Effects of Isosorbide Incorporation into Flexible Polyurethane Foams: Reversible Urethane Linkages and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FPUFs Based on ISB
2.3. Preparation of Polyurethane (PU) Films Based on ISB
2.4. Free Radical Scavenging of PU Films Based on ISB
2.5. Characterization
3. Results and Discussion
3.1. Physical Properties of FPUFs Based on ISB
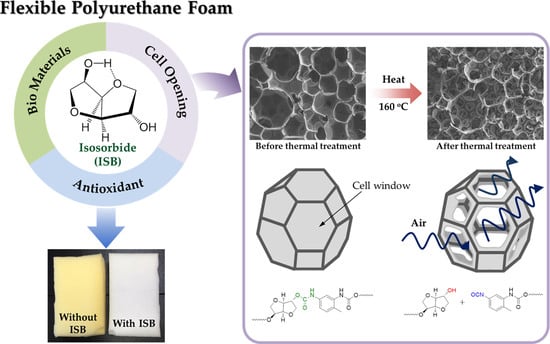
3.2. Cell Opening by the Reversibility of Urethane Units Based on ISB in FPUFs
3.3. Antioxidant Activity of ISB-containing FPUFs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Szycher, M. Szycher’s Handbook of Polyurethanes; CRC Press: New York, NY, USA, 2012; pp. 183–192. [Google Scholar]
- Latinwo, G.K.; Aribike, D.S.; Susu, A.A.; Kareem, S.A. Effects of different filler treatments on the morphology and mechanical properties of flexible polyurethane foam composites. Nat. Sci. 2010, 8, 23–31. [Google Scholar] [CrossRef]
- Konig, A.; Fehrenbacher, U.; Hirth, T.; Kroke, E. Flexible Polyurethane Foam with the Flame-retardant Melamine. J. Cell. Plast. 2008, 44, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, J.; Hu, Z.; Zhu, F.; Huang, Y. Correlation between the acoustic and porous cell morphology of polyurethane foam: Effect of interconnected porosity. Mater. Des. 2012, 41, 319–325. [Google Scholar] [CrossRef]
- Kattiyaboot, T.; Thongpin, C. Effect of Natural Oil Based Polyols on the Properties of Flexible Polyurethane Foams Blown by Distilled Water. Energy Procedia 2016, 89, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Verdejo, R.; Stämpfli, R.; Alvarez-Lainez, M.; Mourad, S.; Rodriguez-Perez, M.A.; Brühwiler, P.A.; Shaffer, M. Enhanced acoustic damping in flexible polyurethane foams filled with carbon nanotubes. Compos. Sci. Technol. 2009, 69, 1564–1569. [Google Scholar] [CrossRef]
- Zaretsky, E.; Asaf, Z.; Ran, E.; Aizik, F. Impact response of high density flexible polyurethane foam. Int. J. Impact Eng. 2012, 39, 1–7. [Google Scholar] [CrossRef]
- Gwon, J.G.; Kim, S.K.; Kim, J.H. Sound absorption behavior of flexible polyurethane foams with distinct cellular structures. Mater. Des. 2016, 89, 448–454. [Google Scholar] [CrossRef]
- Lin, Y.; Hsieh, F. Water-blown flexible polyurethane foam extended with biomass materials. J. Appl. Polym. Sci. 1997, 65, 695–703. [Google Scholar] [CrossRef]
- Hodlur, R.; Rabinal, M. Self assembled graphene layers on polyurethane foam as a highly pressure sensitive conducting composite. Compos. Sci. Technol. 2014, 90, 160–165. [Google Scholar] [CrossRef]
- Zhang, X.; Davis, H.; Macosko, C. A new cell opening mechanism in flexible polyurethane foam. J. Cell. Plast. 1999, 35, 458–476. [Google Scholar] [CrossRef]
- Yasunaga, K.; Neff, R.; Zhang, X.; Macosko, C. Study of cell opening in flexible polyurethane foam. J. Cell. Plast. 1996, 32, 427–448. [Google Scholar] [CrossRef]
- Herrington, R.; Hock, K. Flexible Polyurethane Foams; Dow Chemical Company: Midland, TX, USA, 1997. [Google Scholar]
- Neff, R.A.; Macosko, C.W. Simultaneous measurement of viscoelastic changes and cell opening during processing of flexible polyurethane foam. Rheol. Acta 1996, 35, 656–666. [Google Scholar] [CrossRef]
- Singh, A.P.; Bhattacharya, M. Viscoelastic changes and cell opening of reacting polyurethane foams from soy oil. Polym. Eng. Sci. 2004, 44, 1977–1986. [Google Scholar] [CrossRef]
- Li, W.; Ryan, A.J.; Meier, I.K. Effect of chain extenders on the morphology development in flexible polyurethane foam. Macromolecules 2002, 35, 6306–6312. [Google Scholar] [CrossRef]
- Aneja, A.; Wilkes, G.L. Exploring macro-and microlevel connectivity of the urea phase in slabstock flexible polyurethane foam formulations using lithium chloride as a probe. Polymer 2002, 43, 5551–5561. [Google Scholar] [CrossRef]
- Park, J.H.; Minn, K.S.; Lee, H.R.; Yang, S.H.; Yu, C.B.; Pak, S.Y.; Oh, C.S.; Song, Y.S.; Kang, Y.J.; Youn, J.R. Cell openness manipulation of low density polyurethane foam for efficient sound absorption. J. Sound Vibrat. 2017, 406, 224–236. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdał, D.; Zaccheria, F.; Ravasio, N. The role of catalysis in the synthesis of polyurethane foams based on renewable raw materials. Catal. Today 2014, 223, 148–156. [Google Scholar] [CrossRef]
- Zhang, X.; Macosko, C.; Davis, H.; Nikolov, A.; Wasan, D. Role of silicone surfactant in flexible polyurethane foam. J. Colloid Interface Sci. 1999, 215, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Rossmy, G.; Kollmeier, H.J.; Lidy, W.; Schator, H.; Wiemann, M. Mechanism of the Stabilization of Flexible Polyether Polyurethane Foams by Silicone-Based Surfactants. J. Cell. Plast. 2016, 17, 319–327. [Google Scholar] [CrossRef]
- Rath, A.; Apichatachutapan, W.; Gummaraju, R.; Neff, R.; Heyman, D. Effect of average particle size and distribution on the performance of copolymer polyols. J. Cell. Plast. 2003, 39, 387–415. [Google Scholar] [CrossRef]
- Ahn, W.; Lee, J.-M. Open-Cell Rigid Polyurethane Foam Using Lithium Salt of 12-Hydroxystearic acid as a Cell Opening Agent. Polym. Korea 2018, 42, 919–924. [Google Scholar] [CrossRef]
- Dounis, D.V.; Wilkes, G.L. Structure-property relationships of flexible polyurethane foams. Polymer 1997, 38, 2819–2828. [Google Scholar] [CrossRef]
- Zou, J.; Lei, Y.; Liang, M.; Zou, H. Effect of nano-montmorillonite as cell opener on cell morphology and resilient performance of slow-resilience flexible polyurethane foams. J. Polym. Res. 2015, 22, 201. [Google Scholar] [CrossRef]
- Vaughan, B.R.; Wilkes, G.L.; Dounis, D.V.; McLaughlin, C. Effect of vegetable-based polyols in unimodal glass-transition polyurethane slabstock viscoelastic foams and some guidance for the control of their structure-property behavior. I. J. Appl. Polym. Sci. 2011, 119, 2683–2697. [Google Scholar] [CrossRef]
- Javni, I.; Song, K.; Lin, J.; Petrovic, Z.S. Structure and properties of flexible polyurethane foams with nano- and micro-fillers. J. Cell. Plast. 2011, 47, 357–372. [Google Scholar] [CrossRef]
- Kaushiva, B.D.; Dounis, D.V.; Wilkes, G.L. Influences of copolymer polyol on structural and viscoelastic properties in molded flexible polyurethane foams. J. Appl. Polym. Sci. 2000, 78, 766–786. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Wendt, B.L. Design and formulation of soybean oil derived flexible polyurethane foams and their underlying polymer structure/property relationships. Polymer 2013, 54, 2511–2520. [Google Scholar] [CrossRef]
- Ugarte, L.; Saralegi, A.; Fernández, R.; Martín, L.; Corcuera, M.A.; Eceiza, A. Flexible polyurethane foams based on 100% renewably sourced polyols. Ind. Crop. Prod. 2014, 62, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Prociak, A.; Malewska, E.; Kurańska, M.; Bąk, S.; Budny, P. Flexible polyurethane foams synthesized with palm oil-based bio-polyols obtained with the use of different oxirane ring opener. Ind. Crop. Prod. 2018, 115, 69–77. [Google Scholar] [CrossRef]
- Lan, Z.; Daga, R.; Whitehouse, R.; McCarthy, S.; Schmidt, D. Structure–properties relations in flexible polyurethane foams containing a novel bio-based crosslinker. Polymer 2014, 55, 2635–2644. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Rusu, D.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Development of water-blown bio-based thermoplastic polyurethane foams using bio-derived chain extender. J. Appl. Polym. Sci. 2013, 128, 292–303. [Google Scholar] [CrossRef]
- Oltmanns, J.U.; Palkovits, S.; Palkovits, R. Kinetic investigation of sorbitol and xylitol dehydration catalyzed by silicotungstic acid in water. Appl. Catal. A Gen. 2013, 456, 168–173. [Google Scholar] [CrossRef]
- Zou, J.; Cao, D.; Tao, W.; Zhang, S.; Cui, L.; Zeng, F.; Cai, W. Sorbitol dehydration into isosorbide over a cellulose-derived solid acid catalyst. RSC Adv. 2016, 6, 49528–49536. [Google Scholar] [CrossRef]
- Fleche, G.; Huchette, M. Isosorbide. Preparation, properties and chemistry. Starch-Stärke 1986, 38, 26–30. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Dussenne, C.; Delaunay, T.; Wiatz, V.; Wyart, H.; Suisse, I.; Sauthier, M. Synthesis of isosorbide: An overview of challenging reactions. Green Chem. 2017, 19, 5332–5344. [Google Scholar] [CrossRef]
- Kasmi, N.; Roso, M.; Hammami, N.; Majdoub, M.; Boaretti, C.; Sgarbossa, P.; Vianello, C.; Maschio, G.; Modesti, M.; Lorenzetti, A. Microwave-assisted synthesis of isosorbide-derived diols for the preparation of thermally stable thermoplastic polyurethane. Des. Monomers Polym. 2017, 20, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Smiga-Matuszowicz, M.; Janicki, B.; Jaszcz, K.; Lukaszczyk, J.; Kaczmarek, M.; Lesiak, M.; Sieron, A.L.; Simka, W.; Mierzwinski, M.; Kusz, D. Novel bioactive polyester scaffolds prepared from unsaturated resins based on isosorbide and succinic acid. Mater. Sci. Eng. C 2014, 45, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2016, 5, 774–783. [Google Scholar] [CrossRef]
- Park, S.-A.; Choi, J.; Ju, S.; Jegal, J.; Lee, K.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Copolycarbonates of bio-based rigid isosorbide and flexible 1,4-cyclohexanedimethanol: Merits over bisphenol-A based polycarbonates. Polymer 2017, 116, 153–159. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Noordover, B.A.; van Staalduinen, V.G.; Duchateau, R.; Koning, C.E.; van Benthem, R.A.; Mak, M.; Heise, A.; Frissen, A.E.; van Haveren, J. Co-and terpolyesters based on isosorbide and succinic acid for coating applications: Synthesis and characterization. Biomacromolecules 2006, 7, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Ravey, M.; Pearce, E.M. Flexible polyurethane foam. I. Thermal decomposition of a polyether-based, water-blown commercial type of flexible polyurethane foam. J. Appl. Polym. Sci. 1997, 63, 47–74. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K. Blocked isocyanates: From analytical and experimental considerations to non-polyurethane applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef]
- Nasar, A.S.; Kalaimani, S. Synthesis and studies on forward and reverse reactions of phenol-blocked polyisocyanates: An insight into blocked isocyanates. RSC Adv. 2016, 6, 76802–76812. [Google Scholar] [CrossRef]
- Kalaimani, S.; Nasar, A.S. Catalysis of deblocking and cure reactions of easily cleavable phenol blocked polyisocyanates with poly(polytetrahydrofuran carbonate) diol. Eur. Polym. J. 2017, 91, 221–231. [Google Scholar] [CrossRef]
- Elwell, M.J.; Mortimer, S.; Ryan, A.J. A synchrotron SAXS study of structure development kinetics during the reactive processing of flexible polyurethane foam. Macromolecules 1994, 27, 5428–5439. [Google Scholar] [CrossRef]
- Blair, G.R.; McEvoy, J.; de Priamus, M.R.; Dawe, B.; Pask, R.; Wright, C. The Effect of Visible Light on the Variability of Flexible Foam Compression Sets; Centre of the Polyurethane Industry, American Chemistry Council: Washington, DC, USA, 2007. [Google Scholar]
- Newman, C.R.; Forciniti, D. Modeling the ultraviolet photodegradation of rigid polyurethane foams. Ind. Eng. Chem. Res. 2001, 40, 3346–3352. [Google Scholar] [CrossRef]
- Jung, B.-O.; Chung, S.-J.; Lee, S.B. Preparation and characterization of eugenol-grafted chitosan hydrogels and their antioxidant activities. J. Appl. Polym. Sci. 2006, 99, 3500–3506. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andaloussi, S.; Langlois, V.; Renard, E. Antibacterial and antioxidant photoinitiated epoxy co-networks of resorcinol and eugenol derivatives. Mater. Today Commun. 2017, 12, 19–28. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Suminokura, T.; Sasaki, T.; Aoki, N. Effect of the cell membrane on mechanical properties of flexible polyurethane foams. Jpn. J. Appl. Phys. 1968, 7, 330. [Google Scholar] [CrossRef]
- Gabbard, J.D. Flexible Water-Blown Polyurethane Foams. U.S. Patent 5,624,968, 29 April 1997. [Google Scholar]
- Wang, W.; Gong, W.; Zheng, B. Preparation of low-density polyethylene foams with high rebound resilience by blending with polyethylene-octylene elastomer. Polym. Eng. Sci. 2013, 53, 2527–2534. [Google Scholar] [CrossRef]
- Li, F.; Hou, J.; Zhu, W.; Zhang, X.; Xu, M.; Luo, X.; Ma, D.; Kim, B.K. Crystallinity and morphology of segmented polyurethanes with different soft-segment length. J. Appl. Polym. Sci. 1996, 62, 631–638. [Google Scholar] [CrossRef]
- Indennidate, L.; Cannoletta, D.; Lionetto, F.; Greco, A.; Maffezzoli, A. Nanofilled polyols for viscoelastic polyurethane foams. Polym. Int. 2010, 59, 486–491. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, T.; Li, L.; Zhang, J.; Liu, X.; Ke, X.; Zhang, A. Hydrogen bond breaking of TPU upon heating: Understanding from the viewpoints of molecular movements and enthalpy. RSC Adv. 2015, 5, 31153–31165. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Zhao, J.; Zhang, Z.; Zhang, J.; Yang, W. Crystallizable and tough aliphatic thermoplastic poly (ether urethane) s synthesized through a non-isocyanate route. RSC Adv. 2014, 4, 43406–43414. [Google Scholar] [CrossRef]
- Allan, D.; Daly, J.; Liggat, J.J. Thermal volatilisation analysis of TDI-based flexible polyurethane foam. Polym. Degrad. Stab. 2013, 98, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Geoffrey, P. Plastics Additives: An AZ Reference; Chapman & Hall Publisher: London, UK, 1998; pp. 55–107. [Google Scholar]
- Bolgar, M.; Hubball, J.; Groeger, J.; Meronek, S. Handbook for the Chemical Analysis of Plastic and Polymer Additives; CRC Press: New York, NY, USA, 2007; p. 61. [Google Scholar]
- Hagberg, E.; Rockafellow, E.M.; Smith, B.; Stensrud, K.F. Hydrogenation of Isohexide Products for Improved Color. U.S. Patent 9,321,784, 26 April 2016. [Google Scholar]
- Matsubara, H.; Suzuki, S.; Hirano, S. An ab initio and DFT study of the autoxidation of THF and THP. Org. Biomol. Chem. 2015, 13, 4686–4692. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.; Tauber, K.; Fuchs, M.; Schmidt, N.G.; Rajagopalan, A.; Faber, K.; Fabian, W.M.; Pfeffer, J.; Haas, T.; Kroutil, W. Aerobic oxidation of isosorbide and isomannide employing TEMPO/laccase. Green Chem. 2014, 16, 2117–2121. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |














| Sample Code | PUF-I0 | PUF-I1 | PUF-I2 | PUF-I3 | PUF-I4 | PUF-I5 |
|---|---|---|---|---|---|---|
| (Composition by wt.) | ||||||
| Polyol part | ||||||
| TF-3000 | 100 | 99.0 | 98.0 | 97.0 | 96.0 | 95.0 |
| ISB | - | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 |
| L-580 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| A-1 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| 33-LV | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| DBTDL | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Distilled water | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Isocyanate part | ||||||
| TDI-80 | 37.7 | 38.8 | 39.9 | 41.0 | 42.1 | 43.2 |
| Isocyanate index | 100 | 100 | 100 | 100 | 100 | 100 |
| Sample Code | PUF-I0 | PUF-I1 | PUF-I2 | PUF-I3 | PUF-I4 | PUF-I5 |
|---|---|---|---|---|---|---|
| CT (s) | 11 | 11 | 13 | 14 | 16 | 17 |
| RT (s) | 72 | 79 | 79 | 76 | 76 | 76 |
| GT (s) | 93 | 101 | 98 | 90 | 86 | 76 |
| Density (kg/m3) | 31 | 31 | 30 | 29 | 30 | 31 |
| Sample Code | PUF-I0 | PUF-I1 | PUF-I2 | PUF-I3 | PUF-I4 | PUF-I5 |
|---|---|---|---|---|---|---|
| Tg (°C) | −36.7 | −37.8 | −37.7 | −37.4 | −31.4 | −31.2 |
| Sample Code | PUF-I0 | PUF-I1 | PUF-I2 | PUF-I3 | PUF-I4 | PUF-I5 |
|---|---|---|---|---|---|---|
| T5% (a) (°C) | 230.2 | 228.5 | 225.3 | 223.7 | 221.3 | 221.3 |
| T10% (b) (°C) | 250.2 | 248.6 | 246.2 | 243.0 | 239.5 | 238.2 |
| Tmax1 (c) (°C) | 273.3 | 274.3 | 274.3 | 274.3 | 273.3 | 274.3 |
| Tmax2 (d) (°C) | 359.7 | 357.8 | 357.8 | 358.7 | 360.5 | 355.8 |
| Sample Code | PUF-I0 | PUF-I1 | PUF-I2 | PUF-I3 | PUF-I4 | PUF-I5 |
|---|---|---|---|---|---|---|
| YI | 46.4 | 45.3 | 35.0 | 29.2 | 23.6 | 19.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-R.; Liang, J.-Y.; Ryu, H.; Song, G.-S.; Lee, D.-S. Effects of Isosorbide Incorporation into Flexible Polyurethane Foams: Reversible Urethane Linkages and Antioxidant Activity. Molecules 2019, 24, 1347. https://doi.org/10.3390/molecules24071347
Shin S-R, Liang J-Y, Ryu H, Song G-S, Lee D-S. Effects of Isosorbide Incorporation into Flexible Polyurethane Foams: Reversible Urethane Linkages and Antioxidant Activity. Molecules. 2019; 24(7):1347. https://doi.org/10.3390/molecules24071347
Chicago/Turabian StyleShin, Se-Ra, Jing-Yu Liang, Hoon Ryu, Gwang-Seok Song, and Dai-Soo Lee. 2019. "Effects of Isosorbide Incorporation into Flexible Polyurethane Foams: Reversible Urethane Linkages and Antioxidant Activity" Molecules 24, no. 7: 1347. https://doi.org/10.3390/molecules24071347