Investigation of the Role of Nano-Titanium on Corrosion and Thermal Performance of Structural Concrete with Macro-Encapsulated PCM
Abstract
:1. Introduction
2. Results and Discussion
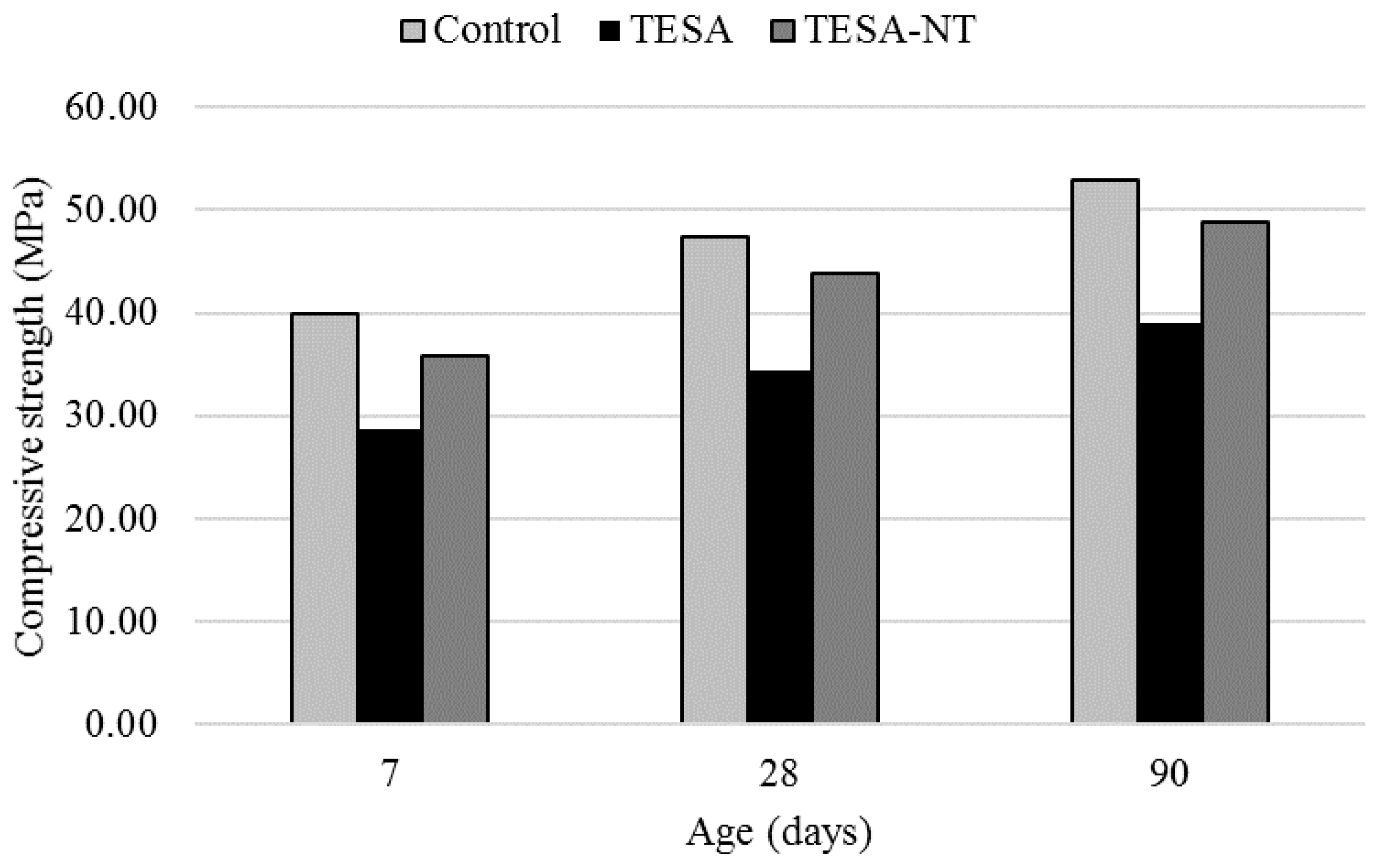
2.1. Compressive Strength
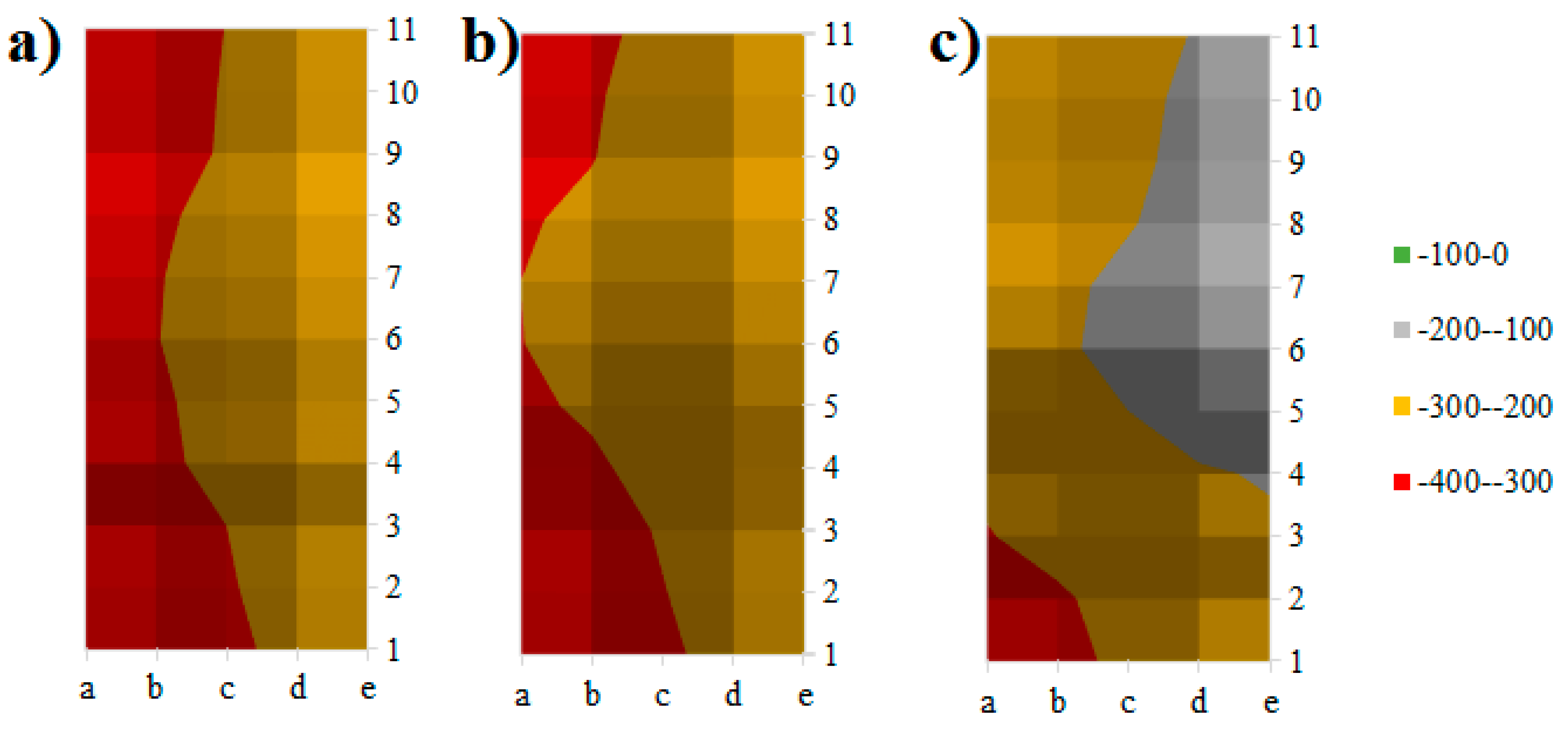
2.2. Corrosion Test
2.3. Thermal Performance
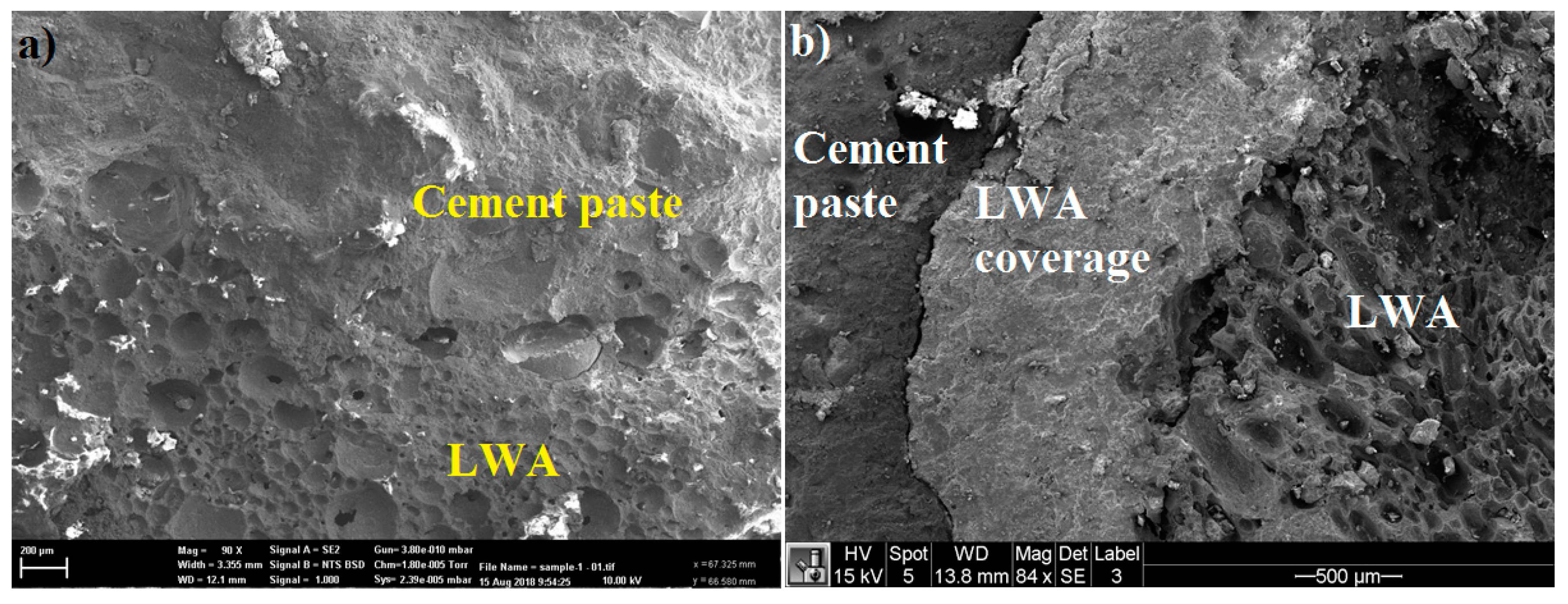
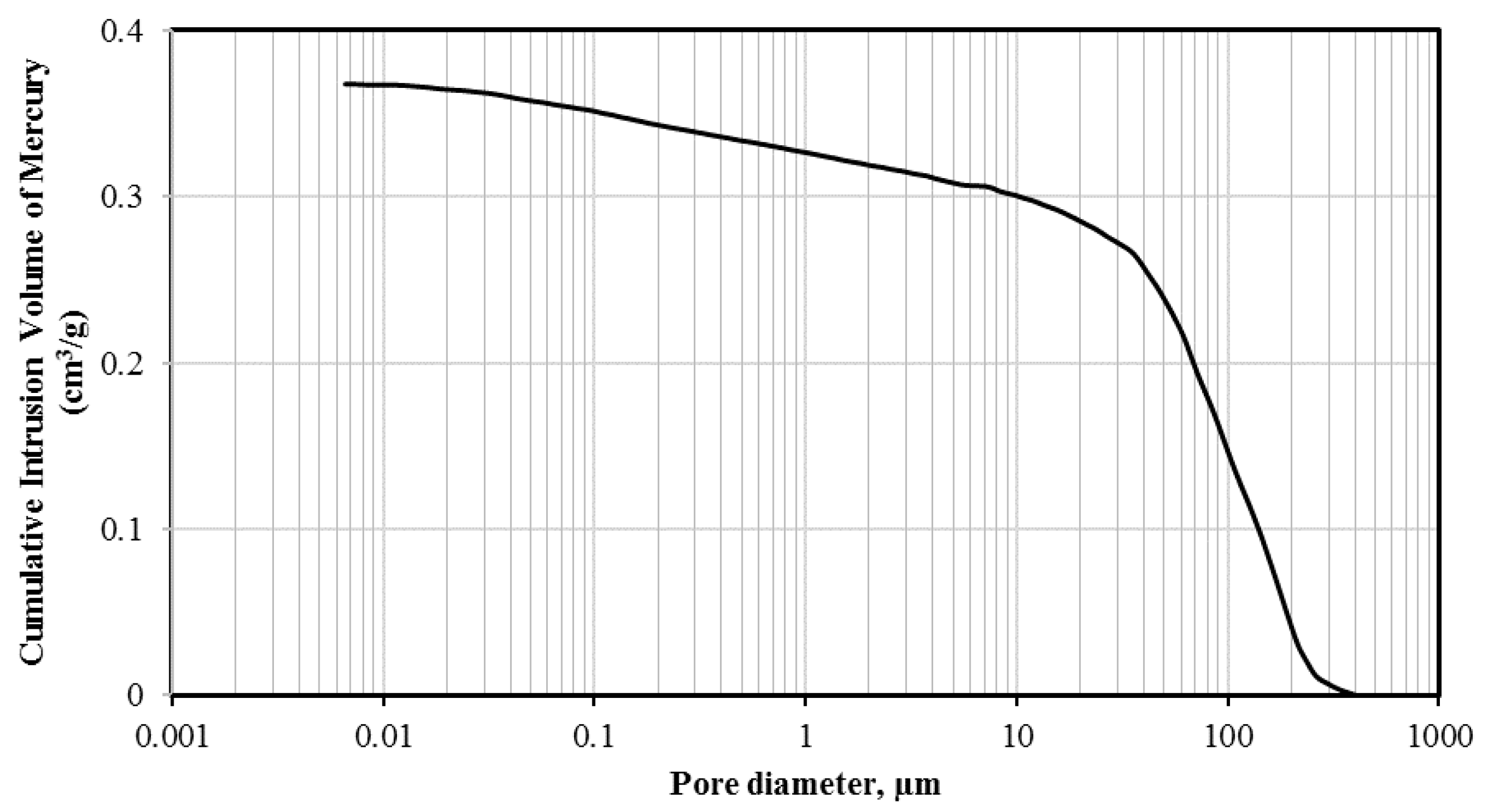
2.4. Microstructure Properties
3. Experimental Program
3.1. Materials
3.2. Mix Proportions and Fabrication of TESA
3.3. Testing Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castella, A.; Barreneche, C.; Graciaa, A.; Fernándezb, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Alkan, C. Enthalpy of melting and solidification of sulfonated paraffins as phase change materials for thermal energy storage. Thermochim. Acta 2006, 451, 126–130. [Google Scholar]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Bilgin, C. Micro/nano encapsulation of some paraffin eutectic mixtures with poly(methyl methacrylate) shell: Preparation, characterization and latent heat thermal energy storage properties. Appl. Energy 2014, 136, 217–227. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyam, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Memon, S.A.; Cui, H.; Lo, T.Y.; Li, Q. Development of structural-functional integrated concrete with macro-encapsulated PCM for thermal energy storage. Appl. Energy 2015, 150, 245–257. [Google Scholar] [CrossRef]
- Jin, X.; Medina, M.A.; Zhang, X. On the importance of the location of PCMs in building walls for enhanced thermal performance. Appl. Energy 2013, 106, 72–78. [Google Scholar] [CrossRef]
- Cui, H.; Memon, S.A.; Liu, R. Development, mechanical properties and numerical simulation of macro encapsulated thermal energy storage concrete. Energy Build. 2015, 96, 162–174. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Kastiukas, G.; Zhou, X.; Castro-Gomes, J. Development and optimisation of phase change material-impregnated lightweight aggregates for geopolymer composites made from aluminosilicate rich mud and milled glass powder. Constr. Build. Mater. 2016, 110, 201–210. [Google Scholar] [CrossRef]
- Waqas, A.; Ud Din, Z. Phase change material (PCM) storage for free cooling of buildings—A review. Renew. Sustain. Energy Rev. 2013, 18, 607–625. [Google Scholar] [CrossRef]
- Madandoust, R.; Mohseni, E.; Mousavi, S.Y.; Namnevis, M. An experimental investigation on the durability of self-compacting mortar containing nano-SiO2, nano-Fe2O3 and nano-CuO. Constr. Build. Mater. 2015, 86, 44–50. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Miyandehi, B.M.; Naseri, F.; Ozbakkaloglu, T.; Jafari, F.; Mohseni, E. Effect of SnO2, ZrO2, and CaCO3 nanoparticles on water transport and durability properties of self-compacting mortar containing fly ash: Experimental observations and ANFIS predictions. Constr. Build. Mater. 2018, 158, 823–834. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Mohseni, E.; AliYazdi, M.; Sarker, P.; Ranjbar, M.M. Effect of nano-CuO and fly ash on the properties of self-compacting mortar. Constr. Build. Mater. 2015, 94, 758–766. [Google Scholar] [CrossRef]
- Mohseni, E.; Khotbehsara, M.M.; Naseri, F.; Monazami, M.; Sarker, P. Polypropylene fiber reinforced cement mortars containing rice husk ash and nano-alumina. Constr. Build. Mater. 2016, 111, 429–439. [Google Scholar] [CrossRef]
- Mohseni, E.; Miyandehi, B.M.; Yang, J.; Yazdi, M.A. Single and combined effects of nano-SiO2, nano-Al2O3 and nano-TiO2 on the mechanical, rheological and durability properties of self-compacting mortar containing fly ash. Constr. Build. Mater. 2015, 84, 331–340. [Google Scholar] [CrossRef]
- Mohseni, E.; Naseri, F.; Amjadi, R.; Khotbehsara, M.M.; Ranjbar, M.M. Microstructure and durability properties of cement mortars containing nano-TiO2 and rice husk ash. Constr. Build. Mater. 2016, 114, 656–664. [Google Scholar] [CrossRef]
- Mohseni, E.; Ranjbar, M.M.; Yazdi, M.A.; Hosseiny, S.S.; Roshandel, E. The effects of silicon dioxide, iron (III) oxide and copper oxide nanomaterials on the properties of self-compacting mortar containing fly ash. Magaz. Concrete Res. 2015, 67. [Google Scholar] [CrossRef]
- Mohseni, E.; Tsavdaridis, K. Effect of Nano-Alumina on Pore Structure and Durability of Class F Fly Ash Self-Compacting Mortar. Am. J. Eng. Appl. Sci. 2016, 9, 323–333. [Google Scholar] [CrossRef]
- Mohseni, E.; Tang, W.; Wang, Z. Structural-functional integrated concrete with macro-encapsulated inorganic PCM. In Proceedings of the 2017 The 2nd International Conference on Energy Engineering and Smart Materials, Lyon, France, 7–9 July 2017. [Google Scholar]
- Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. In ASTM C876-15; ASTM International: West Conshohocken, PA, USA, 2009.
- Naseri, F.; Jafari, F.; Mohseni, E.; Tang, W.; Feizbakhsh, A.; Khatibinia, M. Experimental observations and SVM-based prediction of properties of polypropylene fibres reinforced self-compacting composites incorporating nano-CuO. Constr. Build. Mater. 2017, 143, 589–598. [Google Scholar] [CrossRef]
- Yang, J.; Mohseni, E.; Behforouzc, B.; Khotbehsarab, M.M. An experimental investigation into the effects of Cr2O3 and ZnO2 nanoparticles on the mechanical properties and durability of self-compacting mortar. Int. J. Mater. Res. 2015, 106, 886–892. [Google Scholar] [CrossRef]
- AS1012.9, Methods of Testing Concrete Compressive Strength Tests—Concrete, Mortar and Grout Specimens; Standards Australia: Sydney, Australia, 2014.
- Sadowski, Ł. New non-destructive method for linear polarisation resistance corrosion rate measurement. Arch. Civil. Mechan. Eng. 2010, 10, 109–116. [Google Scholar] [CrossRef]
- Dhouibi, L.; Triki, E.; Raharinaivo, A. The application of electrochemical impedance spectroscopy to determine the long-term effectiveness of corrosion inhibitors for steel in concrete. Cem. Concr. Compos. 2002, 24, 35–43. [Google Scholar] [CrossRef]
- Abouhussien, A.A.; Hassan, A.A. Experimental and empirical time to corrosion of reinforced concrete structures under different curing conditions. Adv. Civil Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Manzur, T.; Baten, B.; Hasan, M.J.; Akter, H.; Tahsin, A.; Hossain, K.M.A. Corrosion behavior of concrete mixes with masonry chips as coarse aggregate. Constr. Build. Mater. 2018, 185, 20–29. [Google Scholar] [CrossRef]
- Lai, W.-L.; Kind, T.; Stoppel, M.; Wiggenhauser, H. Measurement of accelerated steel corrosion in concrete using ground-penetrating radar and a modified half-cell potential method. J. Infrastruct. Syst. 2012, 19, 205–220. [Google Scholar] [CrossRef]
Sample Availability: Not available. |















| Corrected Half-Cell Potential (mV) | Potential for Corrosion (%) |
|---|---|
| >−200 | 10 |
| (−200)–(−350) | 50 |
| <−350 | 90 |
| Aggregate | Volume of Cumulative Mercury Intruded (cm3/g) | ||||||
|---|---|---|---|---|---|---|---|
| Pore Proportions (%) Classified by Diameter (μm) | |||||||
| <0.01 | 0.01–0.1 | 0.1–1 | 1–10 | 10–100 | >100 | ||
| Scoria | 0.3676 | 0.1 | 5 | 7 | 7 | 44 | 37 |
| Mix Code | Cement | Fly Ash | Water | Sand | Coarse Aggregate | TESA | NT | HRWR |
|---|---|---|---|---|---|---|---|---|
| Control | 300 | 70 | 140 | 780 | 1080 | 0 | 0 | 5 |
| TESA | 300 | 70 | 140 | 780 | 0 | 692 | 0 | 5 |
| TESA-NT | 280 | 70 | 140 | 780 | 0 | 692 | 20 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohseni, E.; Tang, W.; Wang, S. Investigation of the Role of Nano-Titanium on Corrosion and Thermal Performance of Structural Concrete with Macro-Encapsulated PCM. Molecules 2019, 24, 1360. https://doi.org/10.3390/molecules24071360
Mohseni E, Tang W, Wang S. Investigation of the Role of Nano-Titanium on Corrosion and Thermal Performance of Structural Concrete with Macro-Encapsulated PCM. Molecules. 2019; 24(7):1360. https://doi.org/10.3390/molecules24071360
Chicago/Turabian StyleMohseni, Ehsan, Waiching Tang, and Shanyong Wang. 2019. "Investigation of the Role of Nano-Titanium on Corrosion and Thermal Performance of Structural Concrete with Macro-Encapsulated PCM" Molecules 24, no. 7: 1360. https://doi.org/10.3390/molecules24071360






