Excellent Temperature-Control Based on Reversible Thermochromic Materials for Light-Driven Phase Change Materials System
Abstract
:1. Introduction
2. Results and Discussion
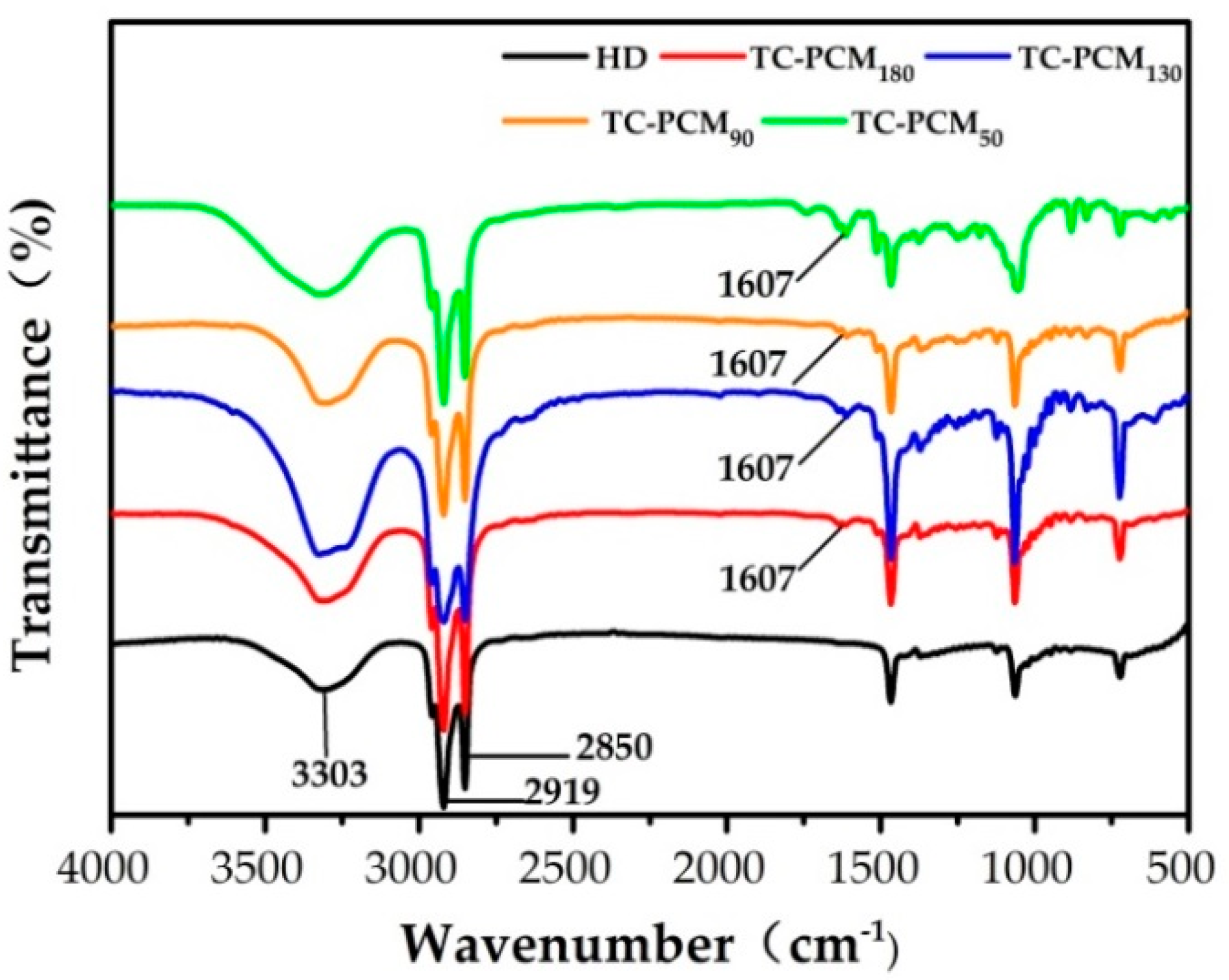
2.1. FT-IR Analysis
2.2. Thermal Property of TC-PCMs
2.3. X-ray Diffraction (XRD) Analysis
2.4. Photo-Thermal Conversion Analysis
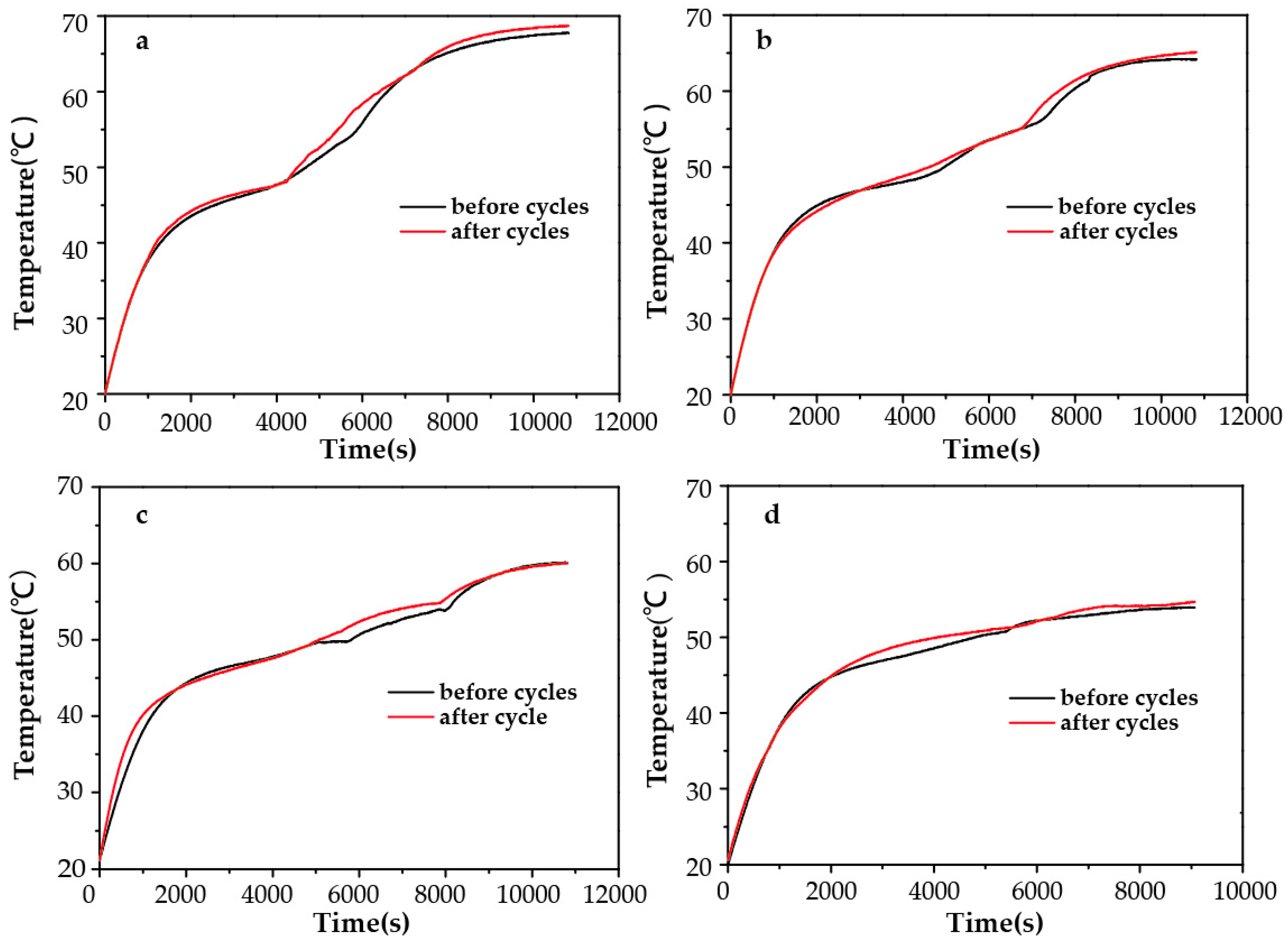
2.5. Cycle Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of TC-PCMs
3.3. Characterization of TC-PCMs
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sust. Energ. Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Gurzadyan, G.G.; Umair, M.M.; Wang, W.; Lu, R.; Zhang, S.; Tang, B. Ultrafast and efficient photothermal conversion for sunlight-driven thermal-electric system. Chem. Eng. J. 2018, 344, 402–409. [Google Scholar] [CrossRef]
- Cui, T.; Xuan, Y.; Li, Q. Design of a novel concentrating photovoltaic–thermoelectric system incorporated with phase change materials. Energ. Convers. Manage. 2016, 112, 49–60. [Google Scholar] [CrossRef]
- Kapsalis, V.; Karamanis, D. Solar thermal energy storage and heat pumps with phase change materials. Appl. Therm. Eng. 2016, 99, 1212–1224. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Tang, B.; Wang, P. Solar-thermal conversion and thermal energy storage of graphene foam-based composites. Nanoscale 2016, 8, 14600–14607. [Google Scholar] [CrossRef]
- Wang, W.; Fan, X.; Qiu, J.; Umair, M.M.; Ju, B.; Zhang, S.; Tang, B. Extracorporeal magnetic thermotherapy materials for self-controlled temperature through phase transition. Chem. Eng. J. 2019, 358, 1279–1286. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energ. 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Sadrameli, S.M. Carbon based material included-shaped stabilized phase change materials for sunlight-driven energy conversion and storage: An extensive review. Sol. Energy 2018, 170, 1130–1161. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Zhang, S. Novel organic solar thermal energy storage materials: Efficient visible light-driven reversible solid–liquid phase transition. J. Mater. Chem. 2012, 22, 18145–18150. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, H.; Zhang, L.; Xu, B.; Xiao, F. Fabrication of novel slurry containing graphene oxide-modified microencapsulated phase change material for direct absorption solar collector. Sol. Energ. Mater. Sol. C. 2018, 188, 73–80. [Google Scholar] [CrossRef]
- Wang, F.; Ling, Z.; Fang, X.; Zhang, Z. Optimization on the photo-thermal conversion performance of graphite nanoplatelets decorated phase change material emulsions. Sol. Energ. Mater. Sol. C. 2018, 186, 340–348. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Zhang, S. Single-walled carbon nanotube/phase change material composites: Sunlight-driven, reversible, form-stable phase transitions for solar thermal energy storage. Adv. Funct. Mater. 2013, 23, 4354–4360. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.; Ni, Z.; Zhang, C.; Lu, C. Synthesis of novel microencapsulated phase change materials with copper and copper oxide for solar energy storage and photo-thermal conversion. Sol. Energ. Mater. Sol. C. 2018, 179, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Qiu, J.; Jin, X.; Umair, M.M.; Lu, R.; Zhang, S.; Tang, B. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity. Appl. Energ. 2019, 237, 83–90. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Zhang, S. Organic, cross-linking, and shape-stabilized solar thermal energy storage materials: A reversible phase transition driven by broadband visible light. Appl. Energ. 2014, 113, 59–66. [Google Scholar] [CrossRef]
- Tang, B.; Wei, H.; Zhao, D.; Zhang, S. Light-heat conversion and thermal conductivity enhancement of PEG/SiO2 composite pcm by in situ Ti4O7 doping. Sol. Energ. Mater. Sol. C. 2017, 161, 183–189. [Google Scholar] [CrossRef]
- Kim, J.U.; Lee, S.; Kang, S.J.; Kim, T.I. Materials and design of nanostructured broadband light absorbers for advanced light-to-heat conversion. Nanoscale 2018, 10, 21555–21574. [Google Scholar] [CrossRef]
- Becker, K.; Lupton, J.M. Efficient light harvesting in dye-endcapped conjugated polymers probed by single molecule spectroscopy. J. Am. Chem. Soc. 2006, 148, 6468–6479. [Google Scholar] [CrossRef]
- Wang, H.; Qian, G.; Wang, M.; Zhang, J.; Luo, Y. Enhanced luminescence of an erbium (iii) ion-association ternary complex with a near-infrared dye. J. Phys. Chem. B 2004, 108, 8084–8088. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Khalid, M.; Rashmi, W.; Chan, A.; Shahbaz, K. Recent progress in solar thermal energy storage using nanomaterials. Renew. Sust. Energ. Rev. 2017, 67, 450–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Xiang, H.; Zhai, G.; Zhu, M. Fabrication of visual textile temperature indicators based on reversible thermochromic fibers. Dyes Pigments 2019, 162, 705–711. [Google Scholar] [CrossRef]
- Malherbe, I.; Sanderson, R.D.; Smit, E. Reversibly thermochromic micro-fibres by coaxial electrospinning. Polymer 2010, 51, 5037–5043. [Google Scholar] [CrossRef]
- Han, G.G.D.; Deru, J.H.; Cho, E.N.; Grossman, J.C. Optically-regulated thermal energy storage in diverse organic phase-change materials. Chem. Comm. 2018, 54, 20722. [Google Scholar]
- Han, G.G.D.; Li, H.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef] [Green Version]
- Harrington, W.N.; Haji, M.R.; Galanzha, E.I.; Nedosekin, D.A. Photoswitchable non-fluorescent thermochromic dye-nanoparticle hybrid probes. Sci. Rep. 2016, 6, 36417. [Google Scholar] [CrossRef]
- Tang, P.; Liu, Y.; Liu, Y.; Meng, H.; Liu, Z.; Li, K.; Wu, D. Thermochromism-induced temperature self-regulation and alternating photothermal nanohelix clusters for synergistic tumor chemo/photothermal therapy. Biomaterials 2019, 188, 12–23. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Dong, N.; Li, Z. Fabrication and characterization of reversible thermochromic wood veneers. Sci. Rep. 2017, 7, 16933. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite phase change materials with good reversible thermochromic ability in delignified wood substrate for thermal energy storage. Appl. Energ. 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Carreto, L.; Almeida, A.R.; Fernandes, A.C. Thermotropic mesomorphism of a model system for the plant epicuticular wax layer. Biophys. J. 2002, 82, 530–540. [Google Scholar] [CrossRef]
- Geng, X.; Li, W.; Yin, Q.; Wang, Y.; Han, N.; Wang, N.; Bian, J.; Wang, J.; Zhang, X. Design and fabrication of reversible thermochromic microencapsulated phase change materials for thermal energy storage and its antibacterial activity. Energy 2018, 159, 857–869. [Google Scholar] [CrossRef]
- Maclaren, D.C.; White, M.A. Design rules for reversible thermochromic mixtures. J. Mater. Sci. 2005, 40, 669–676. [Google Scholar] [CrossRef]
- Ventola, L.; Ramírez, M.; Calvet, T.; Solans, X. Polymorphism of n-alkanols: 1-heptadecanol, 1-octadecanol, 1-nonadecanol, and 1-eicosanol. Chem. Mater. 2002, 14, 508–517. [Google Scholar]
- Van Miltenburg, J.C.; Oonk, H.A.J. Heat capacities and derived thermodynamic functions of 1-octadecanol, 1-nonadecanol, 1-eicosanol, and 1-docosanol between 10 k and 370 k. J. Chem. Eng. Data 2001, 46, 90–97. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Sample | TC (% wt) | Tc (°C) | Tt (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|
| 1-HD | 0.00 | 48.00 | 41.87 | 229.5 | 48.76 | 233.8 |
| TC-PCM30 | 1.89 | 46.23 | 41.12 | 242.9 | 46.04 | 244.5 |
| TC-PCM90 | 1.08 | 47.01 | 40.81 | 244.9 | 46.95 | 240.3 |
| TC-PCM130 | 0.75 | 47.47 | 41.85 | 248.9 | 47.03 | 247.2 |
| TC-PCM180 | 0.55 | 47.45 | 41.81 | 236.3 | 47.13 | 238.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Liu, F.; Umair, M.M.; Jin, X.; Zhang, S.; Tang, B. Excellent Temperature-Control Based on Reversible Thermochromic Materials for Light-Driven Phase Change Materials System. Molecules 2019, 24, 1623. https://doi.org/10.3390/molecules24081623
Ren C, Liu F, Umair MM, Jin X, Zhang S, Tang B. Excellent Temperature-Control Based on Reversible Thermochromic Materials for Light-Driven Phase Change Materials System. Molecules. 2019; 24(8):1623. https://doi.org/10.3390/molecules24081623
Chicago/Turabian StyleRen, Caixia, Fangfang Liu, Malik Muhammad Umair, Xin Jin, Shufen Zhang, and Bingtao Tang. 2019. "Excellent Temperature-Control Based on Reversible Thermochromic Materials for Light-Driven Phase Change Materials System" Molecules 24, no. 8: 1623. https://doi.org/10.3390/molecules24081623






