A Useful Synthesis of 2-Acylamino-1,3,4-oxadiazoles from Acylthiosemicarbazides Using Potassium Iodate and the Discovery of New Antibacterial Compounds
Abstract
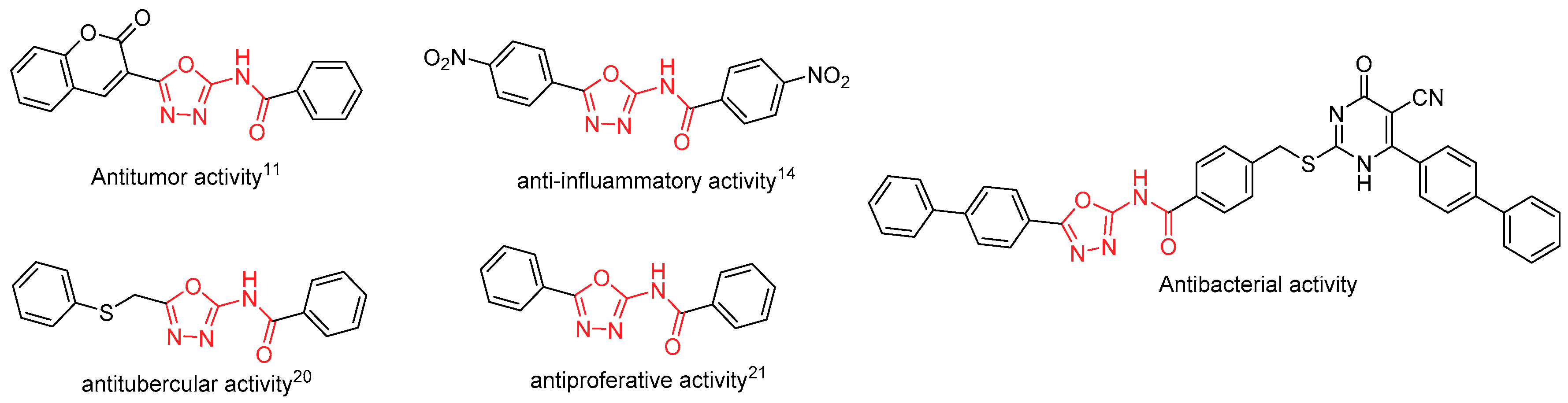
:1. Introduction
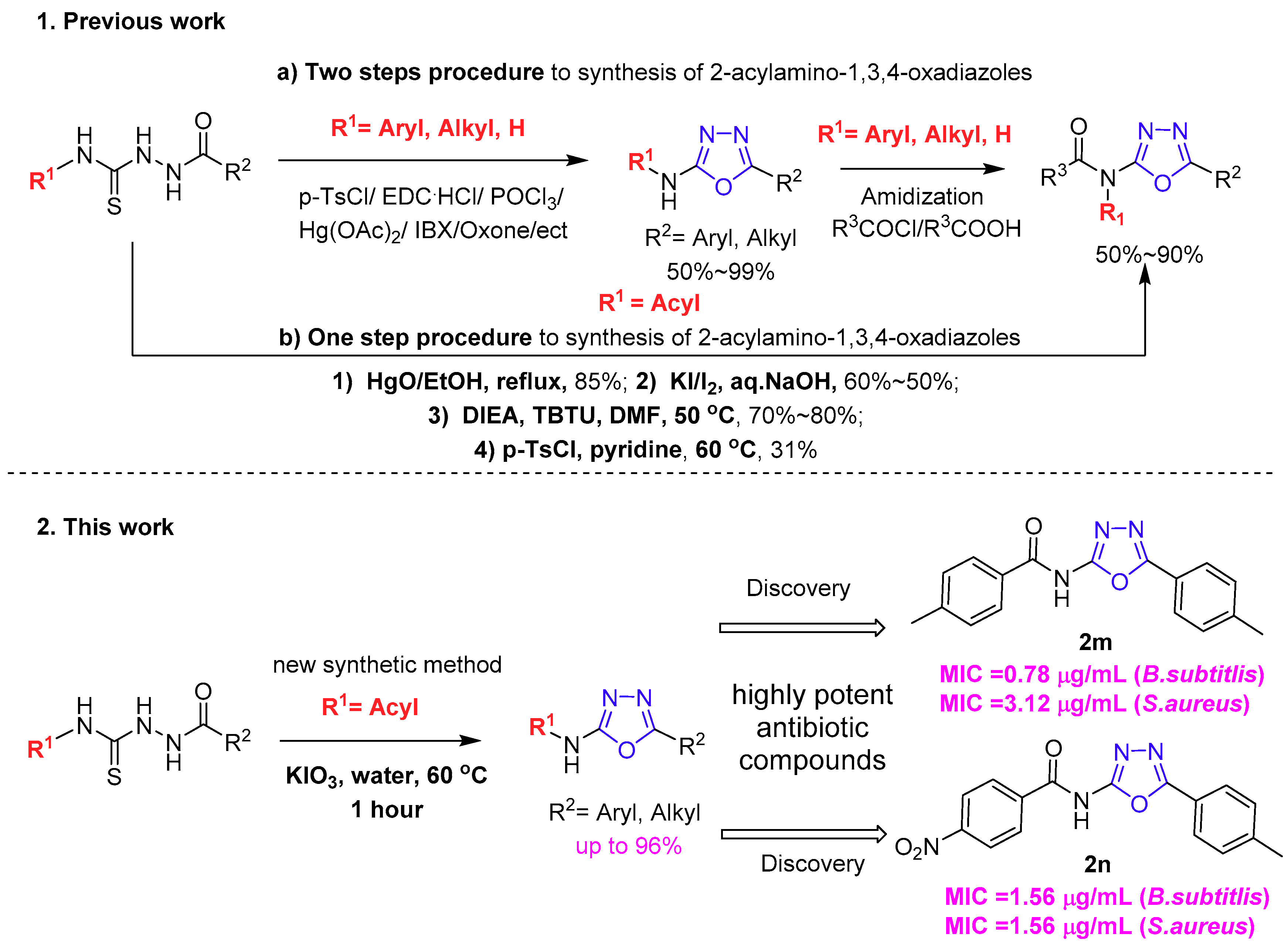
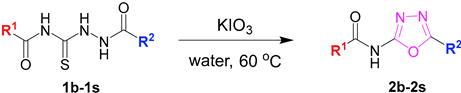
2. Results and Discussion
2.1. Optimization of the Reaction Conditions
2.2. Exploring the Substrates’ Scope
2.3. Evaluation of the Antibacterial Activity
3. Materials and Methods
3.1. Reagent and Methods
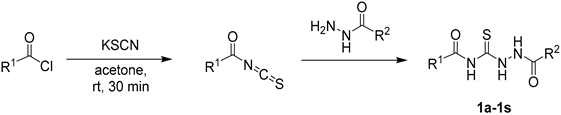
3.2. Synthesis of 1,4-Diacylthiosemicarbazide Substrates (1a–1s)
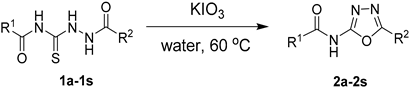
3.3. Synthesis of 2-acylamino-1,3,4-oxadiazole substrates (2a–2s)
3.4. Antibacterial Activity and Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Hong, Z.; Liu, M.; Guo, S.; Yang, D.; Wang, Y.; Lan, T.; Gao, L.; Qi, H.; Gong, P. Design, synthesis, and biological activity of novel tetrahydropyrazolopyridone derivatives as FXa inhibitors with potent anticoagulant activity. Bioorg. Med. Chem. 2017, 25, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Chapleo, C.B.; Myers, M.; Myers, P.L.; Saville, J.F.; Smith, A.C.; Stillings, M.R.; Tulloch, I.F.; Walter, D.S.; Welbourn, A.P. The oxidation of 1-thioaroylsemicarbazides. J. Med. Chem. 1986, 29, 2273–2280. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Karpińska, M.; Matysiak, J.; Niewiadomy, A. Characterization and preliminary anticonvulsant assessment of some 1,3,4-thiadiazole derivatives. Pharmacol. Rep. 2015, 67, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Gonsalves, R.; Shenoy, S. Synthesis and antibacterial studies of a new series of 1, 2-bis (1, 3, 4-oxadiazol-2-yl) ethanes and 1,2-bis (4-amino-1,2,4-triazol-3-yl) ethanes. Eur. J. Med. Chem. 2000, 35, 267–271. [Google Scholar] [CrossRef]
- Cesur, N.; Birteksöz, S.; Ötük, G. Synthesis and biological evaluation of some new thiosemicarbazide, 4-thiazolidinone, 1,3,4-oxadiazole and 1,2,4-triazole-3-thione derivatives bearing imidazo [1, 2-a] pyridine moiety. Acta Pharm. Sci. 2002, 44, 23–41. [Google Scholar]
- Laddi, U.; Desai, S.; Bennur, R.; Bennur, S. Some new 1,3,4-oxadiazoles as antimicrobial agents. Indian J. Heterocycl. Chem. 2002, 11, 319–322. [Google Scholar]
- Rahman, M.A. ZnX2 (X = Cl, Br) catalyzed efficient regioselective synthesis of 1,3,4-oxadiazole and 1,3,4-thiadiazole rings and their antibacterial studies. Ph.D. Thesis, Tennessee State University, Nashville, TN, USA, 2014. [Google Scholar]
- Mishra, P.; Rajak, H.; Mehta, A. Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4-oxadiazoles and their evaluation for antimicrobial activities. J. Gen. Appl. Microbiol. 2005, 51, 133–141. [Google Scholar] [CrossRef]
- Stephens, C.E.; Tanious, F.; Kim, S.; Wilson, W.D.; Schell, W.A.; Perfect, J.R.; Franzblau, S.G.; Boykin, D.W. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 2001, 44, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.J.; Lai, L.H.; Jin, G.Y.; Zhang, Z.X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem. 2002, 50, 3757–3760. [Google Scholar] [CrossRef]
- Bondock, S.; Adel, S.; Etman, H.A.; Badria, F.A. Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur. J. Med. Chem. 2012, 48, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Dawood, K.M.; Gomha, S.M. Synthesis and Anti-cancer Activity of 1,3,4-Thiadiazole and 1,3-Thiazole Derivatives Having 1,3,4-Oxadiazole Moiety. J. Heterocycl. Chem. 2015, 52, 1400–1405. [Google Scholar] [CrossRef]
- Durgashivaprasad, E.; Mathew, G.; Sebastian, S.; Reddy, S.M.; Mudgal, J.; Nampurath, G.K. Novel 2,5-disubstituted-1,3,4-oxadiazoles as anti-inflammatory drugs. Indian J. Pharmacol. 2014, 46, 521–526. [Google Scholar]
- Singh, A.K.; Lohani, M.; Parthsarthy, R. Synthesis, characterization and anti-inflammatory activity of some 1, 3, 4-oxadiazole derivatives. Iran. J. Pharm. Res. 2013, 12, 319–323. [Google Scholar]
- Mullican, M.D.; Wilson, M.W.; Conner, D.T.; Kostlan, C.R.; Schrier, D.J.; Dyer, R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles-1,3,4-oxadiazoles, and-1,2,4-triazoles as orally active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993, 36, 1090–1099. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Wang, Y.; Chen, W.; Huang, Q.; Liu, C.; Song, G. Syntheses and insecticidal activities of novel 2,5-disubstituted 1,3,4-oxadiazoles. J. Fluorine Chem. 2003, 123, 163–169. [Google Scholar] [CrossRef]
- Idoux, J.P.; Gibbs-Rein, K.S.; Gupton, J.T.; Cunningham, G.N. Synthesis and insecticidal activity of some 2, 5-(fluoroalkoxyphenyl)-1,3,4-oxadiazoles and their N,N′-dibenzoylhydrazine precursors. J. Chem. Eng. Data 1988, 33, 385–388. [Google Scholar] [CrossRef]
- Tyagi, M.; Kumar, A. Synthesis of 2-[2’-carbonyl-5’-(heteroarylinomethylene)-1’,3’,4’-thiadiazol-2’-yl/oxadiazole-2’-yl)]-4,5-dihydroimidazolines as hypotensive agents. Oriental J. Chem. 2002, 18, 125–130. [Google Scholar]
- Almasirad, A.; Tabatabai, S.A.; Faizi, M.; Kebriaeezadeh, A.; Mehrabi, N.; Dalvandi, A.; Shafiee, A. Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy) phenyl]-1,3,4-oxadiazoles and 1,2, 4-triazoles. Bioorg. Med.Chem. Lett. 2004, 14, 6057–6059. [Google Scholar] [CrossRef]
- Ekins, S.; Freundlich, J.S.; Hobrath, J.V.; White, E.L.; Reynolds, R.C. Combining computational methods for hit to lead optimization in Mycobacterium tuberculosis drug discovery. Pharm. Res. 2014, 31, 414–435. [Google Scholar] [CrossRef]
- Mochona, B.; Qi, X.; Euynni, S.; Sikazwi, D.; Mateeva, N.; Soliman, K.F. Design and evaluation of novel oxadiazole derivatives as potential prostate cancer agents. Bioorg. Med.Chem. Lett. 2016, 26, 2847–2851. [Google Scholar] [CrossRef] [Green Version]
- Soares de Oliveira, C.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; Filgueiras de Athayde-Filho, P. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef] [Green Version]
- Rivera, N.R.; Balsells, J.; Hansen, K.B. Synthesis of 2-amino-5-substituted-1,3,4-oxadiazoles using 1,3-dibromo-5,5-dimethylhydantoin as oxidant. Tetrahedron Lett. 2006, 47, 4889–4891. [Google Scholar] [CrossRef]
- Dolman, S.J.; Gosselin, F.; O’Shea, P.D.; Davies, I.W. Superior reactivity of thiosemicarbazides in the synthesis of 2-amino-1,3,4-oxadiazoles. J. Org. Chem. 2006, 71, 9548–9551. [Google Scholar] [CrossRef]
- Matsuno, K.; Masuda, Y.; Uehara, Y.; Sato, H.; Muroya, A.; Takahashi, O.; Yokotagawa, T.; Furuya, T.; Okawara, T.; Otsuka, M.; et al. Identification of a New Series of STAT3 Inhibitors by Virtual Screening. ACS Med. Chem. Lett. 2010, 1, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Fülöp, F.; Semega, E.; Dombi, C.; Bernath, G. Synthesis of new heterocyclic compounds as potential pharmaceutical agents. J. Heterocycl. Chem. 1990, 27, 951. [Google Scholar] [CrossRef]
- Küçükgüzel, G.; Kocatepe, A.; De Clercq, E.; Şahin, F.; Güllüce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353–359. [Google Scholar] [CrossRef]
- Coppo, F.T.; Evans, K.A.; Graybill, T.L.; Burton, G. Efficient one-pot preparation of 5-substituted-2-amino-1, 3, 4-oxadiazoles using resin-bound reagents. Tetrahedron Lett. 2004, 45, 3257. [Google Scholar] [CrossRef]
- Yang, S.-J.; Choe, J.-H.; Abdildinova, A.; Gong, Y.-D. A highly efficient diversification of 2-amino/amido-1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives via reagent-based cyclization of thiosemicarbazide intermediate on solid-phase. ACS Comb. Sci. 2015, 17, 732–741. [Google Scholar] [CrossRef]
- Omar, A.; Mohsen, M.; Aboulwafa, O.M. Synthesis and anticonvulsant properties of a novel series of 2-substituted amino-5-aryl-1,3,4-oxadiazole derivatives. J. Heterocycl. Chem. 1984, 21, 1415–1418. [Google Scholar] [CrossRef]
- Kurzer, F.; Doyle, K.M. The oxidation of 1-thioaroylsemicarbazides. J. Chem. Soc. Perk. Trans. 1 1986, 1873–1880. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Z.; Jin, G. Synthesis and biological activity of 1,3,4-oxadiazole-substituted pyridazinones. J. Chem. Res. 2002, 2002, 228–230. [Google Scholar] [CrossRef]
- Shinde, V.N.; Ugarkar, B.G.; Ghorpade, S.R. A convenient synthesis of 5-substituted 2-amino-1,3,4-oxadiazoles from corresponding acylthiosemicarbazides using iodine and Oxone. J. Chem. Res. 2013, 37, 53–54. [Google Scholar] [CrossRef]
- Vachal, P.; Toth, L.M. General facile synthesis of 2,5-diarylheteropentalenes. Tetrahedron Lett. 2004, 45, 7157–7161. [Google Scholar] [CrossRef]
- Chaudhari, P.S.; Pathare, S.P.; Akamanchi, K.G. o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization Initiated Domino Reactions for Synthesis of Azoles. J. Org. Chem. 2012, 77, 3716–3723. [Google Scholar] [CrossRef]
- Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W.; Chang, J. Synthesis of 2-amino-1,3,4-oxadiazoles and 2-amino-1,3,4-thiadiazoles via sequential condensation and I2-mediated oxidative C-O/C-S bond formation. J. Org. Chem. 2014, 80, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Prasanna, C.S.; Poojary, B.; Rao, K.S.; Shridhara, K.; Bhat, U.G. Synthesis and characterization of 1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives containing 2-chloropyridin-5-yl-methyl moiety. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2004, 43B, 2170–2174. [Google Scholar] [CrossRef]
- Xia, Q.; He, Q.; Xu, D.; Sun, D.; Peng, Z. Synthesis, characterization and antibacterial activity of five-membered heterocycles. Huaxue Xuebao 2010, 68, 2414–2420. [Google Scholar]
- Rostom, S.A.F.; Shalaby, M.A.; El-Demellawy, M.A. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem. 2003, 38, 959–974. [Google Scholar] [CrossRef]
- Yale, H.L.; Losee, K. 2-Amino-5-substituted 1,3,4-oxadiazoles and 5-imino-2-substituted Δ2-1,3,4-oxadiazolines. A group of novel muscle relaxants. J. Med. Chem. 1966, 9, 478–483. [Google Scholar] [CrossRef]
- Maghari, S.; Ramezanpour, S.; Darvish, F.; Balalaie, S.; Rominger, F.; Bijanzadeh, H.R. A new and efficient synthesis of 1,3,4-oxadiazole derivatives using TBTU. Tetrahedron 2013, 69, 2075–2080. [Google Scholar] [CrossRef]
- Singh, C.B.; Ghosh, H.; Murru, S.; Patel, B.K. Hypervalent iodine(III)-mediated regioselective N-acylation of 1,3-disubstituted thioureas. J. Org. Chem. 2008, 78, 2924–2927. [Google Scholar] [CrossRef]
- Ghosh, H.; Yella, R.; Nath, J.; Patel, B.K. Desulfurization mediated by hypervalent iodine(III): A novel strategy for the construction of heterocycles. Eur. J. Org. Chem. 2008, 36, 6189–6196. [Google Scholar] [CrossRef]
- Ameryckx, A.; Thabault, L.; Pochet, L.; Leimanis, S.; Poupaert, J.H.; Wouters, J.; Joris, B.; Van Bambeke, F.; Frederick, R. 1-(2-Hydroxybenzoyl)-thiosemicarbazides are promising antimicrobial agents targeting D-alanine-d-alanine ligase in bacterio. Eur. J. Med. Chem. 2018, 159, 324–338. [Google Scholar] [CrossRef]
- Azhari, S.J.; Mlahi, M.R.; Al-Asmy, A.A.; Mostafa, M.M. Synthesis of novel binary and ternary complexes derived from 1-(2-hydroxy benzoyl)-4-phenylthiosemicarbazide (L1) and 2,2′-dipyridyl (L2) with CoII, CuII and ZnII salts. Spectrochim. Acta Part. A 2015, 136, 185–191. [Google Scholar] [CrossRef]
- Prajapat, P. Utility of drug discovery in medicinal and organic chemistry. Mod. Chem. Appl. 2017, 5, 1000e123. [Google Scholar] [CrossRef]
- Nielsen, T.E.; Schreiber, S.L. Towards the optimal screening collection. A synthesis strategy. Angew. Chem. Int. Ed. 2008, 47, 48–56. [Google Scholar] [CrossRef]
- Schreiber, S.L. Organic synthesis toward small-molecule probes and drugs. Proc. Natl. Acad. Sci. USA 2011, 108, 6699–6702. [Google Scholar] [CrossRef] [Green Version]
- Barbachyn, M.R.; Ford, C.W. Oxazolidinone structure-activity relationships leading to linezolid. Angew. Chem. Int. Ed. 2003, 42, 2010–2023. [Google Scholar] [CrossRef]
- Schillaci, D.; Spano, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef]
- Wu, S.; Xia, J.; Wen, G.; Feng, B.; Jia, Y.-Q.; Zhang, W.-X.; Yang, Q.-Y.; Zhang, C.; Qi, Y. Preparation method of 1-benzoyl-4-acyl semicarbazide derivatives as antibacterial drug. Patent CN.108456157 A, 28 August 2018. [Google Scholar]
- Andrews J., M. Determination of minimum inhibitory concentrations. J. Antimicrobial. Chemotherapy 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Fang, L.; Fang, X.; Xing, Y.; Gou, S.; Xi, T. Synthesis and anticancer activity of some novel 2-phenazinamine derivatives. Eur. J. Med. Chem. 2013, 69, 1–9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2m, 2n and 2q are available from the authors. |


| Entry | Oxidant | Solvent | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | K2S2O8 | Water | 100 | N.O. a,c |
| 2 | (NH4)2S2O8 | Water | 100 | N.O. a,c |
| 3 | IBX | Water | 100 | <30 a |
| 4 | Oxone | Water | 100 | <40 a |
| 5 | KIO3 | Water | 100 | 53 b |
| 6 | KIO3 | Water | 80 | 83 b |
| 7 | KIO3 | Water | 40 | 46 b |
| 8 | KIO3 | Water | 60 | 90 b |
| 9 | KIO3 | DCM | 60 | 5 a |
| 10 | KIO3 | Acetone | 60 | N.O. a,c |
| Entry | R1 | R2 | Starting Material | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 |  |  | 1b | 2b | 89 |
| 2 |  |  | 1c | 2c | 65 |
| 3 |  |  | 1d | 2d | 90 |
| 4 | 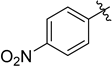 |  | 1e | 2e | 95 |
| 5 |  |  | 1f | 2f | 50 |
| 6 |  |  | 1g | 2g | 57 |
| 7 |  |  | 1h | 2h | 62 |
| 8 |  | 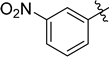 | 1i | 2i | 90 |
| 9 |  |  | 1j | 2j | 96 |
| 10 |  |  | 1k | 2k | 94 |
| 11 |  |  | 1l | 2l | 68 |
| 12 |  |  | 1m | 2m | 70 |
| 13 |  |  | 1n | 2n | 91 |
| 14 |  | 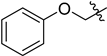 | 1o | 2o | 93 |
| 15 |  |  | 1p | 2p | 91 |
| 16 |  |  | 1q | 2q | 92 |
| 17 |  |  | 1r | 2r | 90 |
| 18 |  |  | 1s | 2s | 93 |
| Comp. ID | MIC (μm/mL) | Comp. ID | MIC (μm/mL) | ||||
|---|---|---|---|---|---|---|---|
| B.subtilis | S. aureus | E. coli | B.subtilis | S. aureus | E. coli | ||
| 1a | 12.5 | 12.5 | >100 | 2a | 3.12 | 6.25 | >100 |
| 1b | 12.5 | 25 | 50 | 2b | 50 | 25 | >100 |
| 1c | 6.25 | 6.25 | 25 | 2c | 6.25 | 25 | 100 |
| 1d | 6.25 | 3.12 | >100 | 2d | 3.12 | 12.5 | >100 |
| 1e | 25 | 100 | >100 | 2e | 1.56 | 3.12 | >100 |
| 1f | 25 | 50 | 12.5 | 2f | 100 | >100 | 100 |
| 1g | 12.5 | 25 | 12.5 | 2g | 100 | >100 | 100 |
| 1h | >100 | >100 | >100 | 2h | 50 | 100 | 100 |
| 1i | 25 | 50 | >100 | 2i | 25 | 100 | >100 |
| 1j | 25 | 25 | >100 | 2j | 6.25 | 25 | >100 |
| 1k | 50 | 100 | >100 | 2k | 1.56 | 6.25 | >100 |
| 1l | 3.12 | 6.25 | >100 | 2l | 1.56 | 6.25 | >100 |
| 1m | 6.25 | 3.12 | >100 | 2m | 0.78 | 3.12 | >100 |
| 1n | 12.5 | 12.5 | >100 | 2n | 1.56 | 1.56 | >100 |
| 1o | 25 | 50 | >100 | 2o | >100 | >100 | >100 |
| 1p | 12.5 | 3.12 | >100 | 2p | 6.25 | 12.5 | >100 |
| 1q | 50 | 100 | >100 | 2q | 0.78 | 3.12 | >100 |
| 1r | 6.25 | 3.12 | >100 | 2r | 1.56 | 6.25 | >100 |
| 1s | 25 | 12.5 | >100 | 2s | 3.12 | 3.12 | >100 |
| LEV | 0.05 | 0.20 | 0.02 | 3a | >100 | >100 | >100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wen, G.; Li, J.; Zhang, W.; Wu, S. A Useful Synthesis of 2-Acylamino-1,3,4-oxadiazoles from Acylthiosemicarbazides Using Potassium Iodate and the Discovery of New Antibacterial Compounds. Molecules 2019, 24, 1490. https://doi.org/10.3390/molecules24081490
Li T, Wen G, Li J, Zhang W, Wu S. A Useful Synthesis of 2-Acylamino-1,3,4-oxadiazoles from Acylthiosemicarbazides Using Potassium Iodate and the Discovery of New Antibacterial Compounds. Molecules. 2019; 24(8):1490. https://doi.org/10.3390/molecules24081490
Chicago/Turabian StyleLi, Tianlei, Gang Wen, Jishun Li, Wenxuan Zhang, and Song Wu. 2019. "A Useful Synthesis of 2-Acylamino-1,3,4-oxadiazoles from Acylthiosemicarbazides Using Potassium Iodate and the Discovery of New Antibacterial Compounds" Molecules 24, no. 8: 1490. https://doi.org/10.3390/molecules24081490






