Extraction of Proanthocyanidins from Chinese Wild Rice (Zizania latifolia) and Analyses of Structural Composition and Potential Bioactivities of Different Fractions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of WRPs
2.1.1. Single Factor Experimental Analysis
2.1.2. Fitting the Model
2.1.3. Optimization of Extraction Conditions
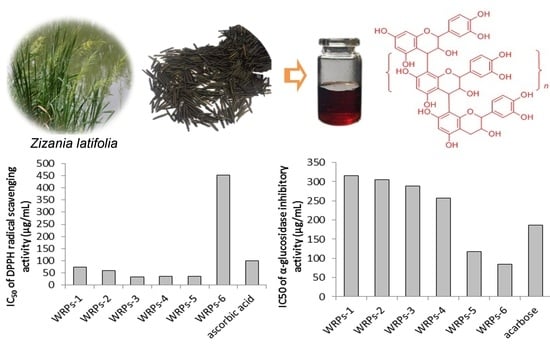
2.2. Proanthocyanidin Content and In Vitro Bioactivities of the Isolated Fractions
2.3. Structural Composition of Fractions WRPs-1–WRPs-6
3. Materials and Methods
3.1. Plant Materials and Chemicals
3.2. Experimental Design to Set Up the Proanthocyanidins Extraction
3.2.1. Ultrasound-Assisted Extraction Procedure
3.2.2. Experimental Design
3.3. Partition and Purification of Crude WRPs
3.4. Fractionation of WRPs
3.5. Acid-Catalysis in the Presence of Phloroglucinol (Phloroglucinolysis)
3.6. UPLC-LTQ-Orbitrap-MS Analysis
3.7. Determination of Proanthocyanidin Content
3.8. DPPH Radical Scavenging Assay
3.9. α-Glucosidase Inhibition Assay
3.10. Pancreatic Lipase Inhibition Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sumczynski, D.; Koubová, E.; Šenkárová, L.; Orsavová, J. Rice flakes produced from commercial wild rice: Chemical compositions, vitamin B compounds, mineral and trace element contents and their dietary intake evaluation. Food Chem. 2018, 264, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Du, Y.M.; Liu, X.M.; Chu, C.; Shi, J.; Zhang, H.B.; Liu, Y.H.; Zhang, Z.F. Morphological characteristics, nutrients, and bioactive compounds of Zizania latifolia, and health benefits of its seeds. Molecules 2018, 23, 1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, P.; Agellon, L.B.; Zhai, C.K. Wild rice (Zizania latifolia (Griseb) Turcz) improves the serum lipid profile and antioxidant status of rats fed with a high fat/cholesterol diet. Brit. J. Nutr. 2009, 102, 1723–1727. [Google Scholar] [CrossRef]
- Sumczynski, D.; Kotásková, E.; Orsavová, J.; Valášek, P. Contribution of individual phenolics to antioxidant activity and in vitro digestibility of wild rices (Zizania aquatica L.). Food Chem. 2017, 218, 107–115. [Google Scholar] [CrossRef]
- Han, S.F.; Zhang, H.; Zhai, C.K. Protective potentials of wild rice (Zizania latifolia (Griseb) Turcz) against obesity and lipotoxicity induced by a high-fat/cholesterol diet in rats. Food Chem. Toxicol. 2012, 50, 2263–2269. [Google Scholar] [CrossRef]
- Zhai, C.K.; Lu, C.M.; Zhang, X.Q.; Sun, G.J.; Lorenz, K.J. Comparative study on nutritional value of Chinese and North American wild rice. J. Food. Compos. Anal. 2001, 14, 371–382. [Google Scholar] [CrossRef]
- Chu, M.J.; Liu, X.M.; Yan, N.; Wang, F.Z.; Du, Y.M.; Zhang, Z.F. Partial purification, identification, and quantitation of antioxidants from wild rice (Zizania latifolia). Molecules 2018, 23, 2782. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant activity of commercial wild rice and identification of flavonoid compounds in active fractions. J. Agric. Food Chem. 2009, 57, 7543–7551. [Google Scholar] [CrossRef]
- Zhou, P.Y.; Zhang, L.M.; Li, W.; Zhang, S.T.; Luo, L.X.; Wang, J.; Sun, B.S. In vitro evaluation of the anti-digestion and antioxidant effects of grape seed procyanidins according to their degrees of polymerization. J. Funct. Foods 2008, 49, 85–95. [Google Scholar] [CrossRef]
- Li, S.Y.; Xiao, J.; Chen, L.; Hu, C.L.; Chen, P.; Xie, B.J.; Sun, Z.D. Identification of A-series oligomeric procyanidins from pericarp of Litchi chinensis by FT-ICR-MS and LC-MS. Food Chem. 2012, 135, 31–38. [Google Scholar] [CrossRef]
- Zhang, S.T.; Li, L.X.; Cui, Y.; Luo, L.X.; Li, Y.Y.; Zhou, P.Y.; Sun, B.S. Preparative high-speed counter-current chromatography separation of grape seed proanthocyanidins according to degree of polymerization. Food Chem. 2017, 219, 399–407. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Cardioprotective properties of grape seed proanthocyanidins: An update. Trends Food Sci. Technol. 2016, 57, 31–39. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, Y.Y.; Peng, J.M.; Li, C.M. Inhibitory effect of persimmon tannin on pancreatic lipase and the underlying mechanism in vitro. J. Agric. Food Chem. 2018, 66, 6013–6021. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Mo, K.L.; Fei, S.M.; Zu, Y.G.; Yang, L. Efficient approach for the extraction of proanthocyanidins from Cinnamomum longepaniculatum leaves using ultrasonic irradiation and an evaluation of their inhibition activity on digestive enzymes and antioxidant activity in vitro. J. Sep. Sci. 2017, 40, 3100–3113. [Google Scholar] [CrossRef]
- Zhang, H.W.; Yang, Y.M.; Ma, C.M. Structures and antioxidant and intestinal disaccharidase inhibitory activities of A-type proanthocyanidins from peanut skin. J. Agric. Food Chem. 2013, 61, 8814–8820. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.H.; Chen, P.X.; Zhang, H.; Liu, R.H.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and alpha-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Odabaş, H.İ.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crop. Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Jakobek, L.; García-Villalb, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Karonen, M.; Leikas, A.; Loponen, J.; Sinkkonen, J.; Ossipov, V.; Pihlaja, K. Reversed-phase HPLC-ESI/MS analysis of birch leaf proanthocyanidins after their acidic degradation in the presence of nucleophiles. Phytochem. Anal. 2007, 18, 378–386. [Google Scholar] [CrossRef]
- Jerez, M.; Tourino, S.; Sineiro, J.; Lluis Torres, J.; Jose Nunez, M. Procyanidins from pine bark: Relationships between structure, composition and antiradical activity. Food Chem. 2007, 104, 518–527. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lin, G.M.; Lin, H.Y.; Chang, S.T. Characteristics of proanthocyanidins in leaves of Chamaecyparis obtusa var. formosana as strong α-glucosidase inhibitors. J. Sci. Food Agric. 2018, 98, 3806–3814. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Li, S.Y.; Yang, Y.J.; Li, J.S.; Zhu, Z.Z.; Lorenzo, J.M.; Barba, F.J. Increasing yield and antioxidative performance of Litchi pericarp procyanidins in baked food by ultrasound-assisted extraction coupled with enzymatic treatment. Molecules 2018, 23, 2089. [Google Scholar] [CrossRef]
- Lin, G.M.; Lin, H.Y.; Hsu, C.Y.; Chang, S.T. Structural characterization and bioactivity of proanthocyanidins from indigenous cinnamon (Cinnamomum osmophloeum). J. Sci. Food Agric. 2016, 96, 4749–4759. [Google Scholar] [CrossRef]
- Wei, S.D.; Zhou, H.C.; Lin, Y.M. Antioxidant activities of fractions of polymeric procyanidins from stem bark of Acacia confusa. Int. J. Mol. Sci. 2011, 12, 1146–1160. [Google Scholar] [CrossRef]
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F.Y. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Holt, H.; Kennedy, J.A. Interaction between grape-derived proanthocyanidins and cell wall material. 2. Implications for vinification. J. Agric. Food Chem. 2010, 58, 10736–10746. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chang, S.T. Antioxidant activities and xanthine oxidase inhibitory effects of phenolic phytochemicals from Acacia confusa twigs and branches. J. Agric. Food Chem. 2010, 58, 1578–1583. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.L.; Zou, P.; Zhang, C.S.; Li, Y.Q. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohyd. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Rahman, M.J.; Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 27.15 | 14 | 1.94 | 168.10 | <0.0001 * |
| X1 | 7.74 | 1 | 7.74 | 670.94 | <0.0001 * |
| X2 | 0.13 | 1 | 0.13 | 11.00 | 0.0051 * |
| X3 | 0.12 | 1 | 0.12 | 10.00 | 0.0069 * |
| X4 | 0.07 | 1 | 0.07 | 5.83 | 0.0300 * |
| X1X2 | 7.78 × 10−4 | 1 | 7.78 × 10−4 | 0.067 | 0.7989 |
| X1X3 | 0.35 | 1 | 0.35 | 30.53 | <0.0001 * |
| X1X4 | 0.08 | 1 | 0.08 | 6.80 | 0.0206 * |
| X2X3 | 7.30 × 10−3 | 1 | 7.30 × 10−3 | 0.63 | 0.4396 |
| X2X4 | 0.27 | 1 | 0.27 | 23.00 | 0.0003 * |
| X3X4 | 0.14 | 1 | 0.14 | 11.94 | 0.0039 * |
| X12 | 12.23 | 1 | 12.23 | 105.89 | <0.0001 * |
| X22 | 9.17 | 1 | 9.17 | 79.41 | <0.0001 * |
| X32 | 0.41 | 1 | 0.41 | 35.66 | <0.0001 * |
| X42 | 0.57 | 1 | 0.57 | 48.97 | <0.0001 * |
| Residual | 0.16 | 14 | 0.012 | - | - |
| Lack of fit | 0.14 | 10 | 0.014 | 2.88 | 0.1596 |
| Pure error | 0.02 | 4 | 4.92 × 10−3 | - | - |
| Cor total | 27.31 | 28 | - | - | - |
| Fraction | Proanthocyanidin Content (mg/g Extract) | IC50/DPPH (μg/mL) | IC50/α-glucosidase (μg/mL) | IC50/pancreatic lipase (μg/mL) |
|---|---|---|---|---|
| WRPs-1 | 524.19 ± 3.56 e | 74.91 ± 0.83 c | 316.07 ± 1.08 a | >2000 |
| WRPs-2 | 639.92 ± 5.77 c | 59.57 ± 1.52 d | 304.17 ± 2.46 b | >2000 |
| WRPs-3 | 863.81 ± 8.02 a | 34.29 ± 0.78 e | 289.04 ± 3.11 c | >2000 |
| WRPs-4 | 679.34 ± 4.55 b | 36.73 ± 0.96 e | 257.20 ± 3.85 d | >2000 |
| WRPs-5 | 629.16 ± 6.82 c | 35.44 ± 1.02 e | 117.72 ± 1.45 f | >2000 |
| WRPs-6 | 567.20 ± 5.76 d | 451.85 ± 2.47 a | 84.01 ± 0.74 g | 1054.01 ± 6.67 a |
| ascorbic acid | - | 100.05 ± 0.94 b | - | - |
| acarbose | - | - | 186.31 ± 1.04 e | - |
| orlistat | - | - | - | 15.45 ± 0.14 b |
| Retention Time (min) | [M + H]+ (m/z) | Fragment Ion (m/z) | Compound | ||
|---|---|---|---|---|---|
| Measured | Calculated | Error (ppm) | |||
| 2.71 | 431.0970 | 431.0973 | −0.67 | 305.0655, 263.0478 | EGC-Ph |
| 3.35 | 415.1024 | 415.1024 | −0.06 | 288.9869, 271.1326, 263.0480 | C-Ph |
| 3.67 | 415.1025 | 415.1024 | 0.27 | 288.9869, 271.1326, 263.0480 | EC-Ph |
| 4.18 | 307.0818 | 307.0812 | 1.89 | 181.0490 | EGC |
| 5.30 | 291.0862 | 291.0863 | −0.56 | 273.0747, 165.0544 | C |
| 6.00 | 291.0862 | 291.0863 | −0.56 | 273.0747, 165.0544 | EC |
| Fraction | Terminal Unit (%) | Extension Unit (%) | mDP | ||||
|---|---|---|---|---|---|---|---|
| C | EC | EGC | C-Ph | EC-Ph | EGC-Ph | ||
| WRPs-1 | 15.15 ± 0.30 a | 11.83 ± 0.42 a | 9.55 ± 0.33 a | 15.87 ± 0.31 d | 44.03 ± 0.53 e | 3.57 ± 0.11 b | 2.66 ± 0.04 f |
| WRPs-2 | 8.95 ± 0.32 b | 9.62 ± 0.11 b | 4.35 ± 0.05 b | 22.89 ± 0.26 a | 49.91 ± 0.46 d | 3.28 ± 0.09 c | 4.32 ± 0.06 e |
| WRPs-3 | 9.69 ± 0.47 b | 6.55 ± 0.13 c | 4.55 ± 0.04 b | 20.43 ± 0.24 b | 55.62 ± 0.59 c | 3.16 ± 0.12 cd | 4.81 ± 0.09 d |
| WRPs-4 | 7.52 ± 0.48 c | 6.60 ± 0.32 c | 3.19 ± 0.17 c | 16.79 ± 0.45 c | 63.03 ± 0.80 b | 2.87 ± 0.08 e | 5.78 ± 0.12 c |
| WRPs-5 | 6.01 ± 0.28 d | 4.83 ± 0.09 d | 4.45 ± 0.12 b | 16.92 ± 0.31 c | 64.71 ± 0.65 b | 3.08 ± 0.15 d | 6.54 ± 0.23 b |
| WRPs-6 | 4.21 ± 0.12 e | 3.80 ± 0.06 e | 1.70 ± 0.04 d | 15.79 ± 0.21 d | 70.63 ± 0.69 a | 3.87 ± 0.10 a | 10.30 ± 0.46 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, M.-J.; Du, Y.-M.; Liu, X.-M.; Yan, N.; Wang, F.-Z.; Zhang, Z.-F. Extraction of Proanthocyanidins from Chinese Wild Rice (Zizania latifolia) and Analyses of Structural Composition and Potential Bioactivities of Different Fractions. Molecules 2019, 24, 1681. https://doi.org/10.3390/molecules24091681
Chu M-J, Du Y-M, Liu X-M, Yan N, Wang F-Z, Zhang Z-F. Extraction of Proanthocyanidins from Chinese Wild Rice (Zizania latifolia) and Analyses of Structural Composition and Potential Bioactivities of Different Fractions. Molecules. 2019; 24(9):1681. https://doi.org/10.3390/molecules24091681
Chicago/Turabian StyleChu, Mei-Jun, Yong-Mei Du, Xin-Min Liu, Ning Yan, Feng-Zhong Wang, and Zhong-Feng Zhang. 2019. "Extraction of Proanthocyanidins from Chinese Wild Rice (Zizania latifolia) and Analyses of Structural Composition and Potential Bioactivities of Different Fractions" Molecules 24, no. 9: 1681. https://doi.org/10.3390/molecules24091681