Topical Application of Cinnamaldehyde Promotes Faster Healing of Skin Wounds Infected with Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
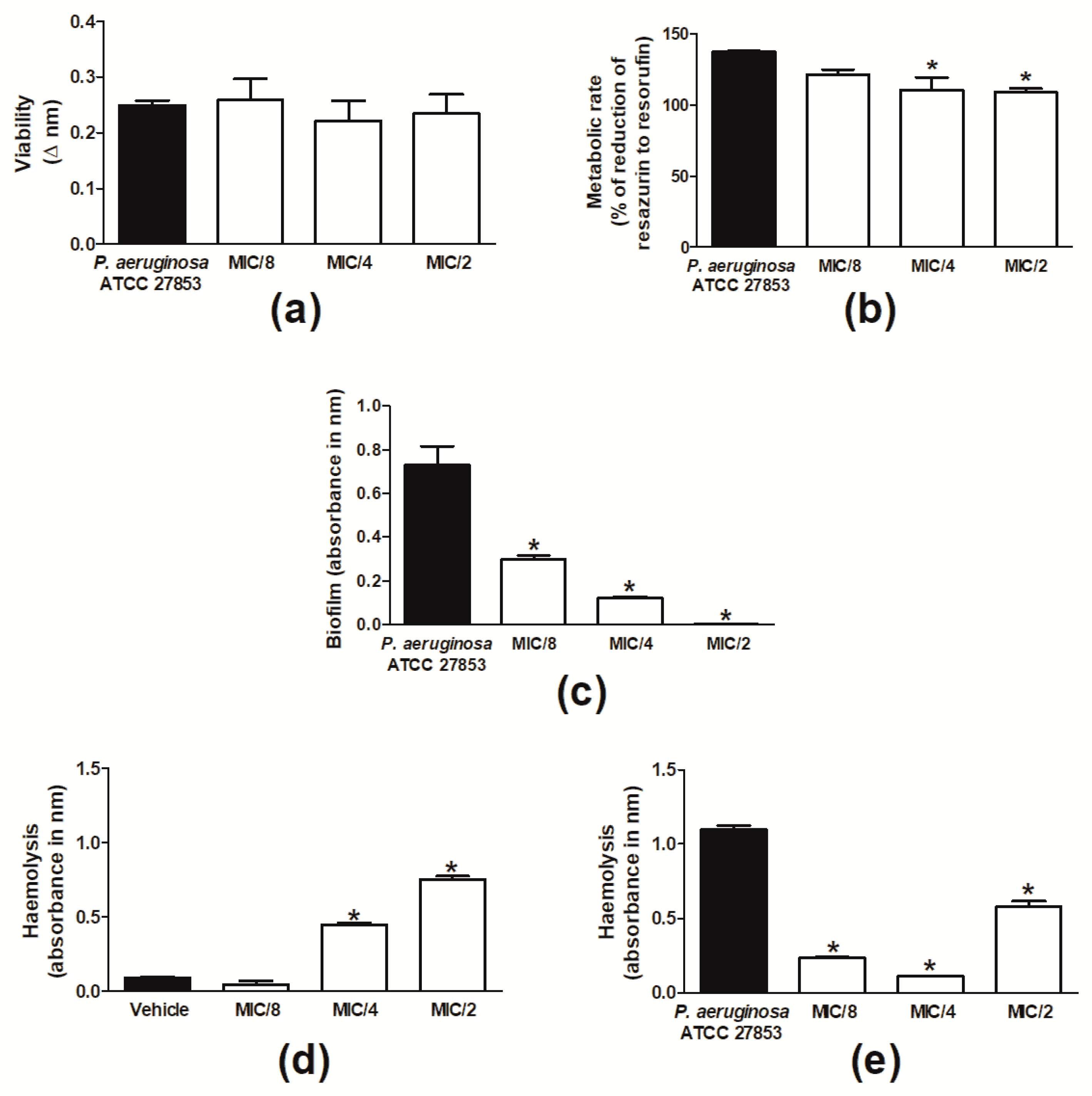
2.1. Cinnamaldehyde Is Antimicrobial against P. aeruginosa Strains
2.2. Cinnamaldehyde Reduces the P. aeruginosa Population in Skin Wounds and Accelerates Their Healing
2.3. Cinnamaldehyde-Induced Wound Healing Is Prevented by Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonism in Mice Infected with P. aeruginosa
2.4. Cinnamaldehyde Reduces the Production of Key Inflammatory Mediators in the Wound Beds of P. aeruginosa-Infected Mice
2.5. Cinnamaldehyde’s Inhibitory Effects on the Production of Inflammatory Mediators in P. aeruginosa-Infected Skin Wounds Partially Depends on TRPA1 Activation
3. Discussion
4. Materials and Methods
4.1. Bacterial Cultures
4.2. In Vitro Studies
4.2.1. Determination of MIC and MBC and Analysis of Bacterial Tolerance to Drug
4.2.2. Biofilm Formation
4.2.3. Haemolysis
4.3. In Vivo Experiments
4.3.1. Animals
4.3.2. Induction of Skin Wounds
4.3.3. Pharmacological Treatments
4.3.4. Analysis of Wound Bacterial Colonization
4.3.5. Sample Preparation for Analysis of Inflammatory Mediators in Wounds
4.3.6. Wound Levels of Nitric Oxide
4.3.7. VEGF and Cytokine Measurements
4.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and management of lower-extremity ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazăr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Gjodsbol, K.; Christensen, J.J.; Karlsmark, T.; Jorgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Wolff, H.; Hansson, C. Differential proteinase expression by Pseudomonas aeruginosa derived from chronic leg ulcers. Acta Derm. Venereol. 2001, 81, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Vatcheva-Dobrevska, R.; Mulet, X.; Ivanov, I.; Zamorano, L.; Dobreva, E.; Velinov, T.; Kantardjiev, T.; Oliver, A. Molecular epidemiology and multidrug resistance mechanisms of Pseudomonas aeruginosa isolates from Bulgarian hospitals. Microb. Drug Resist. 2013, 19, 355–361. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef]
- Buntinx, L.; Chang, L.; Amin, A.; Morlion, B.; de Hoon, J. Development of an in vivo target-engagement biomarker for TRPA1 antagonists in humans. Br. J. Clin. Pharmacol. 2017, 83, 603–611. [Google Scholar] [CrossRef]
- Cox, S.D.; Markham, J.L. Susceptibility and intrinsic tolerance of Pseudomonas aeruginosa to selected plant volatile compounds. J. Appl. Microbiol. 2007, 103, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Ultrasonic nanoemulsification of food grade trans-cinnamaldehyde: 1,8-Cineol and investigation of the mechanism of antibacterial activity. Ultrason. Sonochem. 2017, 35, 415–421. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomed. 2017, 12, 2813. [Google Scholar] [CrossRef] [PubMed]
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Takasao, N.; Tsuji-Naito, K.; Ishikura, S.; Tamura, A.; Akagawa, M. Cinnamon extract promotes type I collagen biosynthesis via activation of IGF-I signaling in human dermal fibroblasts. J. Agric. Food Chem. 2012, 60, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Han, L.; Fu, P.; Zeng, H.; Lv, C.; Chang, W.; Runyon, R.S.; Ishii, M.; Han, L.; Liu, K.; et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab. Investig. 2018, 98, 783–798. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and inflammation in chronic wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; de Carvalho, M.G.; Catunda, F.E.A., Jr. Antibacterial and antibiofilm activities of Cinnamomum sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Yu, X.; Zhu, M.; Kang, H.; Ma, J.; Wu, M.; Gan, J.; Deng, X.; Liang, H. Structural and molecular mechanism of CdpR involved in quorum-sensing and bacterial virulence in Pseudomonas aeruginosa. PLoS Biol. 2016, 14, e1002449. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair. Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Chen, J.; Hackos, D.H. TRPA1 as a drug target--promise and challenges. Naunyn. Schmiedebergs Arch. Pharmacol. 2015, 388, 451–463. [Google Scholar] [CrossRef]
- Mendes, S.J.F.; Sousa, F.I.A.B.; Pereira, D.M.S.; Ferro, T.A.F.; Pereira, I.C.P.; Silva, B.L.R.; Pinheiro, A.J.M.C.R.; Mouchrek, A.Q.S.; Monteiro-Neto, V.; Costa, S.K.P.; et al. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 2016, 34, 60–70. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [PubMed]
- Startek, J.B.; Talavera, K.; Voets, T.; Alpizar, Y.A. Differential interactions of bacterial lipopolysaccharides with lipid membranes: Implications for TRPA1-mediated chemosensation. Sci. Rep. 2018, 8, 12010. [Google Scholar] [CrossRef]
- Atoyan, R.; Shander, D.; Botchkareva, N.V. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J. Investig. Dermatol. 2009, 129, 2312–2315. [Google Scholar] [CrossRef]
- Yang, Y.S.; Cho, S.I.; Choi, M.G.; Choi, Y.H.; Kwak, I.S.; Park, C.W.; Kim, H.O. Increased expression of three types of transient receptor potential channels (TRPA1, TRPV4 and TRPV3) in burn scars with post-burn pruritus. Acta Derm. Venereol. 2015, 95, 20–24. [Google Scholar] [CrossRef]
- Okada, Y.; Shirai, K.; Reinach, P.S.; Kitano-Izutani, A.; Miyajima, M.; Flanders, K.C.; Jester, J.V.; Tominaga, M.; Saika, S. TRPA1 is required for TGF-β signaling and its loss blocks inflammatory fibrosis in mouse corneal stroma. Lab. Investig. 2014, 94, 1030–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, S.; Nakamura, E.; Endo, T.; Kubo, Y.; Takeuchi, K. Impairment by activation of TRPA1 of gastric epithelial restitution in a wound model using RGM1 cell monolayer. Inflammopharmacology 2007, 15, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Fine, J.-D.; Murrell, D.; Paller, A.; Connors, S.; Keough, K.; Marsh, E.; Folkman, J. Basic fibroblast growth factor: A missing link between collagen VII, increased collagenase, and squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Mol. Med. 1998, 4, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Johnson, D.; Cohen, C.; Brown, L.F. High-level expression of vascular endothelial growth factor and its receptors in an aphthous ulcer. J. Cutan. Med. Surg. 2003, 7, 225–228. [Google Scholar] [CrossRef]
- Kim, M.E.; Na, J.Y.; Lee, J.S. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol. Immunotoxicol. 2018, 40, 219–224. [Google Scholar] [CrossRef]
- Pannee, C.; Chandhanee, I.; Wacharee, L. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells. J. Adv. Pharm. Technol. Res. 2014, 5, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Z.; Fu, Y.; Yang, P.; Qin, Z.; Chen, Y.; Xu, Y. Trans-cinnamaldehyde improves memory impairment by blocking microglial activation through the destabilization of iNOS mRNA in mice challenged with lipopolysaccharide. Neuropharmacology 2016, 110, 503–518. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Arbiser, J.L.; Nowak, R.; Michaels, K.; Skabytska, Y.; Biedermann, T.; Lewis, M.J.; Bonner, M.Y.; Rao, S.; Gilbert, L.C.; Yusuf, N.; et al. Evidence for biochemical barrier restoration: Topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis. Sci. Rep. 2017, 7, 11198. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kaufmann, G.F.; Bowen, J.P.; Arbiser, J.L.; Janda, K.D. Solenopsin A, a venom alkaloid from the fire ant Solenopsis invicta, inhibits quorum-sensing signaling in Pseudomonas aeruginosa. J. Infect. Dis. 2008, 198, 1198–1201. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Ferro, T.A.; Araujo, J.M.; Dos Santos Pinto, B.L.; Dos Santos, J.S.; Souza, E.B.; da Silva, B.L.; Colares, V.L.; Novais, T.M.; Filho, C.M.; Struve, C.; et al. Cinnamaldehyde inhibits Staphylococcus aureus virulence factors and protects against infection in a Galleria mellonella model. Front. Microbiol. 2016, 7, 2052. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Strain | Antibiotic | MAR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMI | AZT | CEP | CET | CIP | GEN | LEV | IMI | MER | PIP/TAZ | POL | ||
| P. aeruginosa ATCC 27853 | S | S | S | S | S | S | S | S | S | S | S | 0 |
| P. aeruginosa 1 | R | R | R | R | R | R | R | R | R | R | S | 0.92 |
| P. aeruginosa 2 | S | S | S | S | S | S | S | S | S | R | S | 0.08 |
| Evaluated Parameters | Range | Score |
|---|---|---|
| Wound healing (% in relation to baseline wound area) | 0–20% | 0 |
| 21–40% | 1 | |
| 41–60% | 2 | |
| 61–80% | 3 | |
| 81–100% | 4 | |
| 101–120% | 5 | |
| 121–140% | 6 | |
| 141–160% | 7 | |
| >160% | 8 | |
| Exudate | No exudate | 0 |
| Light | 2 | |
| Moderate | 3 | |
| Heavy | 4 | |
| Exudate type | No exudate | 0 |
| Blood | 1 | |
| Serosanguineous | 2 | |
| Serous | 3 | |
| Purulent | 4 | |
| Oedema | No oedema | 0 |
| Mild | 1 | |
| Moderate | 2 | |
| Severe | 3 | |
| Surrounding skin tissue colour | Normal | 0 |
| Red | 1 | |
| White or hypopigmented | 2 | |
| Dark red or purple | 3 | |
| Black or hyperpigmented | 4 | |
| Debridement tissue type | Epithelial tissue | 0 |
| Granulation tissue | 1 | |
| Granulation tissue | 2 | |
| Necrotic tissue | 3 | |
| Amount of necrotic tissue (% in relation to the total wound area) | Absence of necrosis | 0 |
| <25% | 1 | |
| 25–49% | 2 | |
| 50–75% | 3 | |
| 76–100% | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, T.A.F.; Souza, E.B.; Suarez, M.A.M.; Rodrigues, J.F.S.; Pereira, D.M.S.; Mendes, S.J.F.; Gonzaga, L.F.; Machado, M.C.A.M.; Bomfim, M.R.Q.; Calixto, J.B.; et al. Topical Application of Cinnamaldehyde Promotes Faster Healing of Skin Wounds Infected with Pseudomonas aeruginosa. Molecules 2019, 24, 1627. https://doi.org/10.3390/molecules24081627
Ferro TAF, Souza EB, Suarez MAM, Rodrigues JFS, Pereira DMS, Mendes SJF, Gonzaga LF, Machado MCAM, Bomfim MRQ, Calixto JB, et al. Topical Application of Cinnamaldehyde Promotes Faster Healing of Skin Wounds Infected with Pseudomonas aeruginosa. Molecules. 2019; 24(8):1627. https://doi.org/10.3390/molecules24081627
Chicago/Turabian StyleFerro, Thiago A.F., Eliene B. Souza, Mariela A.M. Suarez, João F.S. Rodrigues, Domingos M.S. Pereira, Saulo J.F. Mendes, Laoane F. Gonzaga, Márcia C.A.M. Machado, Maria R.Q. Bomfim, João B. Calixto, and et al. 2019. "Topical Application of Cinnamaldehyde Promotes Faster Healing of Skin Wounds Infected with Pseudomonas aeruginosa" Molecules 24, no. 8: 1627. https://doi.org/10.3390/molecules24081627







