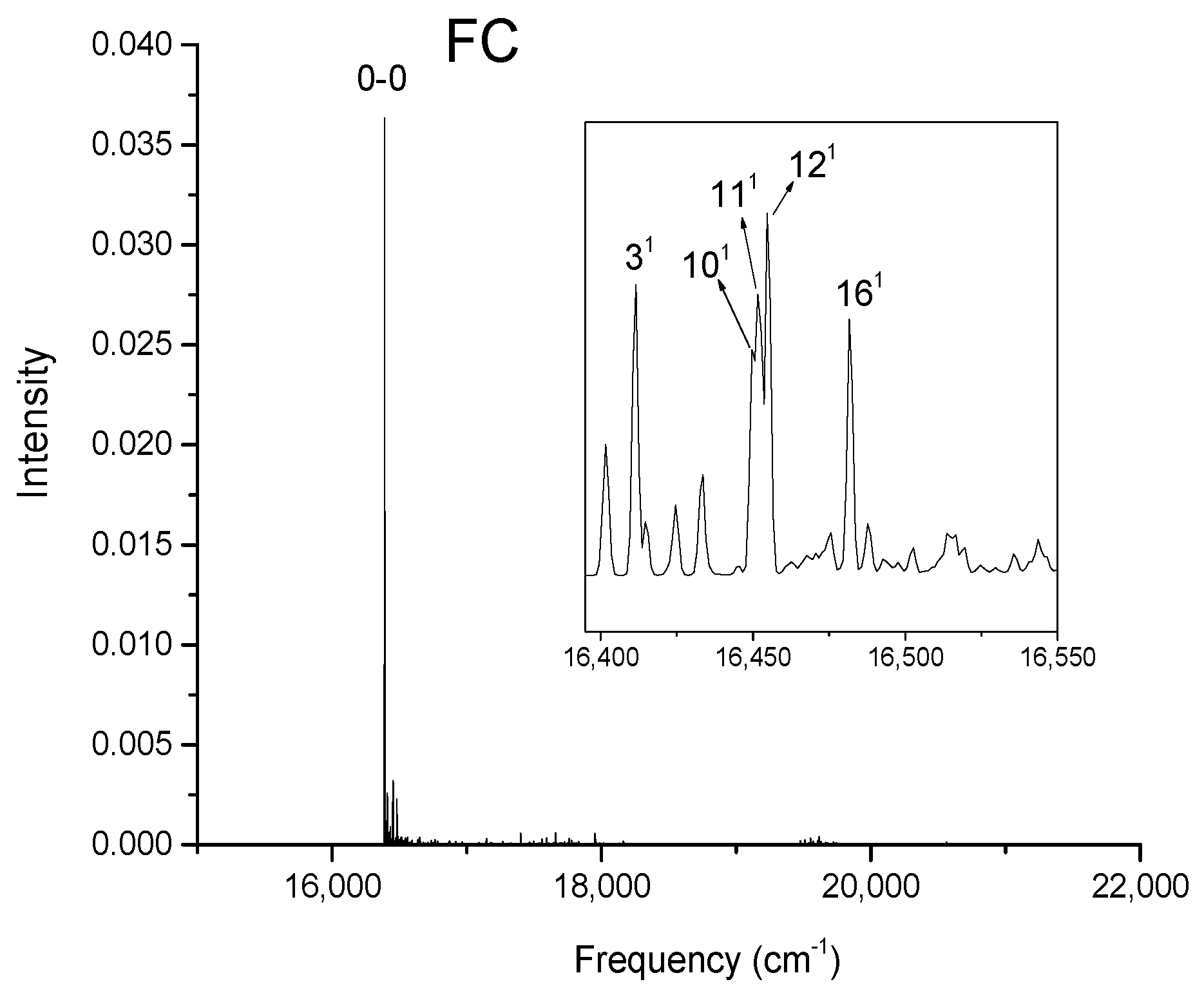
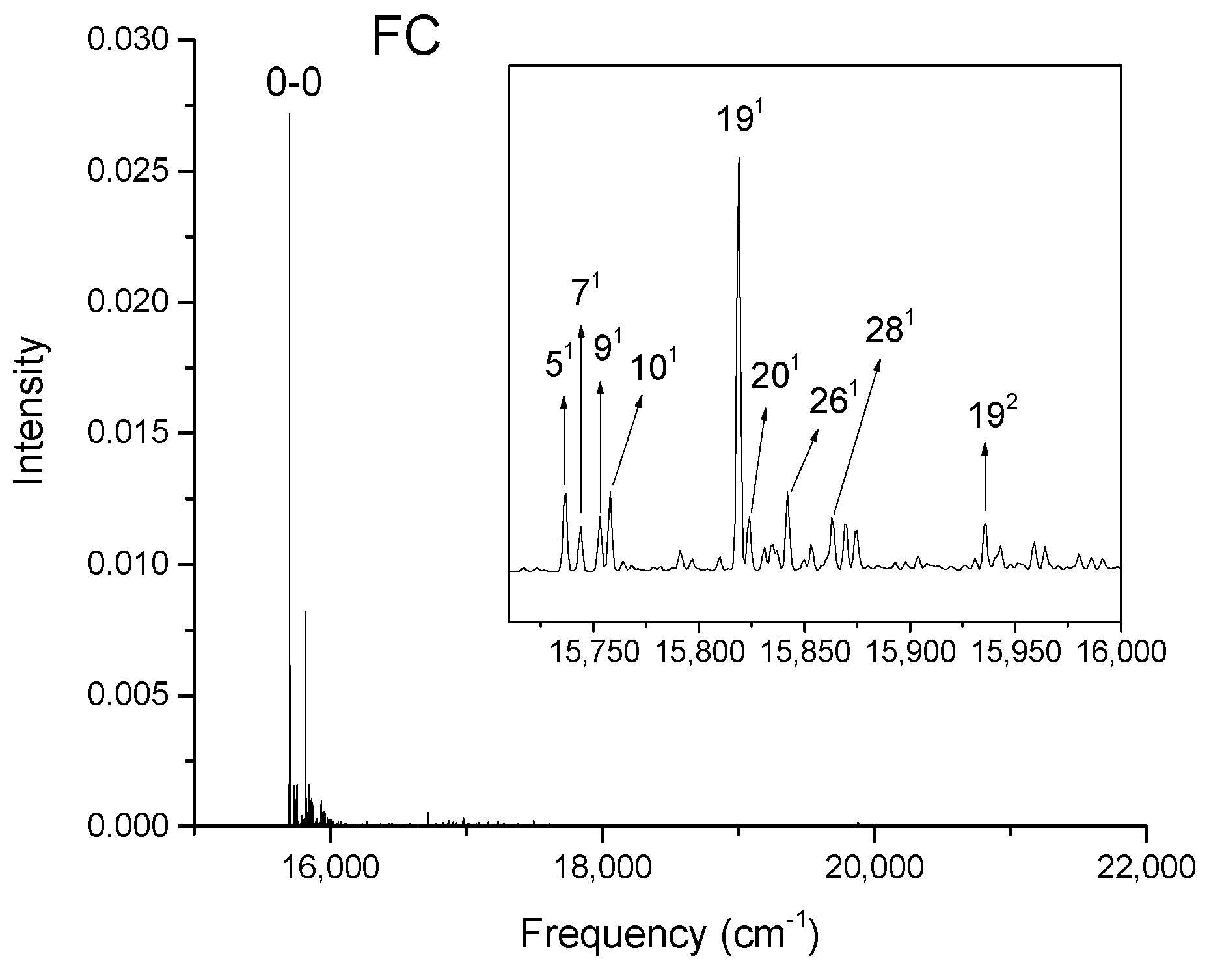
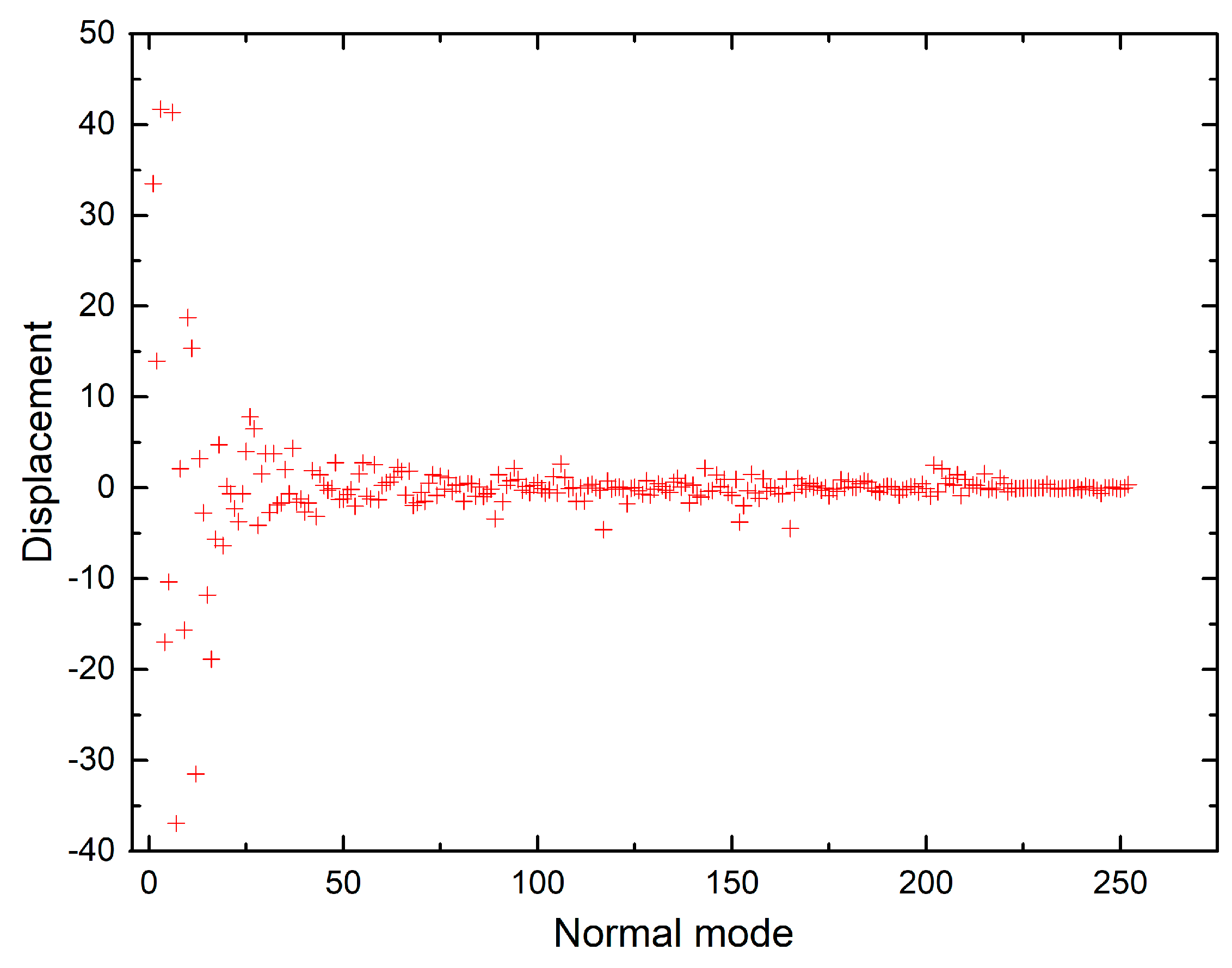
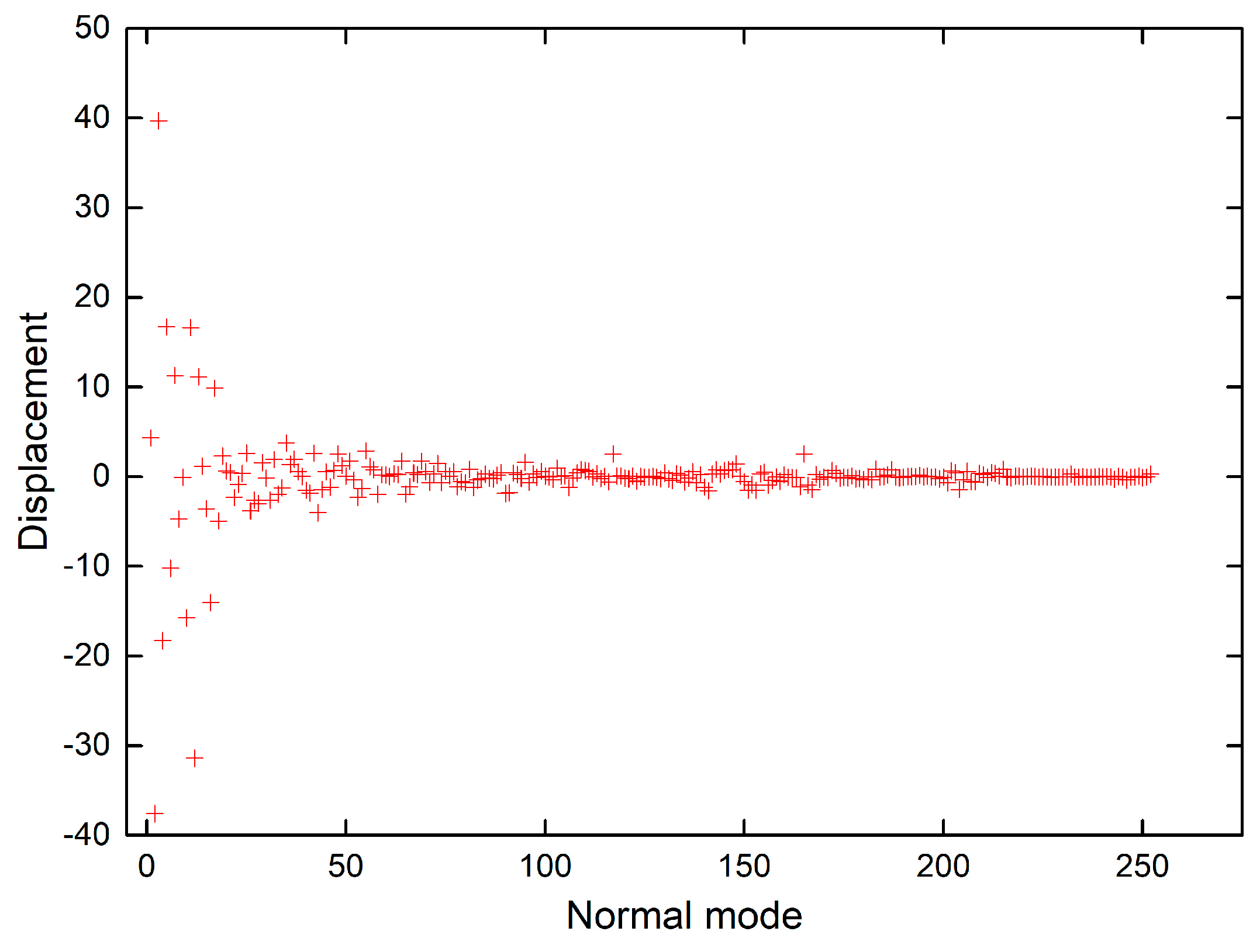
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Melkozernov, A.N.; Blankenship, R.E. Photosynthetic Functions of Chlorophylls. In Chlorophylls and Bacteriochlorophylls Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Amsterdam, The Netherlands, 2006; pp. 397–412. ISBN 978-1-4020-4515-8. [Google Scholar]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Scheer, H. An Overview of Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. In Chlorophylls and Bacteriochlorophylls Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Amsterdam, The Netherlands, 2006; pp. 1–26. ISBN 978-1-4020-4515-8. [Google Scholar]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Voloshin, R.A.; Korol’kova, D.V.; Tomo, T.; Shen, J.-R. Chlorophylls d and f and their role in primary photosynthetic processes of cyanobacteria. Biochemistry 2016, 81, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Nürnberg, D.J.; Morton, J.; Santabarbara, S.; Telfer, A.; Joliot, P.; Antonaru, L.A.; Ruban, A.V.; Cardona, T.; Krausz, E.; Boussac, A.; et al. Photochemistry beyond the red limit in chlorophyll f–containing photosystems. Science 2018, 360, 1210–1213. [Google Scholar] [CrossRef]
- Nabedryk, E.; Leonhard, M.; Mäntele, W.; Breton, J. Fourier Transform Infrared Difference Spectroscopy Shows No Evidence for an Enolization of Chlorophyll a upon Cation Formation either in Vitro or during P700 Photooxidation. Biochemistry 1990, 29, 3242–3247. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Katz, J.J. An Infrared Study of Chlorophyll-Chlorophyll and Chlorophyll-Water Interactions. J. Am. Chem. Soc. 1969, 91, 2661–2677. [Google Scholar] [CrossRef]
- Katz, J.J.; Closs, G.L.; Pennington, F.C.; Thomas, M.R.; Strain, H.H. Infrared Spectra, Molecular Weights, and Molecular Association of Chlorophylls a and b, Methyl Chlorophyllides, and Pheophytins in Various Solvents. J. Am. Chem. Soc. 1963, 85, 3801–3809. [Google Scholar] [CrossRef]
- Weigl, J.W.; Livingston, R. Infrared Spectra of Chlorophyll and Related Compounds1. J. Am. Chem. Soc. 1953, 75, 2173–2176. [Google Scholar] [CrossRef]
- Fujiwara, M.; Tasumi, M. Metal-Sensitive Bands in the Raman and Infrared Spectra of Intact and Metal-Substituted Chlorophyll a. J. Phys. Chem. 1986, 90, 5646–5650. [Google Scholar] [CrossRef]
- Lutz, M. Resonance Raman spectra of chlorophyll in solution. J. Raman Spectrosc. 1974, 2, 497–516. [Google Scholar] [CrossRef]
- Wróbel, D. Resonance Raman spectra of chlorophylls dissolved in liquid crystal matrices. I. The interaction between chlorophylls and a liquid crystalline MBBA + EBBA mixture. Biophys. Chem. 1987, 26, 91–99. [Google Scholar] [CrossRef]
- Diers, J.R.; Zhu, Y.; Blankenship, R.E.; Bocian, D.F. Qy-excitation resonance Raman spectra of chlorophyll a and bacteriochlorophyll c/d aggregates. Effects of peripheral substituents on the low-frequency vibrational characteristics. J. Phys. Chem. 1996, 100, 8573–8579. [Google Scholar] [CrossRef] [PubMed]
- Diers, J.R.; Bocian, D.F. Qy-Excitation Resonance Raman Spectra of Bacteriochlorophyll Observed under Fluorescence-Free Conditions. Implications for Cofactor Structure in Photosynthetic Proteins. J. Am. Chem. Soc. 1995, 117, 6629–6630. [Google Scholar] [CrossRef]
- Donohoe, R.J.; Dyer, R.B.; Swanson, B.I.; Violette, C.A.; Frank, H.A.; Bocian, D.F. Near-Infrared-Excitation Resonance Raman Spectra of the Primary Electron Donor in Photosynthetic Reaction Centers from Rhodobacter sphaeroides. J. Am. Chem. Soc. 1990, 112, 6716–6718. [Google Scholar] [CrossRef]
- Palaniappan, V.; Schenck, C.C.; Bocian, D.F. Low-frequency near-infrared-excitation resonance Raman spectra of (M)H202L mutant reaction centers from Rhodobacter sphaeroides. Implications for the structural, vibronic, and electronic properties of the bacteriochlorin cofactors. J. Phys. Chem. 1995, 99, 17049–17058. [Google Scholar] [CrossRef]
- Palaniappan, V.; Martin, P.C.; Bocian, D.F.; Chynwat, V.; Frank, H.A. Comprehensive Resonance Raman Study of Photosynthetic Reaction Centers from Rhodobacter sphaeroides. Implications for Pigment Structure and Pigment-Protein Interactions. J. Am. Chem. Soc. 1993, 115, 12035–12049. [Google Scholar] [CrossRef]
- Palaniappan, V.; Aldema, M.A.; Frank, H.A.; Bocian, D.F. Qy-Excitation Resonance Raman Scattering from the Special Pair in Rhodobacter sphaeroides Reaction Centers. Implications for Primary Charge Separation. Biochemistry 1992, 31, 11050–11058. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, V.; Bocian, D.F. Near-Infrared-Excitation Resonance Raman Studies of Bacterial Reaction Centers. In The Photosynthetic Bacterial Reaction Center II. Nato ASI Series (Series A: Life Sciences); Breton, J., Verméglio, A., Eds.; Springer: Boston, MA, USA, 1992; pp. 119–126. [Google Scholar]
- Cherepy, N.J.; Shreve, A.P.; Moore, L.J.; Franzen, S.; Boxer, S.G.; Mathies, R.A. Near-infrared resonance raman spectroscopy of the special pair and the accessory bacteriochlorophylls in photosynthetic reaction centers. J. Phys. Chem. 1994, 98, 6023–6029. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Holzwarth, A.R.; Mathies, R.A. Near-Infrared Resonance Raman Spectra of Chloroflexus Aurantiacus Photosynthetic Reaction Centers. Biochemistry 1995, 34, 5288–5293. [Google Scholar] [CrossRef]
- Shreve, A.P.; Cherepy, N.J.; Franzen, S.; Boxer, S.G.; Mathies, R.A. Rapid-flow resonance Raman spectroscopy of bacterial photosynthetic reaction centers. Proc. Natl. Acad. Sci. USA 1991, 88, 11207–11211. [Google Scholar] [CrossRef]
- Mattioli, T.A.; Haley, L.V.; Koningstein, J.A. Resonance Raman spectra of and vibronic coupling in Ni(II) Pheophytin a. Chem. Phys. 1990, 140, 317–329. [Google Scholar] [CrossRef]
- Aronoff, S. The absorption spectra of chlorophyll and related compounds. Chem. Rev. 1950, 47, 175–195. [Google Scholar] [CrossRef]
- Jansen, G.; Noort, M. High resolution spectra of zinc porphin and magnesium porphin in a n-octane matrix at 4.2 K. Effect of the addition of ethanol and other solvents. Spectrochim. Acta Part A Mol. Spectrosc. 1976, 32, 747–753. [Google Scholar] [CrossRef]
- Yang, P.; Qi, D.; You, G.; Shen, W.; Li, M.; He, R. Influence of Duschinsky and Herzberg-Teller effects on S 0→ S 1vibrationally resolved absorption spectra of several porphyrin-like compounds. J. Chem. Phys. 2014, 141, 124304. [Google Scholar] [CrossRef]
- Zamzam, N.; Kaucikas, M.; Nürnberg, D.J.; Rutherford, A.W.; Van Thor, J.J. Femtosecond infrared spectroscopy of chlorophyll f-containing photosystem I. Phys. Chem. Chem. Phys. 2019, 21, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.R. A practical method for the use of curvilinear coordinates in calculations of normal-mode-projected displacements and duschinsky rotation matrices for large molecules. J. Chem. Phys. 2001, 115, 9103–9109. [Google Scholar] [CrossRef]
- Small, G.J. Herzberg-teller vibronic coupling and the duschinsky effect. J. Chem. Phys. 1971, 54, 3300–3306. [Google Scholar] [CrossRef]
- Gall, A.; Pascal, A.A.; Robert, B. Vibrational techniques applied to photosynthesis: Resonance Raman and fluorescence line-narrowing. Biochim. Biophys. Acta Bioenergy 2015, 1847, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Robert, B.; Lutz, M. Proteic Events following Charge Separation in the Bacterial Reaction Center: Resonance Raman Spectroscopy. Biochemistry 1988, 27, 5108–5114. [Google Scholar] [CrossRef]
- Tavitian, B.A.; Nabedryk, E.; Mäntele, W.; Breton, J. Light-induced Fourier transform infrared (FTIR) spectroscopic investigations of primary reactions in photosystem I and photosystem II. FEBS Lett. 1986, 201, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Tomo, T.; Inoue, Y. Fourier transform infrared study of the cation radical of P680 in the photosystem II reaction center: Evidence for charge delocalization on the chlorophyll dimer. Biochemistry 1998, 37, 13614–13625. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Nabedryk, E.; Leibl, W. FTIR study of the primary electron donor of photosystem I (P700) revealing delocalization of the charge in P700+ and localization of the triplet character in 3P700. Biochemistry 1999, 38, 11585–11592. [Google Scholar] [CrossRef]
- Breton, J. Fourier transform infrared spectroscopy of primary electron donors in type I photosynthetic reaction centers. Biochim. Biophys. Acta 2001, 1507, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Inoue, Y.; Satoh, K. FT-IR Studies on the Triplet State of P680 in the Photosystem II Reaction Center: Triplet Equilibrium within a Chlorophyll Dimer. Biochemistry 1993, 32, 7186–7195. [Google Scholar] [CrossRef]
- Groot, M.L.; Pawlowicz, N.P.; van Wilderen, L.J.G.W.; Breton, J.; van Stokkum, I.H.M.; van Grondelle, R. Initial electron donor and acceptor in isolated Photosystem II reaction centers identified with femtosecond mid-IR spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 13087–13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot, M.L.; Breton, J.; Van Wilderen, L.J.G.W.; Dekker, J.P.; Van Grondelle, R. Femtosecond visible/visible and visible/mid-IR pump-probe study of the photosystem II core antenna complex CP47. J. Phys. Chem. B 2004, 108, 8001–8006. [Google Scholar] [CrossRef]
- Di Donato, M.; Van Grondelle, R.; Van Stokkum, I.H.M.; Groot, M.L. Excitation energy transfer in the photosystem II core antenna complex CP43 studied by femtosecond visible/visible and visible/mid-infrared pump probe spectroscopy. J. Phys. Chem. B 2007, 111, 7345–7352. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, K.M.P.; Wang, R.; Hastings, G. Modification of the phylloquinone in the A1 binding site in photosystem I studied using time-resolved FTIR difference spectroscopy and density functional theory. Biochemistry 2006, 45, 1880–1893. [Google Scholar]
- Wang, R.; Parameswaran, S.; Hastings, G. Density functional theory based calculations of the vibrational properties of chlorophyll-a. Vib. Spectrosc. 2007, 44, 357–368. [Google Scholar] [CrossRef]
- O’Malley, P.J. The effect of oxidation and reduction of chlorophyll a on its geometry, vibrational and spin density properties as revealed by hybrid density functional methods. J. Am. Chem. Soc. 2000, 122, 7798–7801. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Fischer, H.; Wenderoth, H. Optisch aktives Hämotricarbonsäureimid aus Chlorophyll. Justus Liebigs Ann. Chem. 1940, 545, 140–147. [Google Scholar] [CrossRef]
- Etinski, M.; Petković, M.; Ristić, M.M.; Marian, C.M. Electron-Vibrational Coupling and Fluorescence Spectra of Tetra-, Penta-, and Hexacoordinated Chlorophylls c1 and c2. J. Phys. Chem. B 2015, 119, 10156–10169. [Google Scholar] [CrossRef]
- Parameswaran, S.; Wang, R.; Hastings, G. Calculation of the vibrational properties of chlorophyll a in solution. J. Phys. Chem. B 2008, 112, 14056–14062. [Google Scholar] [CrossRef]
- Hastings, G.; Wang, R. Vibrational mode frequency calculations of chlorophyll-d for assessing (P740+-P740) FTIR difference spectra obtained using photosystem I particles from Acaryochloris marina. Photosynth. Res. 2008, 95, 55–62. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, H.; Larkum, A.W.D.; Cai, Z.L. Raman properties of chlorophyll d, the major pigment of Acaryochloris marina: Studies using both Raman spectroscopy and density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 527–534. [Google Scholar] [CrossRef]
- Santoro, F.; Lami, A.; Improta, R.; Bloino, J.; Barone, V. Effective method for the computation of optical spectra of large molecules at finite temperature including the Duschinsky and Herzberg-Teller effect: The Qxband of porphyrin as a case study. J. Chem. Phys. 2008, 128. [Google Scholar] [CrossRef]
- Dierksen, M.; Grimme, S. The vibronic structure of electronic absorption spectra of large molecules: A time-dependent density functional study on the influence of “Exact” hartree-fock exchange. J. Phys. Chem. A 2004, 108, 10225–10237. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, W.; Fan, X.; Zheng, X.; Liang, J. Theoretical simulation of the Qx-band absorption and fluorescence spectra of cis-isobacteriochlorin: Including the Duschinsky and Herzberg–Teller effects. Chem. Phys. Lett. 2018, 713, 215–225. [Google Scholar] [CrossRef]
- Santoro, F.; Improta, R.; Lami, A.; Bloino, J.; Barone, V. Effective method to compute Franck-Condon integrals for optical spectra of large molecules in solution. J. Chem. Phys. 2007, 126, 084509. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Mode | Mode frequencies in cm−1 (I) | |||||
|---|---|---|---|---|---|---|
| Chl-a | Chl-f | |||||
| Ground State | Excited State | Mode Number | Ground State | Excited State | Mode Number | |
| ν(C=O) 173-ester | 1857.97 (317.0833) | 1857.41 (315.8875) | 214 | 1856.57 (320.0083) | 1856.55 (318.9635) | 213 |
| νsym(C=O) 131-keto and 133-ester | 1833.18 (211.1643) | 213 | 1837.32 (282.1274) | 212 | ||
| ν(C=O) 133-ester | 1828.18 (135.0542) (with a degree of coupling with the 131-keto) | 1829.35 (144.0902) (with a degree of coupling with the 131-keto) | ||||
| νasym(C=O) 131-keto and 133-ester | 1816.05 (792.9019) | 212 | 1821.06 (625.7565) | 211 | ||
| ν(C=O) 131-keto | 1806.18 (992.1096) (with a degree of coupling with the 133- ester) | 1810.09 (912.2731) (with a degree of coupling with the 133- ester) | ||||
| ν(C=O) 21-formyl | 1785.68 (521.4323) | 1769.05 (541.5630) | 210 | |||
| Vinyl in CH2CH=CH2 at C173 | 1753.38 (1.3698) | 1753.25 (1.3717) | 211 | 1753.14 (1.3259) | 1753.12 (1.2672) | 209 |
| Vinyl at C3 | 1732.52 (17.3765) | 1718.97 (96.2622) | 210 | 1732.89 (31.5954) | 1719.40 (69.5951) | 208 |
| Macrocycle modes | 1698.79 (293.0123) | 1683.44 (79.6537) | 209 | 1689.63 (397.7097) | 1684.46 (26.8216) | 207 |
| 1666.44 (51.7727) | 1672.73 (264.2016) | 208 | 1665.23 (118.5037) | 1668.80 (341.5863) | 206 | |
| 1663.94 (32.1118) | 1649.97 (171.2925) | 207 | 1652.81 (327.7956) | 1657.80 (343.2854) | 205 | |
| 1640.69 (208.8276) | 1637.37 (317.9105) | 206 | 1636.42 (253.8413) | 1635.44 (494.9683) | 204 | |
| 1638.92 (500.0061) | 1618.55 (148.7429) | 205 | 1616.82 (320.7904) | 1609.58 (152.9921) | 203 | |
| 1610.54 (93.0218) | 1604.54 (33.7996) | 204 | 1594.14 (262.3743) | 1582.01 (91.3738) | 202 | |
| 1597.03 (276.4570) | 1575.94 (68.8870) | 203 | 1577.34 (21.7559) | 1567.39 (84.9900) | 201 | |
| 1557.01 (106.3392) | 1550.06 (91.7621) | 202 | 1556.30 (22.8720) | 1551.37 (34.9369) | 200 | |
| Model | Mode frequencies in cm−1 (I) | |||||
|---|---|---|---|---|---|---|
| Chl-a | Chl-f | |||||
| Ground State | Excited State | Mode Number | Ground State | Excited State | Mode Number | |
| ν(C=O) 173-ester | 1844.75 (253.9732) | 1844.21 (253.6782) | 214 | 1843.73 (255.4692) | 1843.28 (255.377) | 213 |
| νsym(C=O) 131-keto and 133-ester | 1811.84 (150.7570) | 213 | 1813.68 (166.3241) | 212 | ||
| ν(C=O) 133-ester | 1804.93 (75.9686) (with a degree of coupling with the 131-keto) | 1806.87 (78.3391) (with a degree of coupling with the 131-keto) | ||||
| νasym(C=O) 131-keto and 133-ester | 1800.32 (688.1421) | 212 | 1802.54 (619.2428) | 211 | ||
| ν(C=O) 131-keto | 1785.77 (959.9775) (with a degree of coupling with the 133-ester) | 1790.34 (907.7671) (with a degree of coupling with the 133-ester) | ||||
| ν(C=O) 21-formyl | 1759.32 (393.4535) | 1735.91 (488.9839) | 210 | |||
| Vinyl in CH2CH=CH2 at C173 | 1730.33 (1.7880) | 1730.20 (1.7956) | 211 | 1730.26 (1.6604) | 1730.06 (1.3955) | 209 |
| Vinyl at C3 | 1700.42 (10.0042) | 1681.87 (24.5299) | 210 | 1698.60 (25.4969) | 1677.08 (81.0752) | 208 |
| Macrocycle modes | 1654.28 (255.6914) | 1639.70 (35.3242) | 209 | 1653.06 (230.3477) | 1638.13 (50.2569) | 207 |
| 1629.30 (14.1135) | 1626.41 (220.3027) | 208 | 1629.98 (17.6638) | 1625.80 (168.9423) | 206 | |
| 1609.28 (54.3863) | 1587.20 (138.3399) | 207 | 1613.48 (227.3701) | 1599.45 (258.5512) | 205 | |
| 1602.48 (132.2188) | 1577.55 (110.8667) | 206 | 1598.51 (65.9290) | 1577.56 (48.6476) | 204 | |
| 1598.75 (153.5159) | 1571.38 (43.2671) | 205 | 1588.30 (162.5164) | 1562.40 (120.3871) | 203 | |
| 1583.42 (100.4916) | 1561.76 (5.0663) | 204 | 1563.88 (79.0122) | 1545.95 (0.7174) | 202 | |
| 1568.51 (25.1269) | 203 | 1549.84 (73.0622) | 201 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamzam, N.; van Thor, J.J. Excited State Frequencies of Chlorophyll f and Chlorophyll a and Evaluation of Displacement through Franck-Condon Progression Calculations. Molecules 2019, 24, 1326. https://doi.org/10.3390/molecules24071326
Zamzam N, van Thor JJ. Excited State Frequencies of Chlorophyll f and Chlorophyll a and Evaluation of Displacement through Franck-Condon Progression Calculations. Molecules. 2019; 24(7):1326. https://doi.org/10.3390/molecules24071326
Chicago/Turabian StyleZamzam, Noura, and Jasper J. van Thor. 2019. "Excited State Frequencies of Chlorophyll f and Chlorophyll a and Evaluation of Displacement through Franck-Condon Progression Calculations" Molecules 24, no. 7: 1326. https://doi.org/10.3390/molecules24071326







