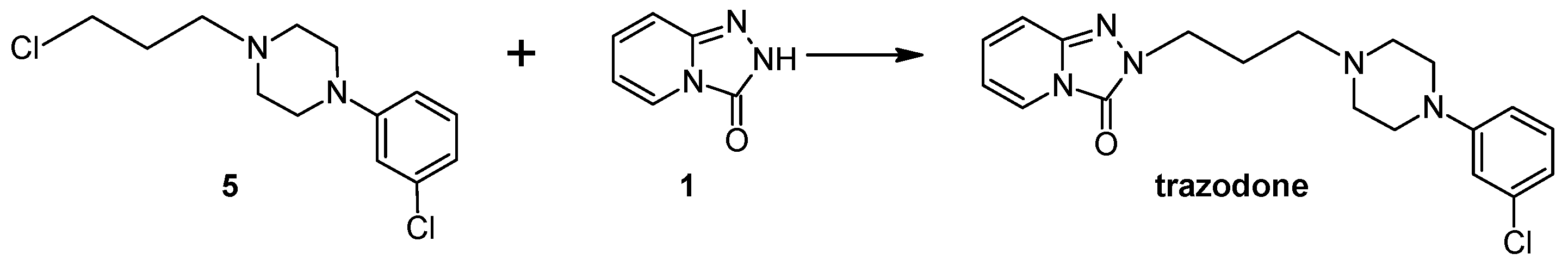
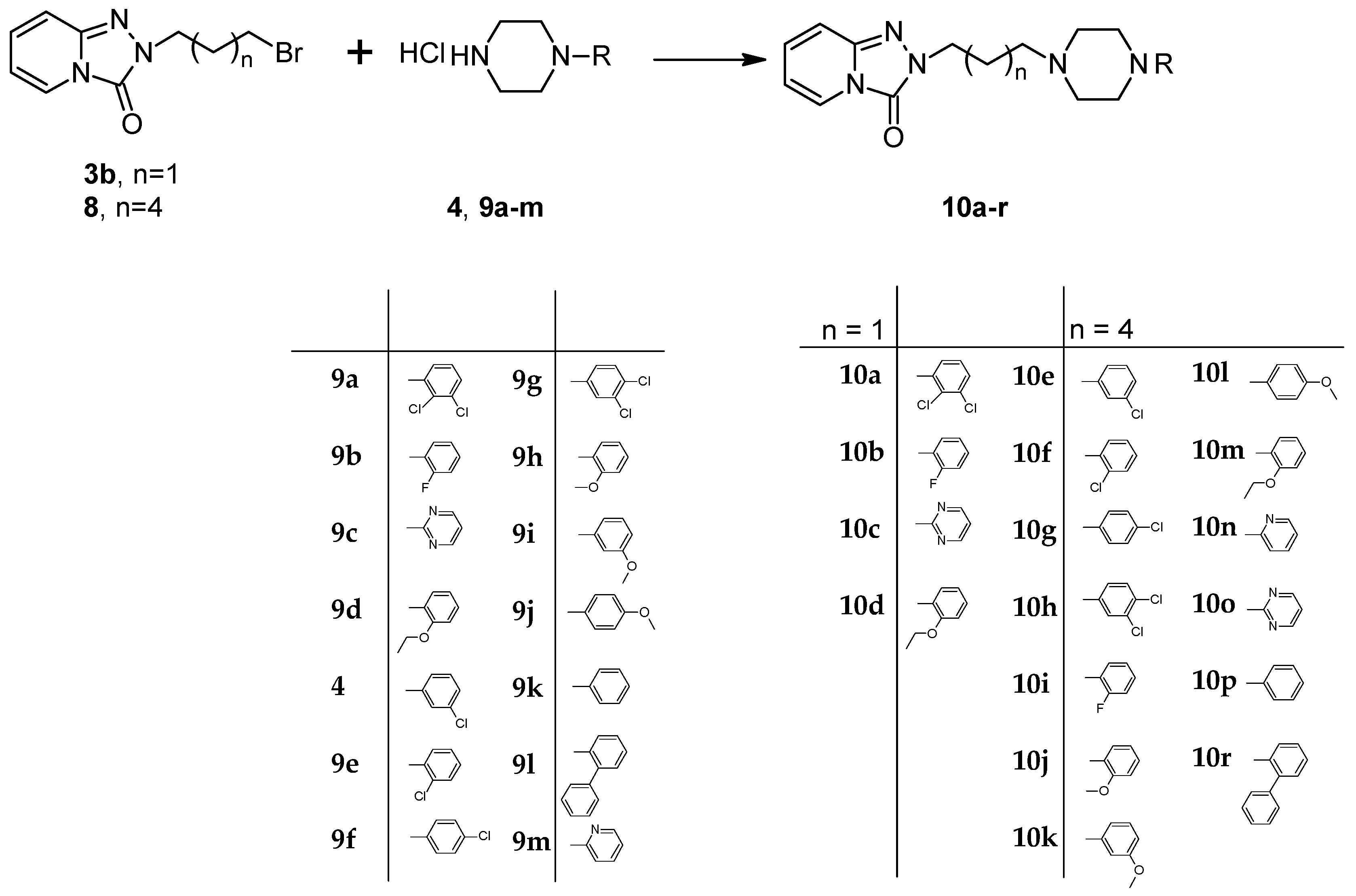
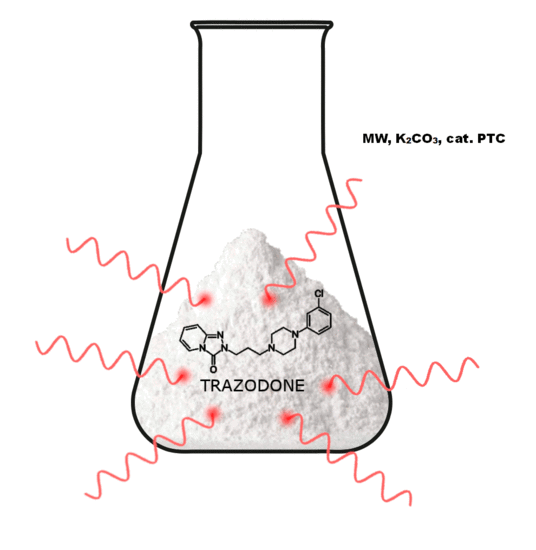
3.10. General Procedure for the Preparation Trazodone Derivatives
Firstly, 529 mg (2.5 mmol) of 2-(3-chloropropyl)-1,2,4-triazolo[4,3-a]pyridin-3-(2H)-one (3a)/745 mg (2.5 mmol) of 2-(3-bromohexyl)-1,2,4-triazolo[4,3-a]pyridin-3-(2H)-one (8), 2.5 mmol of the corresponding arylpiperazine hydrochloride (9a–m), 80 g (0.25 mmol) of TBAB, 1.04 g (7.5 mmol) of K2CO3, and 0.75 cm3 of acetonitrile were placed in a conical flask, after which the mixture was subjected to microwave radiation (Samsung M182DN; 300 W). The progress of the reaction was monitored by TLC (eluent chloroform–methanol 9:1). After the reaction, water was added and the resulting product was filtered off on a Büchner funnel. After drying, the obtained 10a–k were dissolved in acetone and a solution of 2M HCl in dioxane was added until acidic (universal indicator). The precipitated hydrochloride was filtered off on a Büchner funnel.
2-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10a): 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 7.1 Hz, 1H, ArH), 7.17 (ddd, J = 17.0, 8.8, 3.0 Hz, 4H, 1H, ArH), 7.02 (d, J = 8.0 Hz, 1H, 1H, ArH), 6.54 (t, J = 6.5 Hz, 1H, 1H, ArH), 4.18 (t, J = 6.1 Hz, 2H, CHNCO), 3.67 (dd, J = 22.9, 11.2 Hz, 4H, CHpip), 3.37 (d, J = 13.5 Hz, 2H, CHpip), 3.17 (s, 2H, CHpip), 3.06 (d, J = 11.5 Hz, 2H, CHaliph), 2.60 (s, 2H, CHaliph).13C NMR (75 MHz, CDCl3) δ 149.07 (Ar), 148.89 (Ar), 142.14 (Ar), 134.44 (Ar), 130.61 (Ar), 128.09 (Ar), 127.82 (Ar), 126.45 (Ar), 123.92 (Ar), 119.52 (Ar), 115.58 (Ar), 111.10 (Ar), 55.54 (Ctriaz), 52.66 (Cpip, Cpip), 48.15 (Cpip, Cpip), 43.45 (Cpip), 23.55 (Caliph). Fourier-transform (FT)-IR 3000 (C–H Ar, Str), 2954, 2850 (C-HAliph, Str), 1704 (C=O, Str), 1650 (C=N, Str), 1500, 1450 (C=C, Str), 1350 (C–N, Str), 750 (C–Cl, Str). HPLC 97% (tR = 4.33), m/z = 406,19, Rf = 0.87, yield = 25%, melting point (mp) = 225–230 °C.
2-[3-[4-(2-fluorophenyl)piperazin-1-yl]propyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10b): 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 6.9 Hz, 1H, ArH), 7.26 (s, 2H, ArH), 7.12 (d, J = 10.0 Hz, 4H, ArH), 6.54 (s, 1H, ArH), 4.18 (s, 2H, CHNCO), 4.03–3.91 (m, 2H, CHpip), 3.62 (s, 2H, CHpip), 3.58–3.49 (m, 2H, CHpip), 3.46–3.30 (m, 2H, CHpip), 3.26–3.16 (m, 2H, CHaliph), 2.66–2.53 (m, 2H, CHaliph). 13C NMR (75 MHz, dimethyl sulfoxide (DMSO)) δ 155.88 (Ar), 152.63 (Ar), 147.58 (Ar), 140.62 (Ar), 137.71(Ar) 130.04 (Ar), 122.79 (Ar), 119.02 (Ar), 115.72 (Ar), 115.45 (Ar), 114.47 (Ar), 110.31 (Ar), 52.42 (Ctriaz), 50.35 (Cpip, Cpip), 46.41 (Cpip, Cpip), 42.01 (Cpip), 22.38 (Caliph). FT-IR 3000 (C–H Ar, Str), 2946, 2852 (C–HAliph, Str), 1711 (C=O, Str), 1639 (C=N, Str), 1574, 1451 (C=C, Str), 1355 (C–N, Str), 1108 (C–F, Str). HPLC 99% (tR = 3.38), m/z = 356.21; Rf = 0.70, yield = 30%, mp = 146–150 °C.
2-[3-(4-pyrimidin-2-ylpiperazin-1-yl)propyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10c): 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 7.1 Hz, 1H, ArH), 7.26–7.07 (m, 4H, ArH), 7.02 (dd, J = 7.8, 1.7 Hz, 1H, ArH), 6.57–6.50 (m, 1H, ArH), 4.18 (t, J = 6.1 Hz, 2H, CHNCO), 3.67 (dd, J = 22.2, 11.3 Hz, 4H, CHpip), 3.37 (d, J = 12.9 Hz, 2H, CHpip), 3.25–3.15 (m, 2H, CHpip), 3.06 (d, J = 11.3 Hz, 2H, CHaliph), 2.59 (t, J = 14.4 Hz, 2H, CHaliph). 13C NMR (75 MHz, CDCl3) δ 148.85 (Ar), 148.67 (Ar), 141.93 (Ar), 130.41 (Ar), 127.89 (Ar, Ar) 126.23 (Ar), 119.32 (Ar), 115.37 (Ar), 110.90 (Ar), 55.32(Ctriaz), 52.46 (Cpip, Cpip), 47.94 (Cpip, Cpip), 43.24 (Cpip), 23.34 (Caliph). FT-IR 2990 (C-H Ar, Str), 2946, 2850 (C–HAliph, Str), 1706 (C=O, Str), 1636 (C=N, Str), 1601, 1459 (C=C, Str), 1355 (C–N, Str). HPLC 99% (tR = 4.32), m/z = 340.46; Rf = 0.68, yield = 34%, mp = 235–240 °C.
2-[3-[4-(2-ethoxyphenyl)piperazin-1-yl]propyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10d): 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 7.1 Hz, 1H, ArH), 7.18–6.95 (m, 4H, ArH), 6.89 (dd, J = 17.3 7.4 Hz, 2H, ArH), 6.54 (t, J = 6.5 Hz, 1H, ArH), 4.17 (t, J = 6.1 Hz, 2H, CHNCO), 4.08 (q, J = 6.9 Hz, 2H, OCH), 3.65–3.53 (m, 4H, CHpip), 3.16 (s, 4H, CHpip), 2.60 (s, 2H, CHaliph), 1.64 (s, 2H, CHaliph), 1.59 (s, 2H, CHaliph). 13C NMR (75 MHz, CDCl3) δ 151.66 (Ar), 148.87 (Ar), 142.17 (Ar, Ar), 130.62 (Ar), 123.92 (Ar, Ar), 121.47 (Ar), 115.59 (Ar, Ar), 113.05 (Ar), 111.11 (Ar), 64.37 (Coxy), 55.39 (Ctriaz), 51.37 (Cpip, Cpip), 48.08 (Cpip, Cpip), 43.41 (Cpip), 23.53 (Caliph), FT-IR 3001 (C–H Ar, Str), 2941, 2858 (C–HAliph, Str), 1708 (C=O, Str), 1611 (C=N, Str), 1531, 1458 (C=C, Str), 1349 (C–N, Str), 1260, 1021 (C–O, Str). HPLC 98% (tR = 3.73), m/z = 382.27; Rf = 0.66, yield = 33%, mp = 104–111 °C.
2-[6-[4-(3-chlorophenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10e): 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 7.1 Hz, 1H, ArH), 7.61 (s, 1H, ArH), 7.49 (d, J = 8.2 Hz, 1H, ArH), 7.39 (d, J = 8.2 Hz, 1H, ArH), 7.31 (d, J = 8.3 Hz, 1H, ArH), 7.15–7.05 (m, 2H, ArH), 6.51 (dt, J = 7.3, 3.7 Hz, 1H, ArH), 4.45 (d, J = 11.4 Hz, 2H, CHNCO), 4.02 (t, J = 6.7 Hz, 2H, CHpip), 3.83 (s, 2H, CHpip), 3.73–3.63 (m, 4H, CHpip), 3.11 (s, 2H, CHaliph), 2.00–1.81 (m, 4H, CHaliph), 1.46 (s, 4H, CHaliph). 13C NMR (75 MHz, CDCl3) δ 148.62(Ar), 146.74 (Ar), 141.56 (Ar), 135.80 (Ar), 131.06 (Ar), 129.92 (Ar), 125.69 (Ar), 123.72 (Ar), 119.31 (Ar), 116.98 (Ar), 115.37 (Ar), 110.65 (Ar), 57.28 (Ctriaz), 50.21 (Cpip, Cpip), 48.35 (Cpip, Cpip), 45.33 (Cpip), 28.32 (Caliph), 26.06 (Caliph), 25.67 (Caliph), 23.39 (Caliph). FT-IR 2981 (C–H Ar, Str), 2935, 2851 (C–HAliph, Str), 1703 (C=O, Str), 1639 (C=N, Str), 1593, 1451 (C=C, Str), 1354 (C–N, Str), 734 (C–Cl, Str). HPLC 97% (tR = 4.23), m/z = 414.30, Rf = 0.66, yield = 47% (Samsung), yield = 58 (CEM Discover SP reactor), mp = 178–183 °C.
2-[6-[4-(2-chlorophenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10f): 1H NMR (300 MHz, DMSO) δ 8.44 (d, J = 4.8 Hz 1H, ArH), 8.33–8.17 (m, 2H, ArH), 7.93–7.82 (m, 1H, ArH), 7.63 (dd, J = 11.7, 6.5 Hz, 2H, ArH), 7.08 (dd, J = 13.6, 6.4 Hz, 1H, ArH), 6.75 (t, J = 4.7 Hz, 1H, ArH), 3.84 (t, J = 7.3 Hz, 2H, CHNCO), 3.58 (s, 8H, CHpip), 3.04 (m, 2H, CHaliph), 1.84 (m, 4H, CHaliph), 1.52 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 161.06 (Ar), 158.58 (Ar), 136.22 (Ar), 132.01 (Ar), 130.58 (Ar), 130.23 (C Ar), 129.15 (Ar), 120.70 (Ar), 118.62 (Ar), 118.54(Ar), 111.66 (Ar), 104.35 (Ar), 55.87 (Ctriaz), 50.74 (Cpip, Cpip), 42.71 (Cpip, Cpip), 41.81 (Cpip), 27.98 (Caliph), 27.82 (Caliph), 27.61 (Caliph), 26.22 (Caliph). FT-IR 3008 (C–H Ar, Str), 2942, 2856 (C–HAliph, Str), 1738 (C=O, Str), 1616 (C=N, Str), 1538, 1456 (C=C, Str), 1349 (C–N, Str), 738 (C–Cl, Str). HPLC 92% (tR = 4.86), m/z = 452.19; Rf = 0.67, yield = 45%, mp = 145–148 °C.
2-[6-[4-(4-chlorophenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10g): 1H NMR (300 MHz, CDCl3) δ 7.85 (d, J = 8.8 Hz, 2H, ArH), 7.77 (d, J = 7.1 Hz, 1H, ArH), 7.51 (d, J = 8.9 Hz, 2H, ArH), 7.13–7.09 (m, 2H, ArH), 6.52 (dd, J = 7.2, 3.6 Hz, 1H, ArH), 4.78 (m, 2H, CHNCO), 4.21 (m, 2H, CHpip), 4.04 (t, J = 6.8 Hz, 2H, CHpip), 3.75–3.56 (m, 4H, CHpip), 3.15 (m, 2H, CHaliph), 2.03–1.81 (m, 4H, CHaliph), 1.45 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 148.89 (Ar), 148.39 (Ar), 141.35 (Ar), 130.85 (Ar), 129.27 (Ar, Ar), 124.30 (Ar) 124.00 (Ar), 117.93 (Ar, Ar), 115.44 (Ar), 111.30 (Ar), 55.69 (Ctriaz), 50.79 (Cpip, Cpip), 45.64 (Cpip, Cpip), 45.26 (Cpip), 28.44 (Caliph), 25.96 (Caliph), 25.87 (Caliph), 23.29 (Caliph). FT-IR 3034 (C–H Ar, Str), 2934, 2860 (C–HAliph, Str), 1708 (C=O, Str), 1637 (C=N, Str), 1539, 1482 (C=C, Str), 1354 (C–N, Str), 755 (C–Cl, Str). HPLC 98% (tR = 1.63); Rf = 0.50, yield = 44%, mp = 163–167 °C.
2-[6-[4-(3,4-dichlorophenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10h): 1H NMR (300 MHz, DMSO) δ 7.84 (d, J = 7.1 Hz, 1H, ArH), 7.45 (d, J = 8.9 Hz, 2H, ArH), 7.23 (dd, J = 5.9, 3.4 Hz, 2H, ArH), 7.00 (dd, J = 9.1, 2.8 Hz, 1H, ArH), 6.66–6.58 (m, 1H, ArH), 4.67 (s, 2H, CHNCO), 3.89 (dd, J = 12.3, 5.2 Hz, 4H, CHpip), 3.60–3.47 (m, 4H, CHpip), 3.16 (m, 2H, CHaliph), 1.75 (m, 4H, CHaliph), 1.32 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 149.77 (Ar), 148.38 (Ar), 141.35 (Ar), 132.11 (Ar), 131.10 (Ar), 130.84 (Ar), 124.29 (Ar), 121.22 (Ar), 117.44 (Ar), 116.29 (Ar), 115.44 (Ar), 111.30 (Ar), 55.73 (Ctriaz), 50.54 (Cpip), 45.71–44.67 (Cpip, Cpip), 42.66 (Cpip, Cpip), 32.66 (Cpip), 28.44 (Caliph), 25.68 (Caliph), 23.34 (Caliph), 22.55(Caliph). FT-IR 3016 (C–H Ar, Str), 2949, 2860 (C–HAliph, Str), 1738 (C=O, Str), 1642 (C=N, Str), 1542 (C=C, Str), 1365 (C–N, Str), 757 (C–Cl, Str). HPLC 95% (tR = 4.79), m/z = 448.20; Rf = 0.54, yield = 45%, mp = 116–120 °C.
2-[6-[4-(2-fluorophenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10i): 1H NMR (400 MHz, DMSO) δ 7.85 (dt, J = 7.1, 1.1 Hz, 1H, ArH), 7.20–7.03 (m, 5H, ArH), 6.62 (ddd, J = 7.2, 5.0, 2.4 Hz, 1H, ArH), 3.90 (t, J = 6.9 Hz, 2H, CHNCO), 3.55 (t, J = 9.5 Hz, 2H, CHpip), 3.46 (d, J = 11.1 Hz, 2H, CHpip), 3.24–3.15 (m, 4H, CHpip), 3.08 (dd, J = 10.9, 5.5 Hz, 2H, CHaliph), 1.78–1.69 (m, 4H, CHaliph), 1.35–1.29 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 154.21–153.60 (Ar), 148.39 (Ar), 141.35 (Ar), 138.74 (Ar), 130.84 (Ar), 125.46 (Ar), 124.31 (Ar), 120.01 (Ar), 116.71 (Ar), 116.51 (v), 115.45 (Ar), 111.30 (Ar), 55.79 (Ctriaz), 51.22 (Cpip, Cpip), 47.41 (Cpip, Cpip), 45.26 (Cpip), 28.44 (Caliph), 26.05 (Caliph), 25.89 (Caliph), 23.29 (Caliph). FT-IR 3021 (C–H Ar, Str), 2936, 2850 (C–HAliph, Str), 1713 (C=O, Str), 1635 (C=N, Str), 1541, 1500 (C=C, Str), 1355 (C–N, Str), 1141 (C–F, Str). HPLC 94% (tR = 1.46), Rf = 0.51, yield = 45%, mp = 183–187 °C.
2-[6-[4-(2-methoxyphenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10j): 1H NMR (300 MHz, CDCl3) δ 8.26 (d, J = 6.4 Hz, 1H, ArH), 7.77 (d, J = 7.0 Hz, 1H, ArH), 7.55–7.42 (m, 1H, ArH), 7.08 (dd, J = 14.9, 6.3 Hz, 4H, ArH), 6.50 (m, 1H, ArH), 5.16 (m, 3H, OCH), 4.46 (m, 2H, CHNCO), 4.13–3.95 (m, 4H, CHpip), 3.66–3.52 (m, 4H, CHpip), 3.14 (s, 2H, CHAliph), 2.02–1.76 (m, 4H, CHaliph), 1.45 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 152.27 (Ar), 148.39 (Ar), 141.36 (Ar), 139.75 (Ar), 130.85 (Ar), 124.30 (Ar), 124.00 (Ar), 121.30 (Ar), 118.72 (Ar), 115.45 (Ar), 112.41 (Ar), 111.31 (Ar), 55.84 (Coxy), 51.45 (Ctriaz), 47.32 (Cpip), 45.75 (Cpip), 45.27 (Cpip, Cpip), 32.18 (Cpip), 28.44 (Caliph), 26.56 (Caliph), 26.16 (Caliph), 25.79 (Caliph), 23.30 (Caliph). FT-IR 3016 (C–H Ar, Str), 2942, 2860 (C–HAliph, Str), 1708 (C=O, Str), 1640 (C=N, Str), 1540, 1448 (C=C, Str), 1366 (C–N, Str), 1261, 1020 (C–O, Str). HPLC 95% (tR = 3.66), Rf = 0.45, yield = 32%, mp = 140–141 °C.
2-[6-[4-(3-methoxyphenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10k): 1H NMR (300 MHz, CDCl3) δ 7.77 (d, J = 7.1 Hz, 1H, ArH), 7.49 (s, 1H, ArH), 7.42 (d, J = 7.5 Hz, 2H, ArH), 7.11 (d, J = 4.1 Hz, 2H, ArH), 7.01 (d, J = 7.4 Hz, 1H, ArH), 6.51 (dd, J = 7.2, 3.8 Hz, 1H, ArH), 4.78 (s, 3H, OCH), 4.29 (s, 2H, CHNCO), 4.03 (t, J = 6.8 Hz, 2H, CHpip), 3.86 (s, 2H, CHpip), 3.66 (d, J = 10.9 Hz, 4H, CHpip), 3.15 (s, 2H, CHaliph), 1.91 (dd, J = 14.3, 7.3 Hz, 4H, CHaliph), 1.48 (d, J = 24.1 Hz, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 150.21 (Ar), 140.44 (Ar, Ar), 139.99 (Ar), 129.15 (Ar), 120.10 (Ar, Ar), 121.16 (Ar), 119.89 (Ar), 114.42 (Ar), 112.11 (Ar), 111.11 (Ar), 55.44 (Coxy), 50.42 (Ctriaz), 47.39 (Cpip), 45.85 (Cpip, Cpip), 32.21 (Cpip), 28.41 (Caliph), 27.14 (Caliph), 28.12 (Caliph), 25.67 (Caliph), 23.31 (Caliph). FT-IR 2982 (C–H Ar, Str), 2938, 2861 (C–HAliph, Str), 1640 (C=O, Str), 1615 (C=N, Str), 1541, 1491 (C=C, Str), 1356 (C–N, Str), 1271, 1027 (C–O, Str). HPLC 100% (tR = 3.88), Rf = 0.54, yield = 30%, mp = 137–138 °C.
2-[6-[4-(4-methoxyphenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride: (10l) 1H NMR (300 MHz, CDCl3) δ 7.86 (d, J = 9.2 Hz, 2H, ArH), 7.76 (m, J = 7.0 Hz, 1H, ArH), 7.10 (d, J = 2.9 Hz, 2H, ArH), 7.00 (d, J = 9.1 Hz, 2H, ArH), 6.51 (m, 1H, ArH), 4.80 (m, 3H, OCH), 4.45–4.26 (m, 2H, CHNCO), 4.04 (t, J = 6.8 Hz, 2H, CHpip), 3.84 (m, 2H, CHpip), 3.62 (m, 4H, CHpip), 3.13 (s, 2H, CHaliph), 1.91 (m, 4H, CHaliph), 1.73 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 154.37 (Ar), 148.39 (Ar), 143.82 (Ar), 141.36 (Ar), 130.85 (Ar), 124.30 (Ar), 118.65 (Ar), 115.45 (Ar, Ar) 114.88 (Ar, Ar), 111.31 (Ar), 56.98 (Coxy), 55.70 (Ctriaz), 51.03 (Cpip, Cpip), 47.31 (Cpip, Cpip), 45.27 (Cpip), 28.44 (Caliph), 26.50–25.58 (Caliph), 23.31 (Caliph), 13.96 (Caliph). FT-IR 3002 (C–H Ar, Str), 2939, 2840 (C–HAliph, Str), 1708 (C=O, Str), 1640 (C=N, Str), 1541, 1509 (C=C, Str), 1374 (C–N, Str), 1260, 1025 (C–O, Str). HPLC 92% (tR = 1.40), Rf = 0.65, yield = 27%, mp = 144–146 °C.
2-[6-[4-(2-ethoxyphenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10m): 1H NMR (300 MHz, CDCl3) δ 8.20–8.15 (s, 1H, ArH), 7.77 (d, J = 7.1 Hz, 1H, ArH), 7.43 (m, 1H, ArH), 7.08 (dd, J = 15.7, 4.7 Hz, 4H, ArH), 6.51 (s, 1H, ArH), 4.96 (m, 2H, OCH), 4.45 (m, 2H, CHNCO), 4.33 (t, J = 7.3 Hz, 2H, CHpip), 4.02 (t, J = 6.9 Hz, 2H, CHpip), 3.61 (t, J = 13.0 Hz, 4H, CHpip), 3.13 (s, 2H, CHaliph), 1.89 (m, 4H, CHaliph), 1.64 (t, J = 7.0 Hz, 3H, CHaliph), 1.44 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 155.67 (Ar), 147.23 (Ar), 144.81 (Ar), 139.45 (Ar), 137.84 (Ar), 120.12 (Ar), 120.01 (Ar), 116.42 (Ar, Ar), 112.98 (Ar, Ar), 110.31 (Ar), 58.91 (Coxy), 55.74 (Ctriaz), 50.04 (Cpip, Cpip), 48.21 (Cpip, Cpip), 44.18 (Cpip), 29.60 (Caliph), 26.12 (Caliph), 21.13 (Caliph), 13.90 (Caliph), 13.40 (Caliph). FT-IR 2990 (C–H Ar, Str), 2936; 2860 (C–HAliph, Str), 1701 (C=O, Str), 1636 (C=N, Str), 1541, 1491 (C=C, Str), 1356 (C–N, Str), 1251, 1040 (C–O, Str). HPLC 97% (tR = 1.63), Rf = 0.46, yield = 31%, mp = 143–145 °C.
2-[6-[4-(2-pyridyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10n): 1H NMR (300 MHz, DMSO) δ 8.14 (d, J = 4.1 Hz, 1H, ArH), 7.83 (t, J = 7.0 Hz, 2H, ArH), 7.22 (d, J = 3.2 Hz, 2H, ArH), 7.15 (d, J = 8.9 Hz, 1H, ArH), 6.88 (s, 1H, ArH), 6.67–6.57 (m, 1H, ArH), 4.40 (d, J = 13.8 Hz, 2H, CHNCO), 3.88 (dd, J = 16.3, 9.5 Hz, 2H, CHpip), 3.58 (d, J = 11.6 Hz, 2H, CHpip), 3.37 (d, J = 14.1 Hz, 2H, CHpip), 3.22 (s, 2H, CHpip), 3.08 (s, 2H, CHaliph), 1.75 (d, J = 6.8 Hz, 4H, CHaliph), 1.28 (d, J = 27.2 Hz, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 148.38 (Ar), 141.35 (Ar), 130.85 (Ar, Ar), 124.30 (Ar, Ar), 115.44 (Ar), 114.27 (Ar, Ar), 114.16 (Ar), 111.31 (Ar), 55.80 (Ctriaz), 50.29 (Cpip), 45.27 (Cpip), 43.34 (Cpip), 42.34 (Cpip), 28.44 (Caliph), 26.02 (Caliph), 25.88 (Caliph), 23.28 (Caliph). FT-IR 2970 (C–H Ar, Str), 2936, 2861 (C–HAliph, Str), 1701 (C=O, Str), 1633 (C=N, Str), 1536, 1495 (C=C, Str), 1365 (C–N, Str). HPLC 98% (tR = 2.32), m/z = 381.27, Rf = 0.48, yield = 70%, mp = 175–178 °C.
2-[6-(4-pyrimidin-2-ylpiperazin-1-yl)hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10o): 1H NMR (300 MHz, CDCl3) δ 8.61 (d, J = 5.2 Hz, 1H, ArH), 8.29 (s, 1H, ArH), 7.76 (s, 1H, ArH), 7.10 (s, 2H, ArH), 6.99 (s, 1H, ArH), 6.54–6.50 (m, 1H, ArH), 4.28–4.21 (m, 2H, CHNCO), 4.01 (s, 4H, CHpip), 3.70 (s, 4H, CHpip), 3.07–3.00 (m, 2H, CHaliph), 1.95–1.81 (m, 4H, CHaliph), 1.45–1.38 (m, 4H, CHAliph). 13C NMR (75 MHz, CDCl3) δ 157.67 (Ar), 148.81 (Ar), 141.73 (Ar), 130.08 (Ar), 123.96 (Ar), 115.59 (Ar), 111.38 (Ar), 110.82 (Ar), 109.87 (Ar), 57.58 (Ctriaz), 51.54 (Cpip, Cpip), 45.57 (Cpip, Cpip), 41.78 (Cpip), 29.56 (Caliph), 28.11 (Caliph), 26.16 (Caliph), 23.54 (Caliph). FT-IR 3023 (C–H Ar, Str), 2937, 2857 (C–HAliph, Str), 1700 (C=O, Str), 1616 (C=N, Str), 1540 (C=C, Str), 1350 (C–N, Str). HPLC 90%, (tR = 1.42), Rf = 0.35, yield = 45% (Samsung), yield = 61% (CEM Discover SP reactor), mp = 146–148 °C.
2-[6-(4-phenylpiperazin-1-yl)hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10p): 1H NMR (300 MHz, DMSO) δ 7.84 (d, J = 7.0 Hz, 1H, ArH), 7.25 (dd, J = 14.6, 5.8 Hz, 4H, ArH), 7.00 (d, J = 8.1 Hz, 2H, ArH), 6.86 (t, J = 7.2 Hz, 1H, ArH), 6.61 (dd, J = 8.4, 5.8 Hz, 1H, ArH), 3.77 (t, J = 12.0 Hz, 2H, CHNCO), 3.64–3.35 (m, 4H, CHpip), 3.16 (dd, J = 22.3, 9.6 Hz, 4H, CHpip), 3.06 (m, 2H, CHaliph), 1.74 (m, 4H, CHaliph), 1.32 (m, 4H, CHaliph). FT-IR 2987 (C–H Ar, Str), 2937, 2861 (C–HAliph, Str), 1693 (C=O, Str), 1639 (C=N, Str), 1541, 1491 (C=C, Str), 1375 (C–N, Str). HPLC 90% (tR = 3.69), m/z = 380.27, Rf = 0.58, yield = 55%, mp = 133–137 °C.
2-[6-[4-(2-phenylphenyl)piperazin-1-yl]hexyl]-[1,2,4]triazolo[4,3-a]pyridin-3-one hydrochloride (10r): 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 7.2 Hz, CH-, 1H, ArH), 7.52 (d, J = 7.2 Hz, 2H, ArH), 7.42 (t, J = 7.5 Hz, 2H, ArH), 7.37–7.29 (m, 2H, ArH), 7.24–7.22 (m, 1H, ArH), 7.21–7.13 (m, 2H, ArH), 7.09 (d, J = 3.2 Hz, 2H, ArH), 6.54–6.46 (m, 1H, ArH), 3.99 (t, J = 7.0 Hz, 2H, CHNCO), 3.53 (t, J = 12.0 Hz, 2H, CHpip), 3.35 (d, J = 10.9 Hz, 2H, CHpip), 3.10 (d, J = 13.4 Hz, 2H, CHpip), 2.86 (m, 2H, CHpip), 2.69 (m, 2H, CHaliph), 1.81 (m, 4H, CHaliph), 1.39 (m, 4H, CHaliph). 13C NMR (101 MHz, DMSO) δ 148.71 (Ar), 148.37 (Ar), 141.34 (Ar), 140.59 (Ar), 134.47 (Ar), 131.74 (Ar), 130.84 (Ar), 129.03 (Ar, Ar), 128.73 (Ar, Ar), 127.55 (Ar), 124.29 (Ar), 123.96 (Ar), 119.01 (Ar, Ar), 115.44 (Ar), 111.30 (Ar), 55.75 (Ctriaz), 51.25 (Cpip), 47.76 (Cpip, Cpip), 45.25 (Cpip, Cpip), 28.42 (Caliph), 26.01 (Caliph), 25.84 (Caliph), 23.25 (Caliph). FT-IR 3023 (C–H Ar, Str), 2937, 2858 (C–HAliph, Str), 1702 (C=O, Str), 1637 (C=N, Str), 1541, 1500 (C=C, Str), 1351 (C–N, Str). HPLC 95% (tR = 4.99), m/z = 456.94, Rf = 0.65, yield = 61% (Samsung), yield = 66% (CEM Discover SP reactor), oil.