Amphiphilic Porphyrin Aggregates: A DFT Investigation
Abstract
:1. Introduction
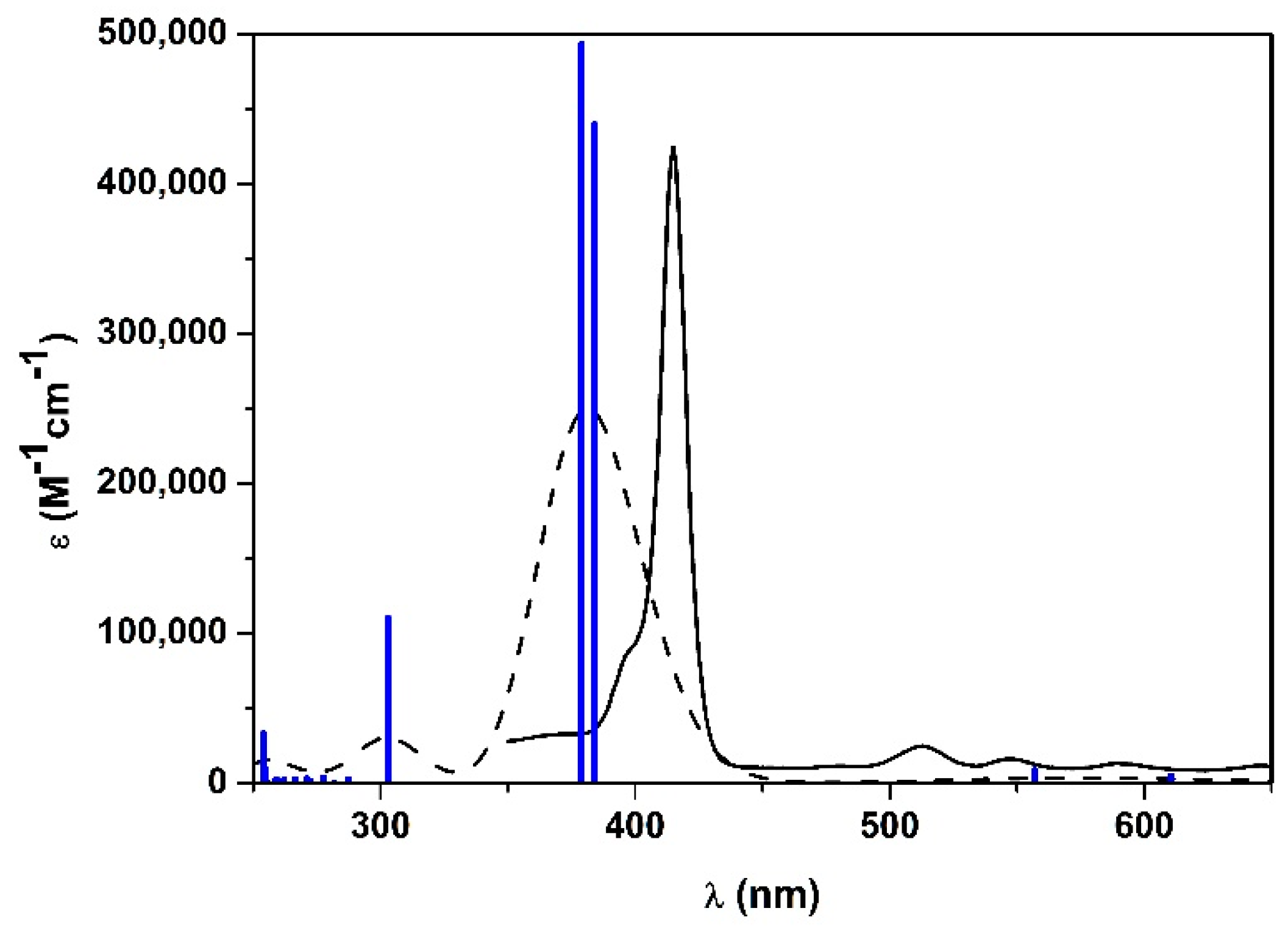
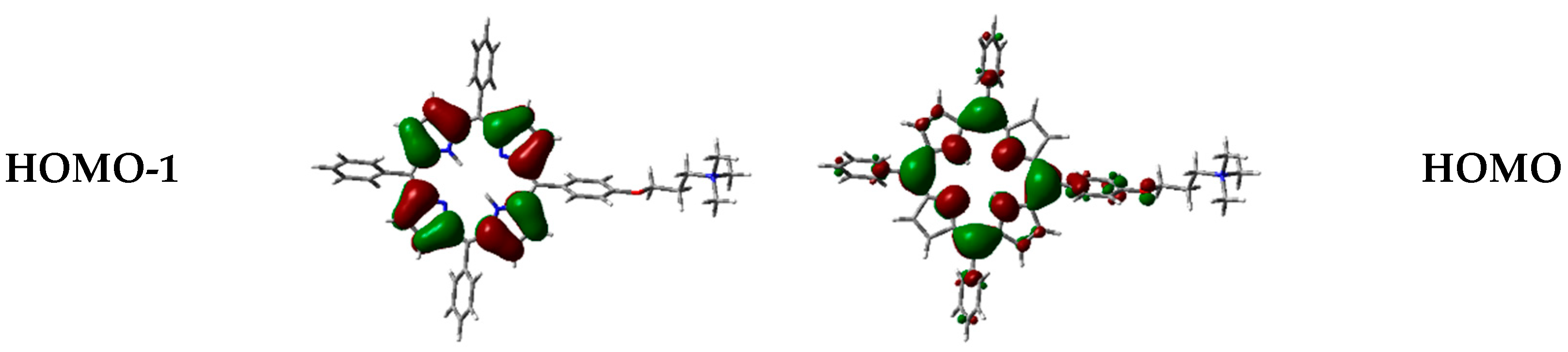
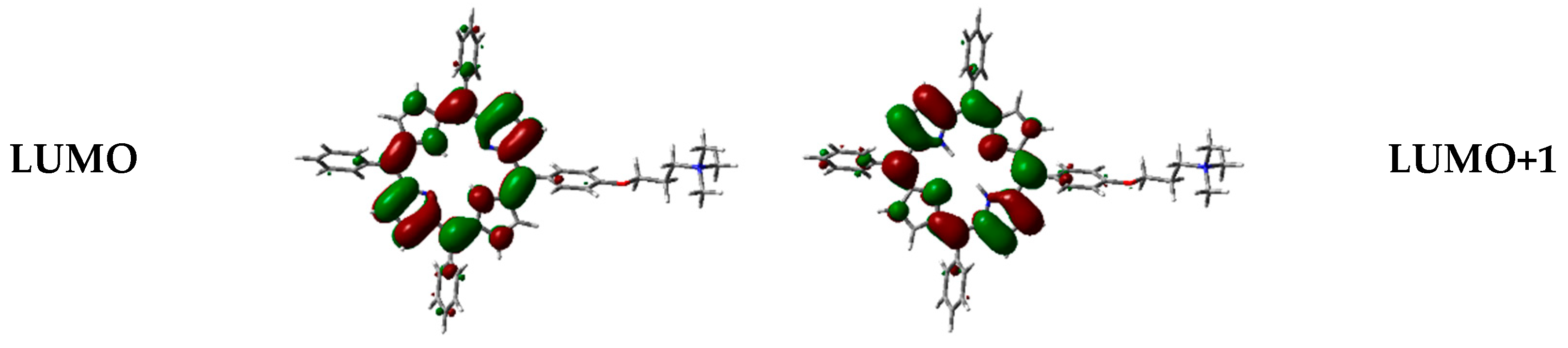
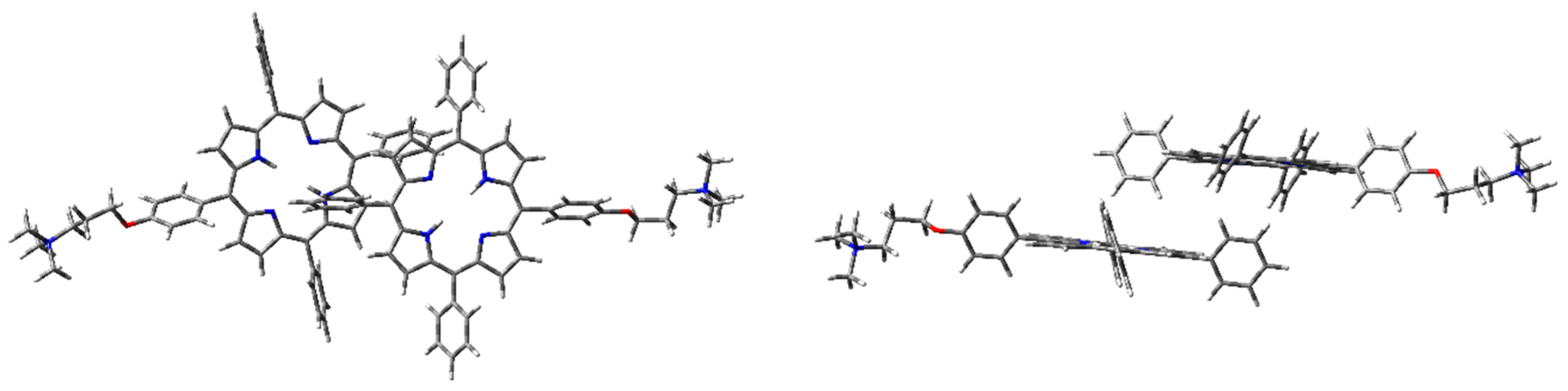
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, P.-P.; Chen, P.-L.; Ma, W.-H.; Liu, M.-H. Morphology-dependent Supramolecular Photocatalytic Performance of Porphyrin Nanoassemblies: From Molecule to Artificial Supramolecular Nanoantenna. J. Mater. Chem. 2012, 22, 20243–20249. [Google Scholar] [CrossRef]
- Meng, J.; Bi, P.; Jia, J.; Sun, X.; Chen, R. Light-Assisted Catalytic Oxidation from Porphyrin J-Aggregate. ChemistrySelect 2017, 2, 4882–4888. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, X.; Wang, R.; Yang, G.; Wang, T.; Jiang, J. Hierarchical Assembly of L- Phenylalanine-Terminated Bolamphiphile with Porphyrin Show Tunable Nanostructures and Photocatalytic Properties. ACS Omega 2018, 3, 10638–10646. [Google Scholar] [CrossRef] [PubMed]
- Purrello, R.; Gurrieri, S.; Lauceri, R. Porphyrin Assemblies as Chemical Sensors. Coord. Chem. Rev. 1999, 190, 683–706. [Google Scholar] [CrossRef]
- Di Natale, C.; Monti, D.; Paolesse, R. Chemical Sensitivity of Porphyrin Assemblies. Mater. Today 2010, 13, 46–52. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Stefanelli, M.; Monti, D.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 42517–42583. [Google Scholar] [CrossRef] [Green Version]
- Elemans, J.A.A.W.; van Hameren, R.; Nolte, R.J.M.; Rowan, A.E. Molecular Materials by Self-Assembly of Porphyrins, Phthalocyanines, and Perylenes. Adv. Mater. 2006, 18, 1251–1266. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-Organized Porphyrinic Materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Jiao, T.; Li, J.; Han, D.; Wang, R.; Tian, G.; Peng, Q. Chiral Nanostructured Composite Films via Solvent-Tuned Self-Assembly and Their Enantioselective Performances. Langmuir 2019, 35, 3337–3345. [Google Scholar] [CrossRef]
- Sabuzi, F.; Tiravia, M.; Vecchi, A.; Gatto, E.; Venanzi, M.; Floris, B.; Conte, V.; Galloni, P. Deposition of tetraferrocenylporphyrins on ITO surfaces for photo-catalytic O2 activation. Dalton Trans. 2016, 45, 14745–14753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchi, A.; Erickson, N.R.; Sabin, J.R.; Floris, B.; Conte, V.; Venanzi, M.; Galloni, P.; Nemykin, V.N. Electronic Properties of Mono-Substituted TetraferrocenylPorphyrins in Solution and on a Gold Surface: Assessment of the Influencing Factors for Photoelectrochemical Applications. Chem. Eur. J. 2015, 21, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchi, A.; Grippo, V.; Floris, B.; Marrani, A.G.; Conte, V.; Galloni, P. π-Interactions as a tool for an easy deposition of meso-tetraferrocenylporphyrin on surfaces. New J. Chem. 2013, 37, 3535–3542. [Google Scholar] [CrossRef]
- Vecchi, A.; Gatto, E.; Floris, B.; Conte, V.; Venanzi, M.; Nemykin, V.N.; Galloni, P. Tetraferrocenylporphyrins as active components of self-assembled monolayers on gold surface. Chem. Commun. 2012, 48, 5145–5147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koti, A.S.R.; Periasamy, N. Self-Assembly of Template-Directed J-Aggregates of Porphyrin. Chem. Mater. 2003, 152, 369–371. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sada, T.; Tateishi, Y.; Suzuki, T.; Sei, Y.; Yamaguchu, K.; Shinkai, S. Template-assisted control of porphyrin aggregation by ladder-type supramolecular assemblies. Tetrahedron Lett. 2005, 46, 5347–5350. [Google Scholar] [CrossRef]
- Bhosale, R.S.; La, D.D.; Al Kobaisi, M.; Bhosale, S.V.; Bhosale, S.V. Melamine and Spermine Mediated Supramolecular Self-Assembly of Octaphosponate Tetraphenyl Porphyrin. ChemistrySelect 2017, 2, 1573–1577. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-Assisted Self-Assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef]
- Pradines, V.; Bijani, C.; Stigliani, J.-L.; Blanzat, M.; Rico-Lattes, I.; Pratviel, G. Cationic Porphyrin–Anionic Surfactant Mixtures for the Promotion of Self-Organized 1:4 Ion Pairs in Water with Strong Aggregation Properties. ChemPhysChem 2015, 16, 3877–3885. [Google Scholar] [CrossRef]
- Simoncini, E.; Caroleo, F.; Ceccacci, F.; Mancini, G.; Stefanelli, M.; Paolesse, R.; Lettieri, R.; Venanzi, M.; Monti, D. Surfactant-induced Chirality on Reluctant Aggregates of a Chiral Amphiphilic Cationic (L)-Proline–Zn(II)Porphyrin Conjugate in Water. RSC Adv. 2014, 4, 55362–55366. [Google Scholar] [CrossRef]
- Stefanelli, M.; Coticone, R.; Sbardella, P.; Ceccacci, F.; Mancini, G.; Mandoj, F.; Paolesse, R.; Venanzi, M.; Monti, D. The aggregation of Amphiphilic (L)-Proline-Porphyrin Derivatives in Ethanol-Water Mixtures Promoted by Chiral Anionic Surfactants. J. Porphyr. Phthalocyanines 2017, 21, 391–397. [Google Scholar] [CrossRef]
- Liu, H.-G.; Feng, X.-S.; Zhang, L.-J.; Ji, G.-L.; Qian, D.-J.; Lee, Y.; Yang, K.-Z. Influences of Hydrophilic and Hydrophobic Substituents on the Organization of Supramolecular Assemblies of Porphyrin Derivatives Formed at the Air/Water Interface. Mater. Sci. Eng. C 2003, 23, 585–592. [Google Scholar] [CrossRef]
- Lettieri, R.; Monti, D.; Zelenka, K.; Trnka, T.; Drasar, P.; Venanzi, M. Glucosylated Steroid-Porphyrins as New Tools for Nanotechnology Applications. New J. Chem. 2012, 36, 1246–1254. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Chen, P.; Rong, Y.; Liu, M. Interfacial Organization of Achiral Porphyrins via Unidirectional Compression: A General Method for Chiroptical Porphyrin Assemblies of Selected Chirality. Phys. Chem. Chem. Phys. 2016, 18, 14023–14029. [Google Scholar] [CrossRef] [PubMed]
- Purrello, R.; Bellacchio, E.; Gurrieri, S.; Lauceri, R.; Raudino, R.; Monsù Scolaro, L.; Santoro, A.M. pH Modulation of Porphyrins Self-Assembly onto Polylysine. J. Phys. Chem. B 1998, 102, 8852–8857. [Google Scholar] [CrossRef]
- Occhiuto, I.G.; Zagami, R.; Trapani, M.; Bolzonello, L.; Romeo, A.; Castriciano, M.A.; Collini, E.; Monsù Scolaro, L. The Role of Counter-Anions in the Kinetics and Chirality of Porphyrin J-aggregates. Chem. Commun. 2016, 52, 11520–11523. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Karikis, K.; Han, J.; Kokotidou, C.; Charisiadis, A.; Folias, F.; Douvas, A.M.; Mitraki, A.; Charalambidis, G.; Yan, X.; et al. A self-assembly study of PNA–porphyrin and PNA–BODIPY hybrids in mixed solvent systems. Nanoscale 2019, 11, 3557–3566. [Google Scholar] [CrossRef]
- Mabesoone, M.F.J.; Markvoort, A.J.; Banno, M.; Yamaguchi, T.; Helmich, F.; Naito, Y.; Yashima, E.; Palmans, A.R.A.; Mejer, E.W. Competing Interactions in Hierarchical Porphyrin Self-Assembly Introduce Robustness in Pathway Complexity. J. Am. Chem. Soc. 2018, 140, 7810–7819. [Google Scholar] [CrossRef]
- Monti, D.; De Rossi, M.; Sorrenti, A.; Laguzzi, G.; Gatto, E.; Stefanelli, M.; Venanzi, M.; Luvidi, L.; Mancini, G.; Paolesse, R. Supramolecular Chirality in Solvent-Promoted Aggregation of Amphiphilic Porphyrin Derivatives: Kinetic Studies and Comparison between Solution Behavior and Solid-State Morphology by AFM Topography. Chem. Eur. J. 2010, 16, 860–870. [Google Scholar] [CrossRef]
- Zelenka, K.; Trnka, T.; Tislerova, I.; Monti, D.; Cinti, S.; Naitana, M.L.; Schiaffino, L.; Venanzi, M.; Laguzzi, G.; Luvidi, L.; et al. Spectroscopic, Morphological and Mechanistic Investigation of the Solvent-Promoted Aggregation of Porphyrins Modified in meso-Positions by Glucosylated Steroids. Chem. Eur. J. 2011, 17, 13743–13753. [Google Scholar] [CrossRef] [Green Version]
- Lorecchio, C.; Venanzi, M.; Mazzuca, C.; Lettieri, R.; Palleschi, A.; Nguyen Thi, T.H.; Cardova, L.; Drasar, P.; Monti, D. Tuning the chiroptical and morphological properties of steroidal-porphyrin aggregates: A mechanistic, structural, and MM investigation. Org. Biomol. Chem. 2014, 12, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Caroleo, F.; Stefanelli, M.; Magna, G.; Venanzi, M.; Paolesse, R.; Sennato, S.; Carbone, M.; Monti, D. Kinetic and Spectroscopic Studies on the Chiral Self-Aggregation of Amphiphilic Zinc and Copper (L)-Prolinate-Tetraarylporphyrin Derivatives in Different Aqueous Media. Org. Biomol. Chem. 2019, 17, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Monsù Scolaro, L.; Castriciano, M.; Romeo, A.; Mazzaglia, A.; Mallamace, F.; Micali, N. Nucleation Effects in the Aggregation of Water-Soluble Porphyrin Aqueous Solutions. Physica A 2002, 304, 158–169. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 7261–7277. [Google Scholar] [CrossRef] [PubMed]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.; Venanzi, M.; Russo, M.; Bussetti, G.; Goletti, C.; Montalti, M.; Zaccheroni, N.; Prodi, L.; Rella, R.; Manera, M.G.; et al. Spontaneous deposition of amphiphilic porphyrin films on glass. New J. Chem. 2004, 28, 1123–1128. [Google Scholar] [CrossRef]
- Bergendahl, L.T.; Paterson, M.J. Excited states of porphyrin and porphycene aggregates: Computational insights. Comput. Theor. Chem. 2014, 1040–1041, 274–276. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The Cation- π Interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef]
- Diaz Suarez, E.; Camargo Dalmatti Alves Lima, F.; Moura Dias, P.; Constantino, V.R.L.; Petrilli, H.M. Theoretical UV-Vis spectra of tetracationic porphyrin: Effects of environment on electronic spectral properties. J. Mol. Model. 2019, 25, 264. [Google Scholar] [CrossRef]
- Sánchez-Bojorgea, N.A.; Zaragoza-Galáan, G.; Flores-Holguín, N.R.; Chávez-Rojo, M.A.; Castro-García, C.; Rodríguez-Valdez, L.M. Theoretical analysis of the electronic properties in Zinc-porphyrinsderivatives. J. Mol. Struct. 2019, 1191, 259–270. [Google Scholar] [CrossRef]
- Stefanelli, M.; Ricci, A.; Chiarini, M.; Lo Sterzo, C.; Berionni Berna, B.; Pomarico, G.; Sabuzi, F.; Galloni, P.; Fronczek, F.R.; Smith, K.M.; et al. β-Arylethynyl substituted silver corrole complexes. Dalton Trans. 2019, 48, 13589–13598. [Google Scholar] [CrossRef] [PubMed]
- Tiravia, M.; Sabuzi, F.; Cirulli, M.; Pezzola, S.; Di Carmine, G.; Cicero, D.O.; Floris, B.; Conte, V.; Galloni, P. 3,7-Bis(N-methyl-N-phenylamino)phenothiazinium Salt: Improved Synthesis and Aggregation Behavior in Solution. Eur. J. Org. Chem. 2019, 3208–3216. [Google Scholar] [CrossRef]
- Paizs, B.; Suhai, S. Comparative Study of BSSE Correction Methods at DFT and MP2 Levels of Theory. J. Comput. Chem. 1998, 19, 575–584. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- da Costa, L.M.; Stoyanov, S.R.; Gusarov, S.; Tan, X.; Gray, M.R.; Stryker, J.M.; Tykwinski, R.; de M. Carneiro, J.W.; Seidl, P.R.; Kovalenko, A. Density Functional Theory Investigation of the Contributions of π−π Stacking and Hydrogen-Bonding Interactions to the Aggregation of Model Asphaltene Compounds. Energy Fuels 2012, 26, 2727–2735. [Google Scholar] [CrossRef]
- Ghosh, M.; Siddlingeshwar, S.B.; Thomas, A.; Sinha, S. Photoinduced electron transfer in non-covalent free-base octaethylporphyrin and 2-nitrofluorene donor-acceptor system: A combined experimental and quantum chemical study. J. Mol. Liq. 2019, 277, 656–662. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Sample Availability: Samples of the compounds 1H2 are available from the authors. |






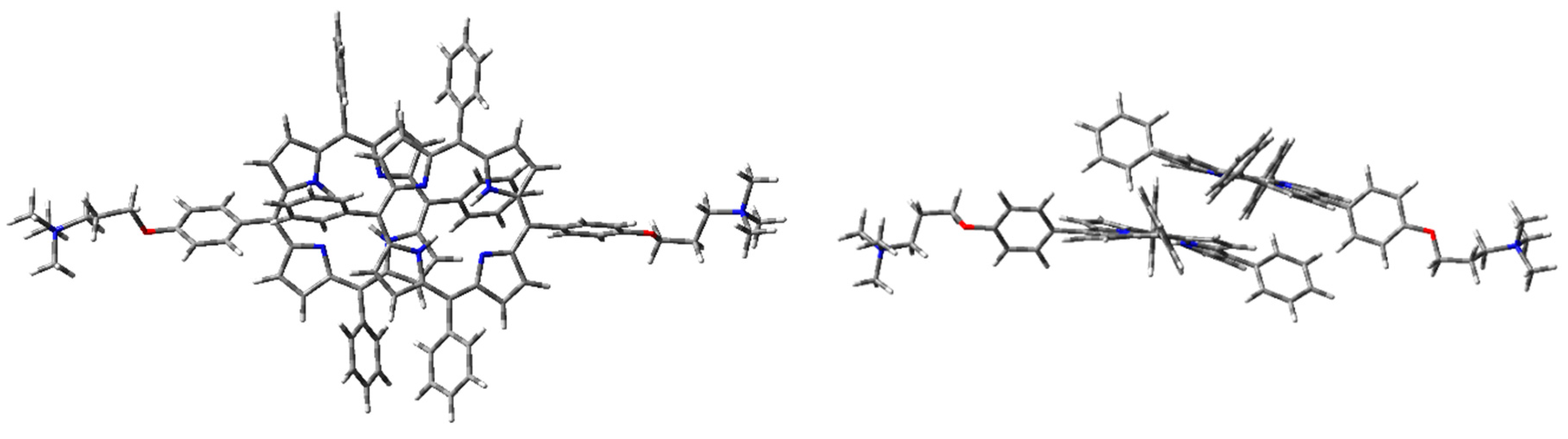
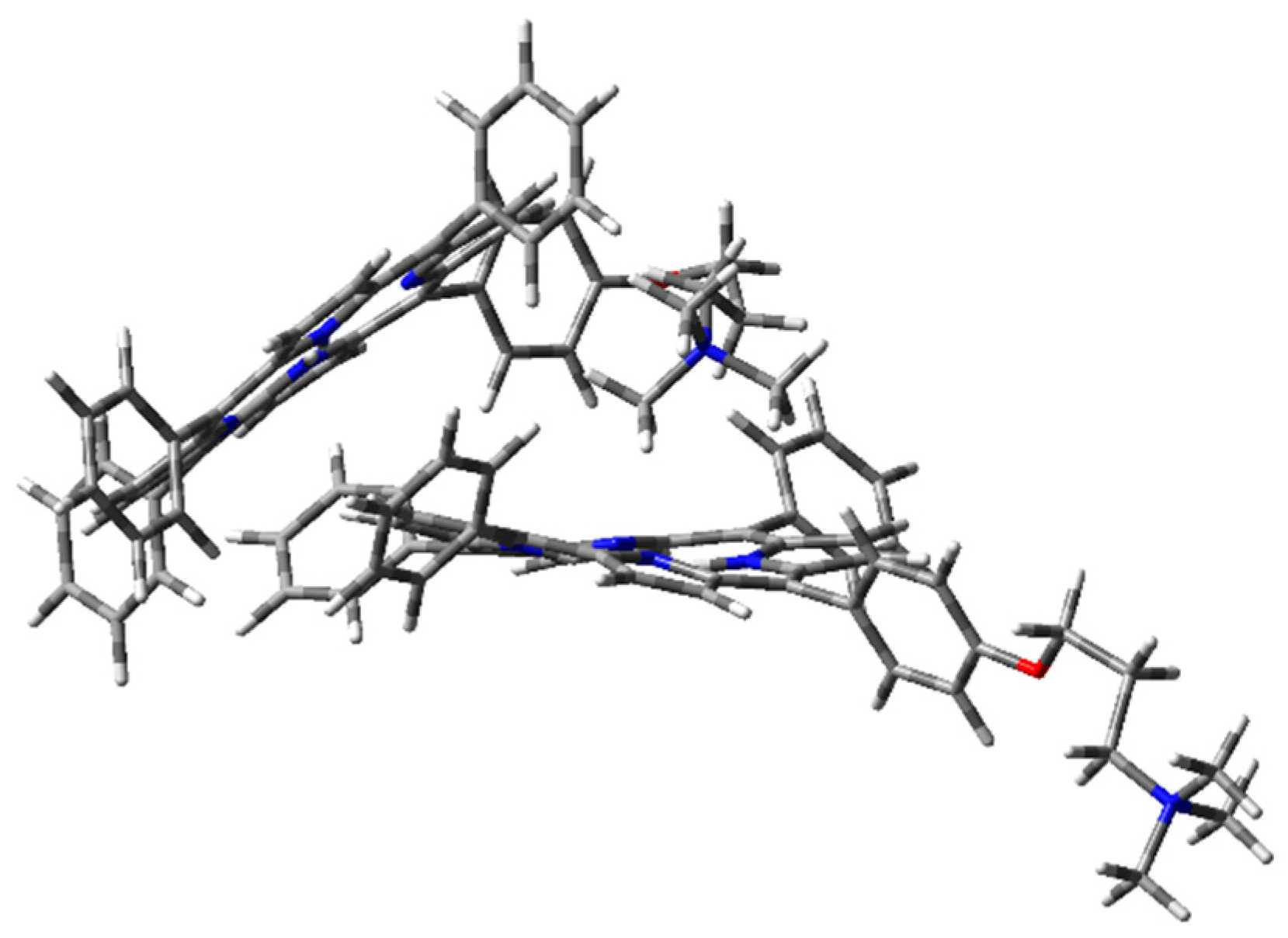
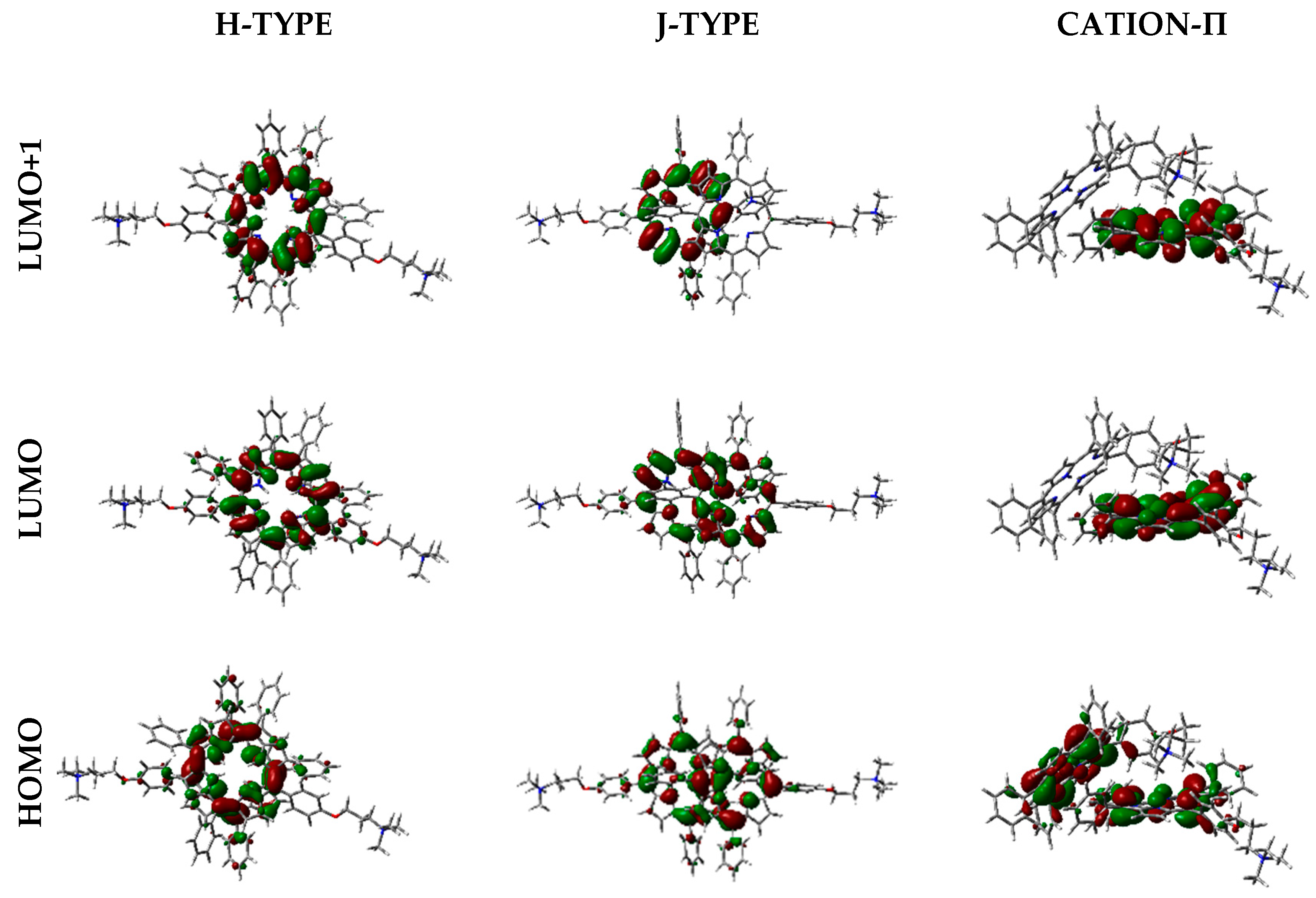

| Modeled Dimeric Structure | ΔECAM-B3LYP (kcal·mol−1) | ΔEWB97XD (kcal·mol−1) |
|---|---|---|
| H | 5.7 | 12.1 |
| J | 3.5 | 16.1 |
| Cation-π | - | - |
| Dimeric Structure | ΔEWB97XD (kcal·mol−1) |
|---|---|
| H | - |
| J | 7.2 |
| Cation-π | 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabuzi, F.; Stefanelli, M.; Monti, D.; Conte, V.; Galloni, P. Amphiphilic Porphyrin Aggregates: A DFT Investigation. Molecules 2020, 25, 133. https://doi.org/10.3390/molecules25010133
Sabuzi F, Stefanelli M, Monti D, Conte V, Galloni P. Amphiphilic Porphyrin Aggregates: A DFT Investigation. Molecules. 2020; 25(1):133. https://doi.org/10.3390/molecules25010133
Chicago/Turabian StyleSabuzi, Federica, Manuela Stefanelli, Donato Monti, Valeria Conte, and Pierluca Galloni. 2020. "Amphiphilic Porphyrin Aggregates: A DFT Investigation" Molecules 25, no. 1: 133. https://doi.org/10.3390/molecules25010133







