Distribution of Polysulfide in Human Biological Fluids and Their Association with Amylase and Sperm Activities
Abstract
:1. Introduction
2. Results and Discussions
2.1. Determination of Polysulfide Level in Biological Fluids
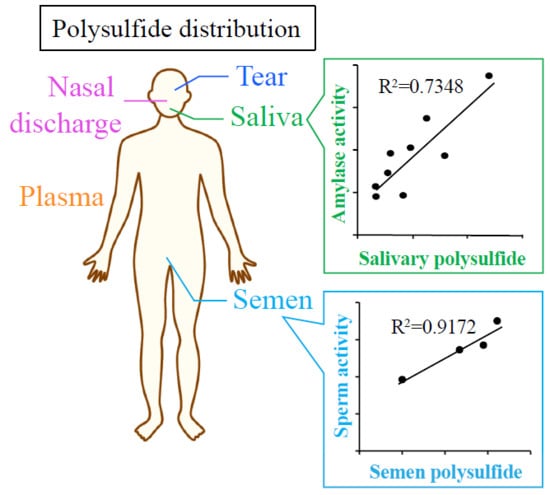
2.2. Analysis of Protein Content of Biological Fluids
2.3. Effect of Age, Gender Difference, and BMI on Polysulfide Levels in Biological Fluids
2.4. Relationship Between Sperm Activity and Polysulfide Levels in Semen
2.5. The Circadian Rhythm of Polysulfide Level in Plasma
3. Materials and Methods
3.1. Materials
3.2. Sample Collection
3.3. Measuring Polysulfide by EMSP
3.4. Measuring Activities of Sperm in Semen
3.5. Determination of Thiol Contents in Plasma
3.6. Measuring Anti-Oxidative Activity Against AAPH Radical
3.7. Detection of Sulfane Sulfur by a Fluorescence Probe
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eaton, P. Protein thiol oxidation in health and disease: Techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic. Biol. Med. 2006, 40, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Reddie, K.G.; Carroll, K.S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 746–754. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Reactions of electrophiles with nucleophilic thiolate sites: Relevance to pathophysiological mechanisms and remediation. Free Radic. Res. 2016, 50, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Fritz, K.S.; Petersen, D.R. An overview of the chemistry and biology of reactive aldehydes. Free Radic. Biol. Med. 2013, 59, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, M.T.; Foster, M.W.; Benhar, M.; Stamler, J.S. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic. Biol. Med. 2009, 46, 119–126. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Nat. Acad. Sci. 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruhlke, M.C.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef]
- Iciek, M.G.; Wlodek, L. Biosynthesis and biological properties of compounds containing highly reactive, reduced sulfane sulfur. Polish J. Pharmacol. 2001, 53, 215–226. [Google Scholar]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitino, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S Signals Through Protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Dóka, É.; Pader, I.; Bíró, A.; Johansson, K.; Cheng, Q.; Ballagó, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E. A novel persulfide detection method reveals protein persulfide-and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ishima, Y.; Shibata, A.; Chuang, V.T.G.; Sawa, T.; Ihara, H.; Watanabe, H.; Xian, M.; Ouchi, Y.; Shimizu, T.; et al. Quantitative determination of polysulfide in albumins, plasma proteins and biological fluid samples using a novel combined assays approach. Anal. Chim. Acta 2017, 969, 18–25. [Google Scholar] [CrossRef]
- Seymour Gray, C.H. Electrophoretic Analysis of Human Semen. Proc. Society Exp. Biol. Med. 1942, 50, 351–353. [Google Scholar] [CrossRef]
- Vitorino, R.; Lobo, M.J.; Ferrer-Correira, A.J.; Dubin, J.R.; Tomer, K.B.; Domingues, P.M.; Amado, F.M. Identification of human whole saliva protein components using proteomics. Proteomics 2004, 4, 1109–1115. [Google Scholar] [CrossRef]
- Casado, B.; Pannell, L.K.; Iadarola, P.; Baraniuk, J.N. Identification of human nasal mucous proteins using proteomics. Proteomics 2005, 5, 2949–2959. [Google Scholar] [CrossRef]
- Pan, Q.; Angelina, A.; Marrone, M.; Stark, W.J.; Akpek, E.K. Autologous serum eye drops for dry eye. Cochrane Database Syst. Rev. 2017, 2, CD009327. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-depth analysis of the human tear proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef]
- Kido, J.; Bando, M.; Hiroshima, Y.; Iwasaka, H.; Yamada, K.; Ohgami, N.; Nambu, T.; Kataoka, M.; Yamamoto, T.; Shinohara, Y.; et al. Analysis of proteins in human gingival crevicular fluid by mass spectrometry. J. Periodontal Res. 2012, 47, 488–499. [Google Scholar] [CrossRef]
- Colares, V.L.P.; Lima, S.N.L.; Sousa, N.C.F.; Araujo, M.C.; Pereira, D.M.S.; Mendes, S.J.F.; Teixeira, S.A.; Monteiro, C.A.; Bandeca, M.C.; Siqueira, W.L.; et al. Hydrogen peroxide-based products alter inflammatory and tissue damage-related proteins in the gingival crevicular fluid of healthy volunteers: A randomized trial. Sci. Rep. 2019, 9, 3457. [Google Scholar] [CrossRef]
- Jodar, M.; Sendler, E.; Krawetz, S.A. The protein and transcript profiles of human semen. Cell Tissue Res. 2015, 363, 85–96. [Google Scholar] [CrossRef]
- Giuseppe Arienti, C.S.; Enrico, C.; Rosaria, V.; Carlo, A. Distribution of lipid and protein in human semen fractions. Clin. Chim. Acta 1999, 289, 111–120. [Google Scholar] [CrossRef]
- Dzunkova, M.; Martinez-Martinez, D.; Gardlik, R.; Behuliak, M.; Jansakova, K.; Jimenez, N.; Vazquez-Castellanos, J.F.; Marti, J.M.; D’Auria, G.; Bandara, H.; et al. Oxidative stress in the oral cavity is driven by individual-specific bacterial communities. NPJ Biofilms Microb. 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Warnet, J.M.; Baudouin, C.; Rat, P. Cytotoxicity of contact lens multipurpose solutions: Role of oxidative stress, mitochondrial activity and P2X7 cell death receptor activation. Eur. J. Pharm. Sci. 2008, 33, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-Loaded Contact Lenses: Eye Protection Against Oxidative Stress. Basic Investig. 2015, 34, 693–697. [Google Scholar]
- Wakamatsu, T.H.; Dogru, M.; Tsubota, K. Tearful relations: Oxidative stress, inflammation and eye diseases. Arquivos Brasileiros de Oftalmologia 2008, 71, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid. Med. Cellul. Long. 2010, 3, 2–12. [Google Scholar] [CrossRef]
- Jones, D.P.; Mody Jr, V.C.; Carlson, J.L.; Lynn, M.J.; Sternberg Jr, P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Rad. Biol. Med. 2002, 33, 1290–1300. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kanemori, T.; Kanemaru, M.; Takai, N.; Mizuno, Y.; Yoshida, H. Performance evaluation of salivary amylase activity monitor. Biosensors Bioelectron. 2004, 20, 491–497. [Google Scholar] [CrossRef]
- Nater, U.; Lamarca, R.; Florin, L.; Moses, A.; Langhans, W.; Koller, M.; Ehlert, U. Stress-induced changes in human salivary alpha-amylase activity—associations with adrenergic activity. Psychoneuroendocrinology 2006, 31, 49–58. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef]
- Kroll, J.L.; Werchan, C.A.; Reeves, A.G.; Bruemmer, K.J.; Lippert, A.R.; Ritz, T. Sensitivity of salivary hydrogen sulfide to psychological stress and its association with exhaled nitric oxide and affect. Physiol. Behav. 2017, 179, 99–104. [Google Scholar] [CrossRef]
- Tomazic, S.J.; Klibanov, A.M. Mechanisms of irreversible thermal inactivation of Bacillus alpha-amylases. J. Biol. Chem. 1988, 263, 3086–3091. [Google Scholar] [PubMed]
- Keskes-Ammar, L.; Feki-Chakroun, N.; Rebai, T.; Sahnoun, Z.; Ghozzi, H.; Hammami, S.; Zghal, K.; Fki, H.; Damak, J.; Bahloul, A. Sperm Oxidative Stress and the Effect of an Oral Vitamin E and Selenium Supplement on Semen Quality in Infertile Men. Archiv. Androl. 2009, 49, 83–94. [Google Scholar] [CrossRef]
- Zhu, Z.; Ren, Z.; Fan, X.; Pan, Y.; Lv, S.; Pan, C.; Lei, A.; Zeng, W. Cysteine protects rabbit spermatozoa against reactive oxygen species-induced damages. PLoS ONE 2017, 12, e0181110. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, Z.Z.; Chua, J.M.; Wong, P.C.; Bian, J. Hydrogen sulfide protects testicular germ cells against heat-induced injury. Nitric Oxide 2015, 46, 165–171. [Google Scholar] [CrossRef]
- Cocuzza, M.; Athayde, K.S.; Agarwal, A.; Sharma, R.; Pagani, R.; Lucon, A.M.; Srougi, M.; Hallak, J. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008, 71, 490–494. [Google Scholar] [CrossRef]
- Jin, S.; Tan, B.; Teng, X.; Meng, R.; Jiao, X.; Tian, D.; Xiao, L.; Xue, H.; Guo, Q.; Duan, X.; et al. Diurnal Fluctuations in Plasma Hydrogen Sulfide of the Mice. Front Pharmacol. 2017, 8, 682. [Google Scholar] [CrossRef]
- Kontush, A.; Reich, A.; Baum, K.; Spranger, T.; Finckh, B.; Kohlschütter, A.; Beisiegel, U. Plasma ubiquinol-10 is decreased in patients with hyperlipidaemia. Atherosclerosis 1997, 129, 119–126. [Google Scholar] [CrossRef]
- Chen, W.; Liu, C.; Peng, B.; Zhao, Y.; Pacheco, A.; Xian, M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem. Sci. 2013, 4, 2892–2896. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |




| n (male) | 9 (5) |
|---|---|
| Age (years) | 28.44 ± 7.62 |
| BMI | 20.85 ± 2.86 |
| Polysulfides (μM) | |
| Plasma | 7469.4 ± 656.68 |
| Tear | 953.55 ± 244.98 |
| Saliva | 40.854 ± 27.348 |
| Nasal discharge | 397.61 ± 399.84 |
| Semen (n = 4) | 594.68 ± 244.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, M.; Ishima, Y.; Chuang, V.T.G.; Sakai, M.; Osafune, H.; Ando, H.; Shimizu, T.; Okuhira, K.; Watanabe, H.; Maruyama, T.; et al. Distribution of Polysulfide in Human Biological Fluids and Their Association with Amylase and Sperm Activities. Molecules 2019, 24, 1689. https://doi.org/10.3390/molecules24091689
Ikeda M, Ishima Y, Chuang VTG, Sakai M, Osafune H, Ando H, Shimizu T, Okuhira K, Watanabe H, Maruyama T, et al. Distribution of Polysulfide in Human Biological Fluids and Their Association with Amylase and Sperm Activities. Molecules. 2019; 24(9):1689. https://doi.org/10.3390/molecules24091689
Chicago/Turabian StyleIkeda, Mayumi, Yu Ishima, Victor T. G. Chuang, Maki Sakai, Hiroki Osafune, Hidenori Ando, Taro Shimizu, Keiichiro Okuhira, Hiroshi Watanabe, Toru Maruyama, and et al. 2019. "Distribution of Polysulfide in Human Biological Fluids and Their Association with Amylase and Sperm Activities" Molecules 24, no. 9: 1689. https://doi.org/10.3390/molecules24091689