Chemical Kinetic Model of Multicomponent Gasoline Surrogate Fuel with Nitric Oxide in HCCI Combustion
Abstract
:1. Introduction
2. Results and Discussion
2.1. DIB Submechanism
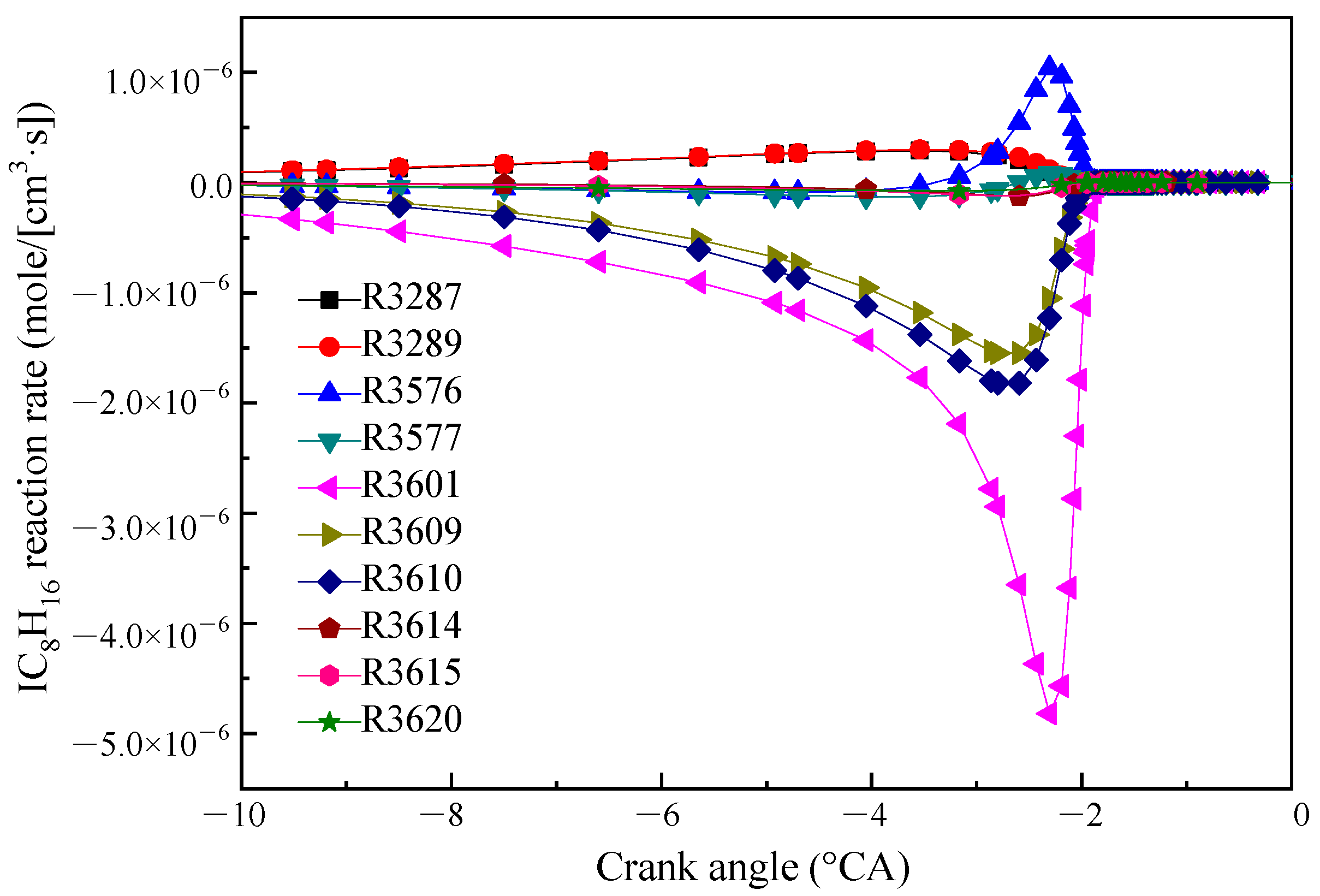
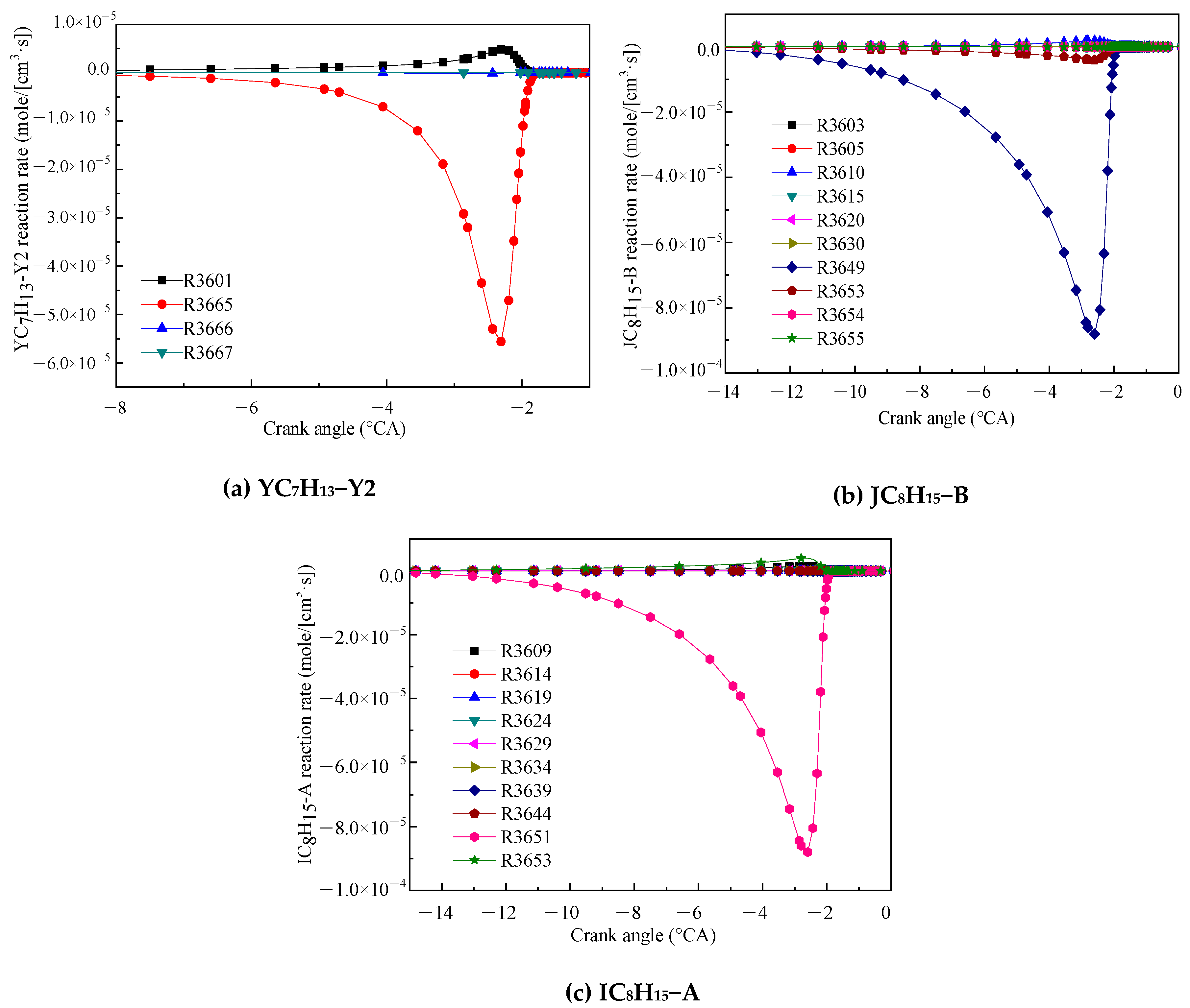
2.1.1. Analysis of the Reaction Path of IC8H16
2.1.2. Temperature Sensitivity Analysis of IC8H16
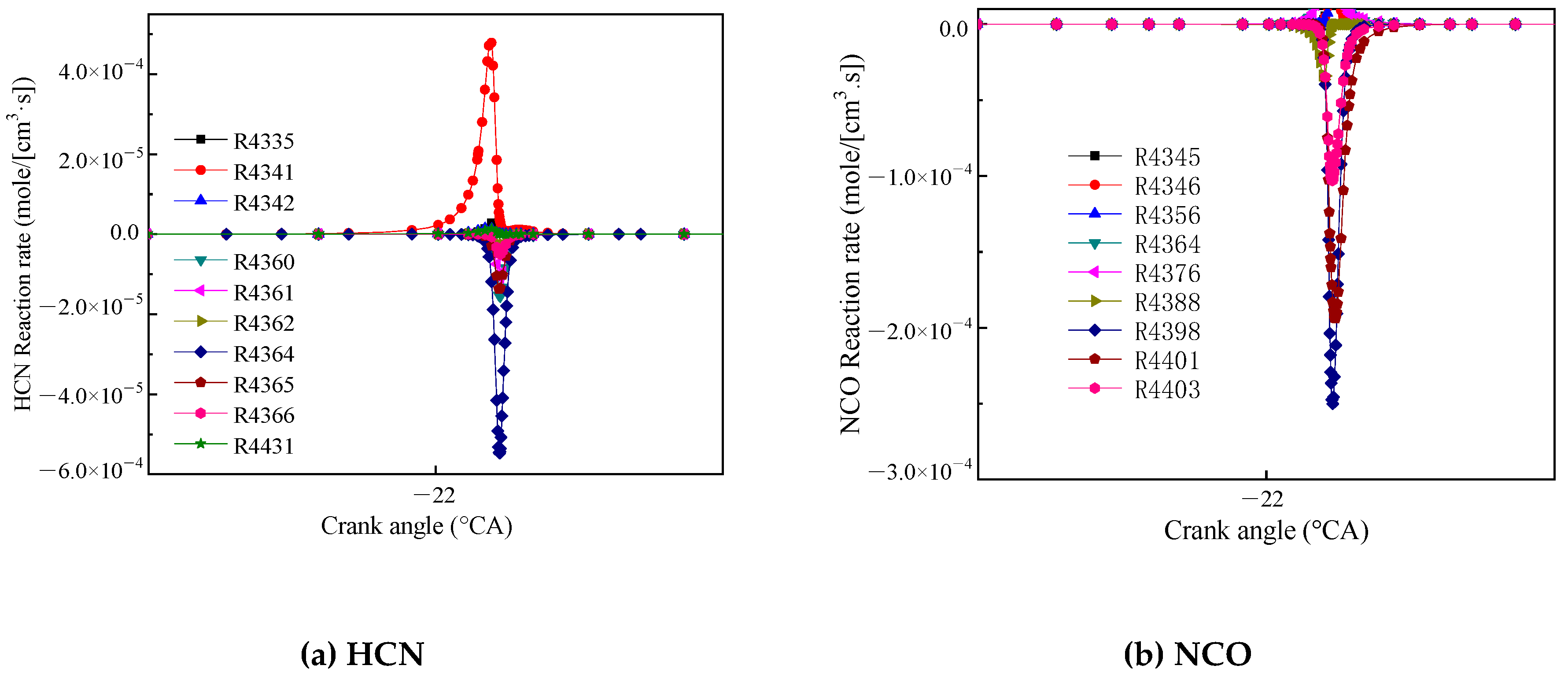
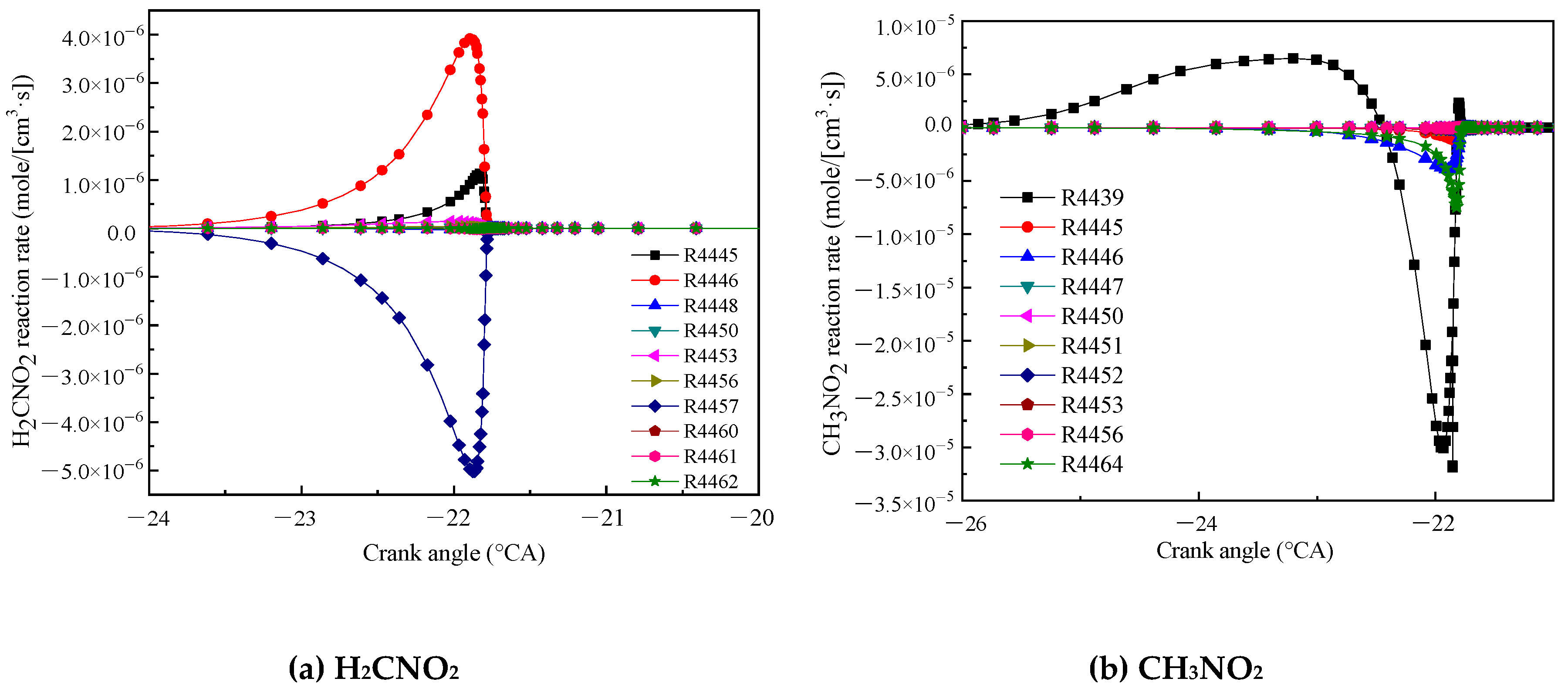
2.2. NO Submechanism
2.3. Validation of the Mechanism
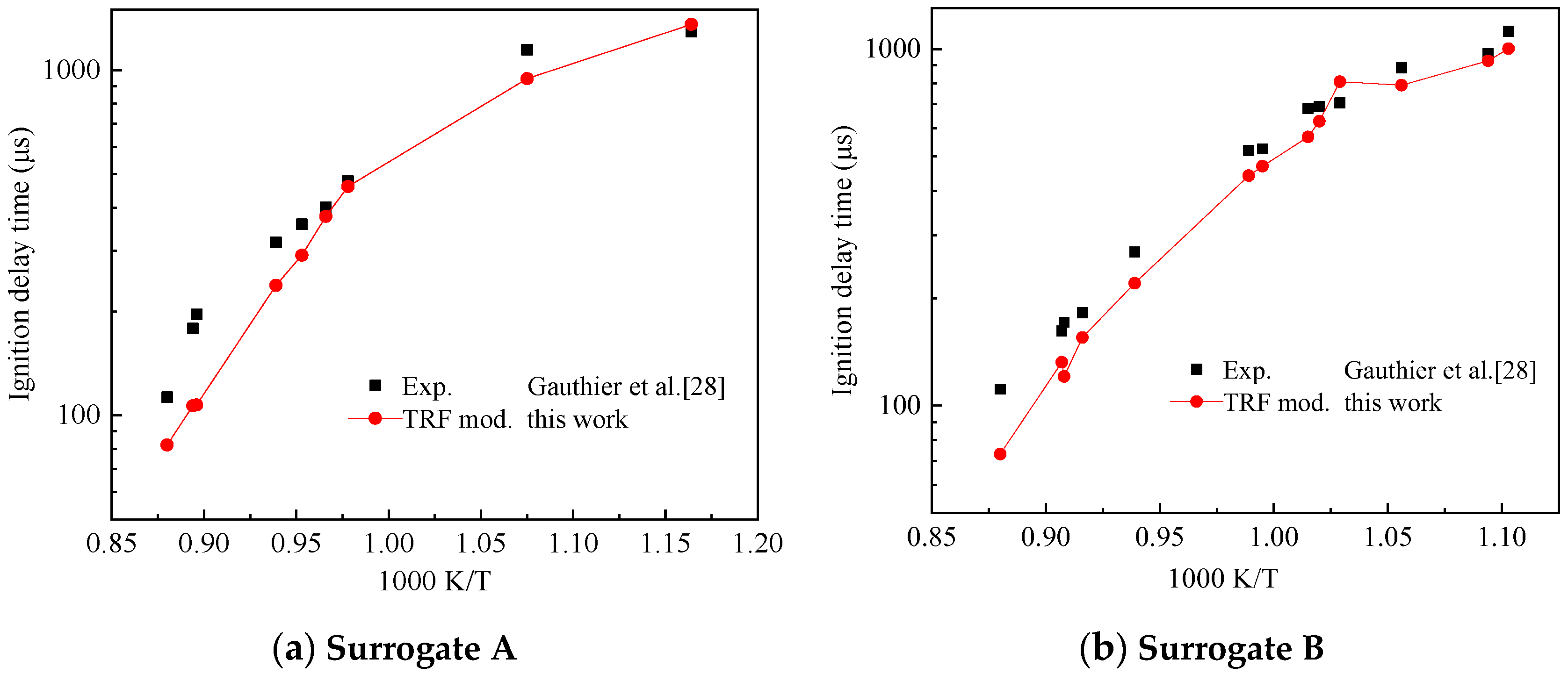
2.3.1. TRF Submechanism Verification
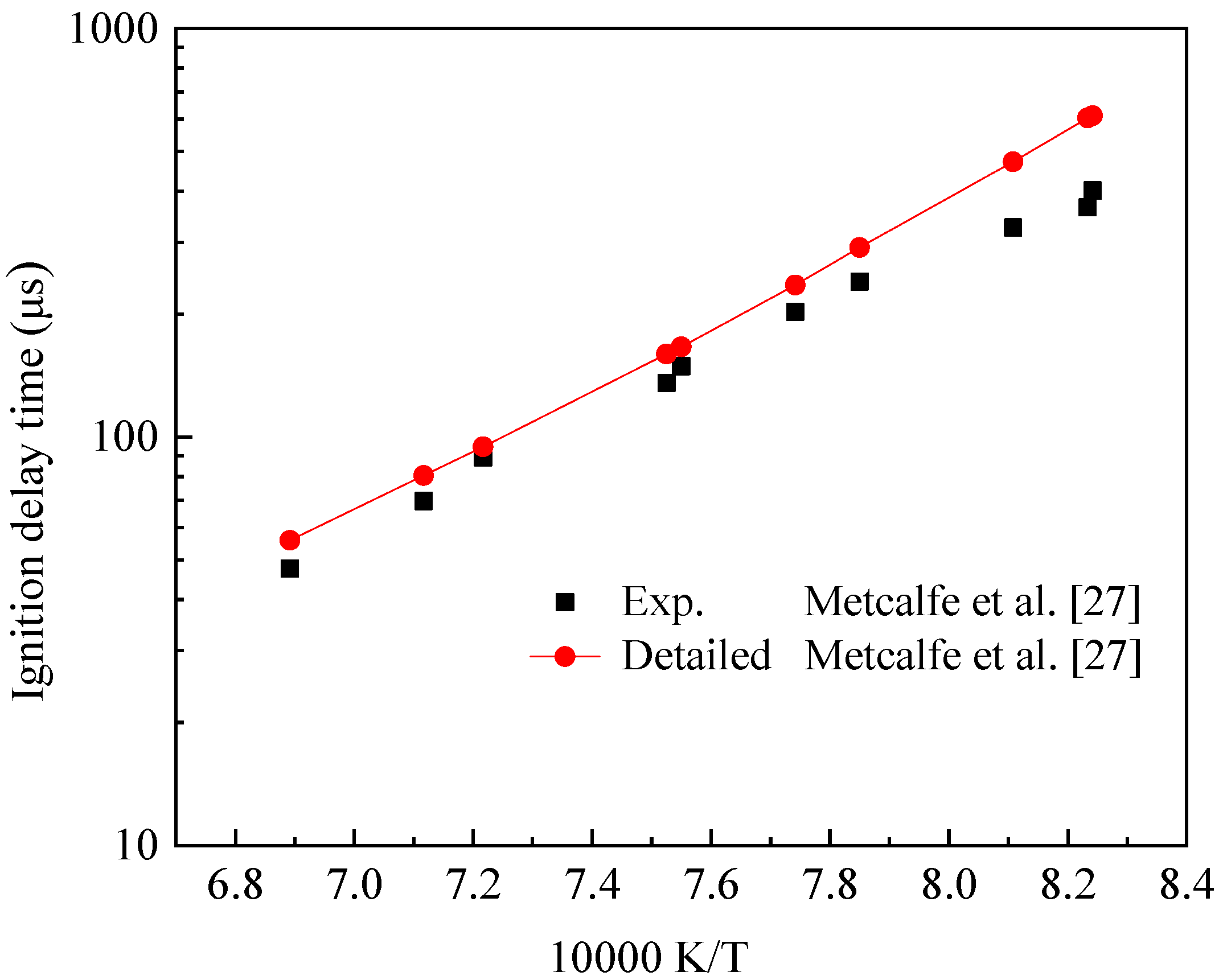
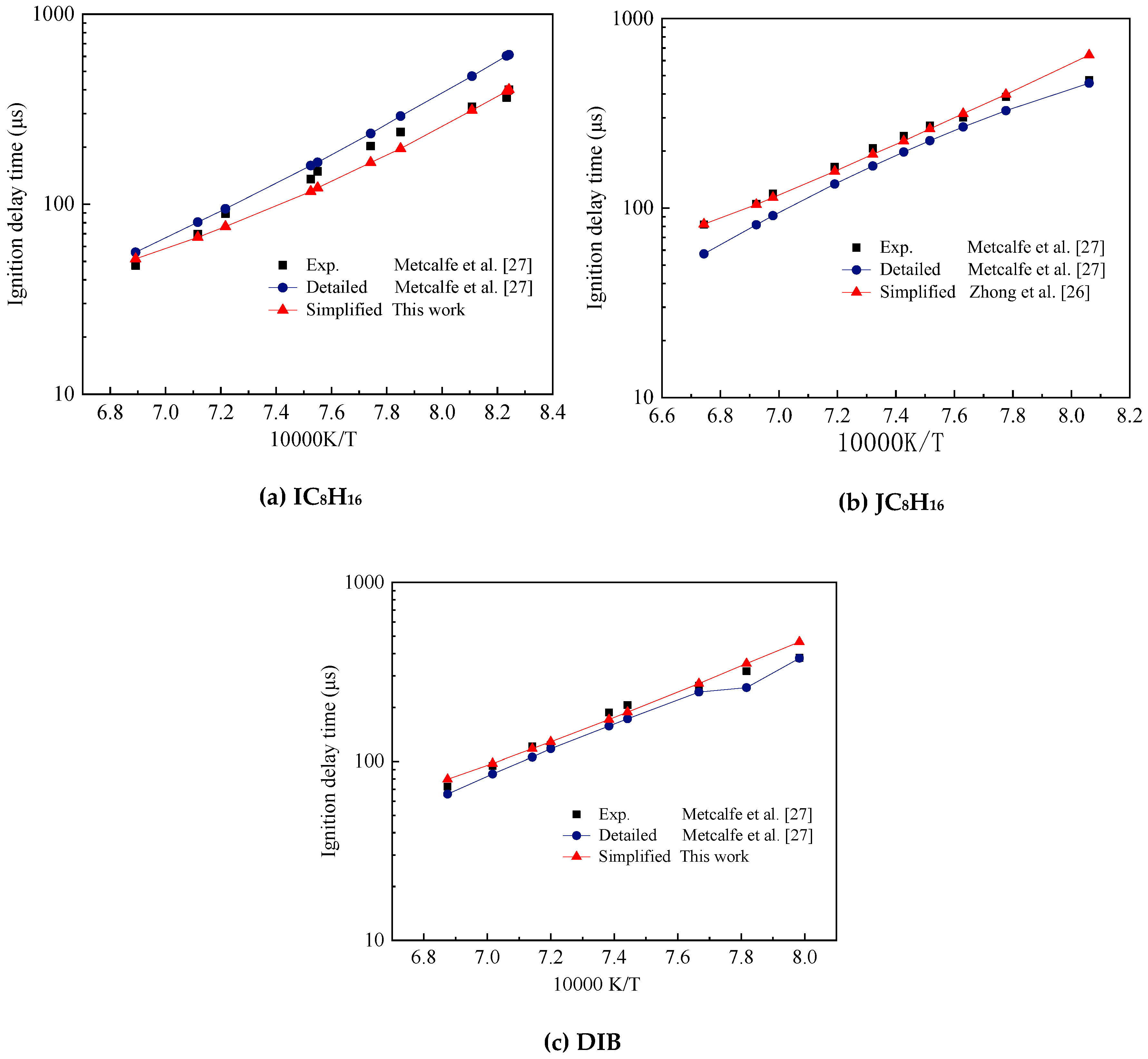
2.3.2. DIB Submechanism Verification
2.3.3. TDRF-Simplified Mechanism Verification
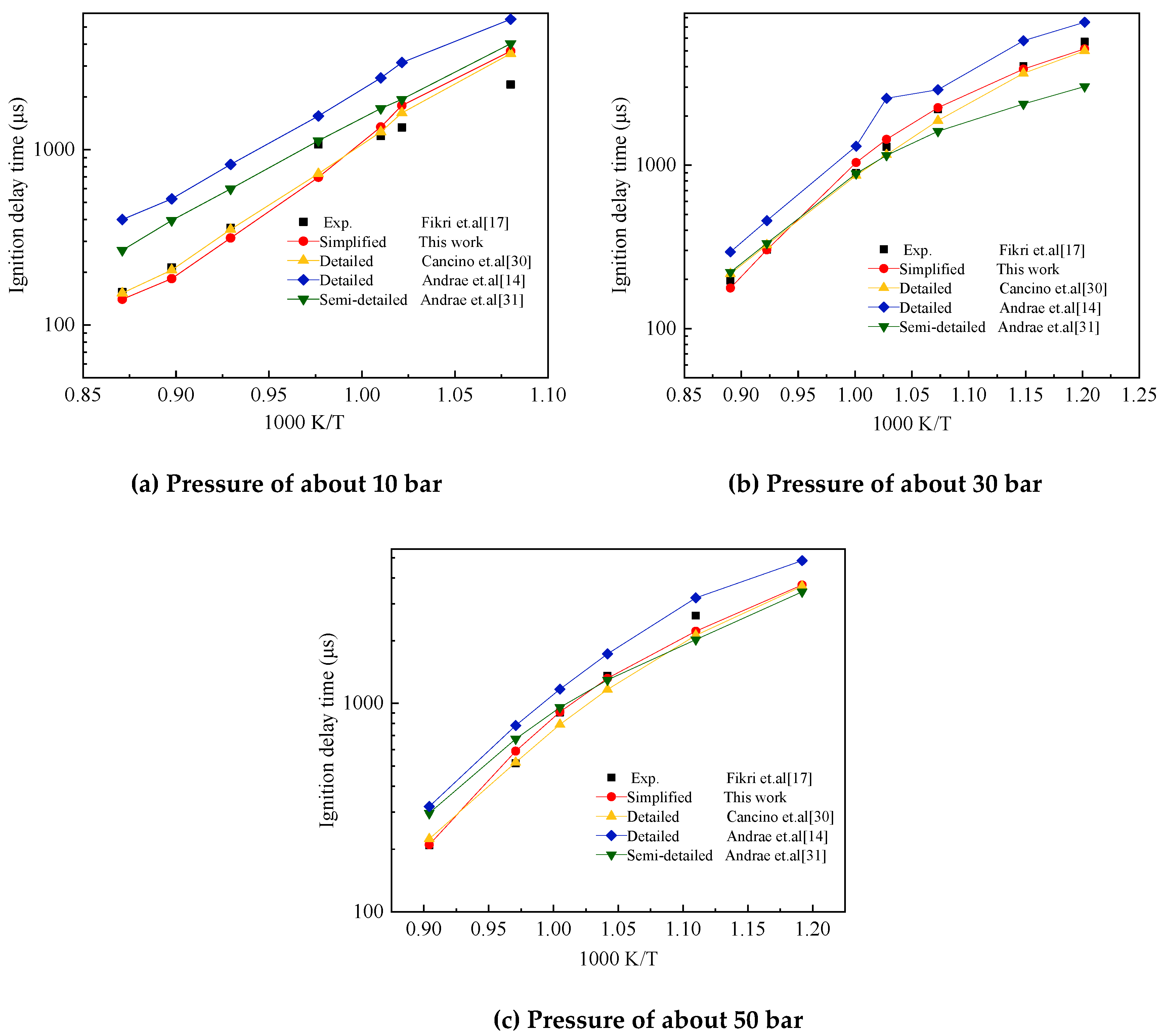
2.3.4. TDRF–NO-Simplified Mechanism Verification
3. Materials and Methods
3.1. Zero-Dimensional Simulation Software
3.2. Source of Kinetic Mechanisms
3.3. Simplified Calculation Method
3.3.1. Rate of Production Analysis
3.3.2. Sensitivity Analysis
3.4. Mechanism Verification Method
4. Conclusions
- (1)
- A complete simplified IC8H16 mechanism was obtained by analyzing the reaction path and temperature sensitivity of IC8H16. The simplified mechanism of IC8H16 was coupled with the simplified mechanism of JC8H16 to construct a DIB submechanism. The new mechanism was compared with the experimental data and detailed mechanism, and comparison results show that the consistency of the three models was better.
- (2)
- The NO submechanism was based on our previous research of NO submechanism and related reactions of NO and DIB were added to construct a NO submechanism containing 33 reactions.
- (3)
- The TRF mechanism was derived from the TRF part of the TDRF mechanism that we constructed before and the simplified mechanism of TDRF was formed by coupling the simplified mechanism of DIB. The ignition delay time was verified under the condition of a shock tube. The model verification results were in good agreement with the experimental data.
- (4)
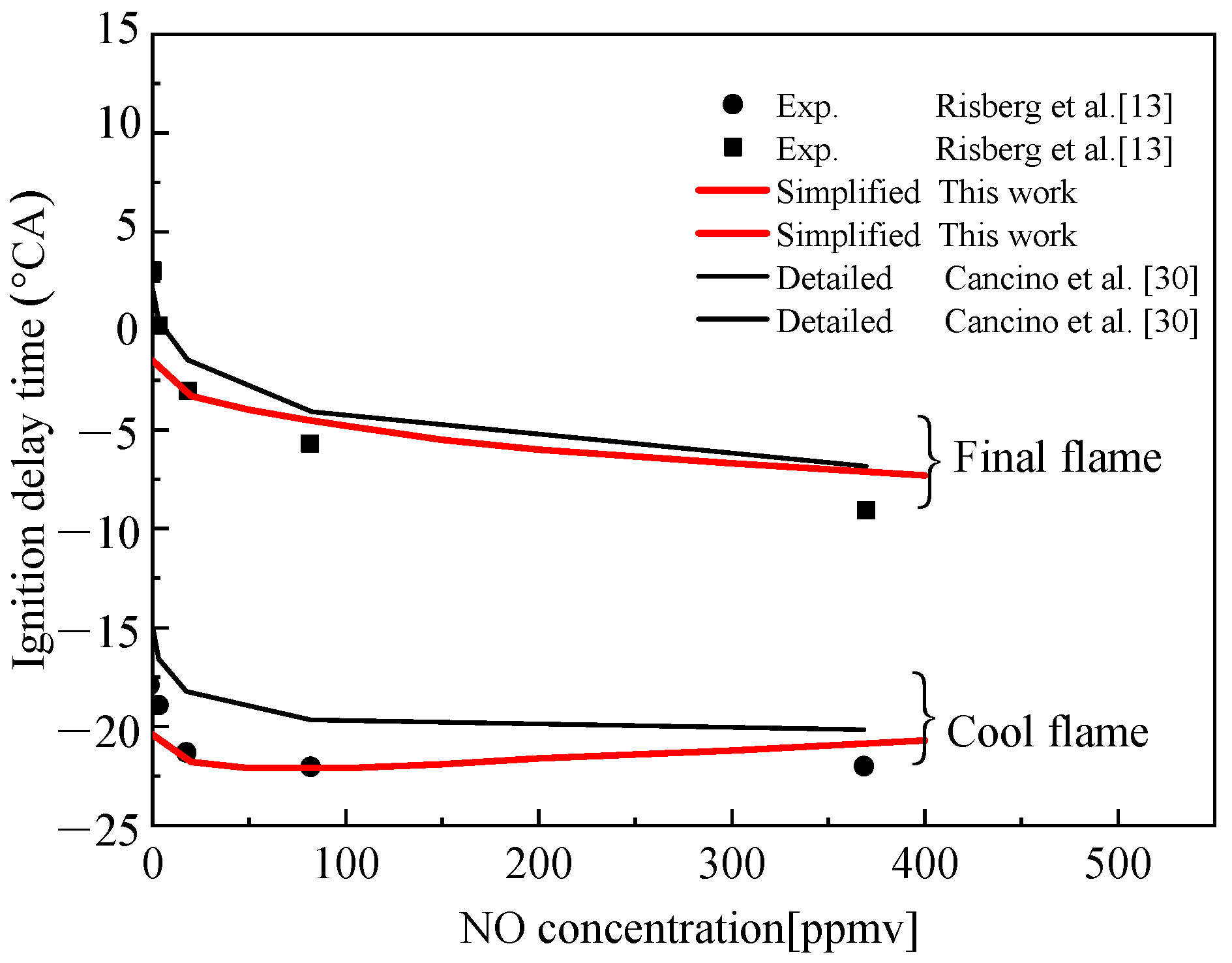
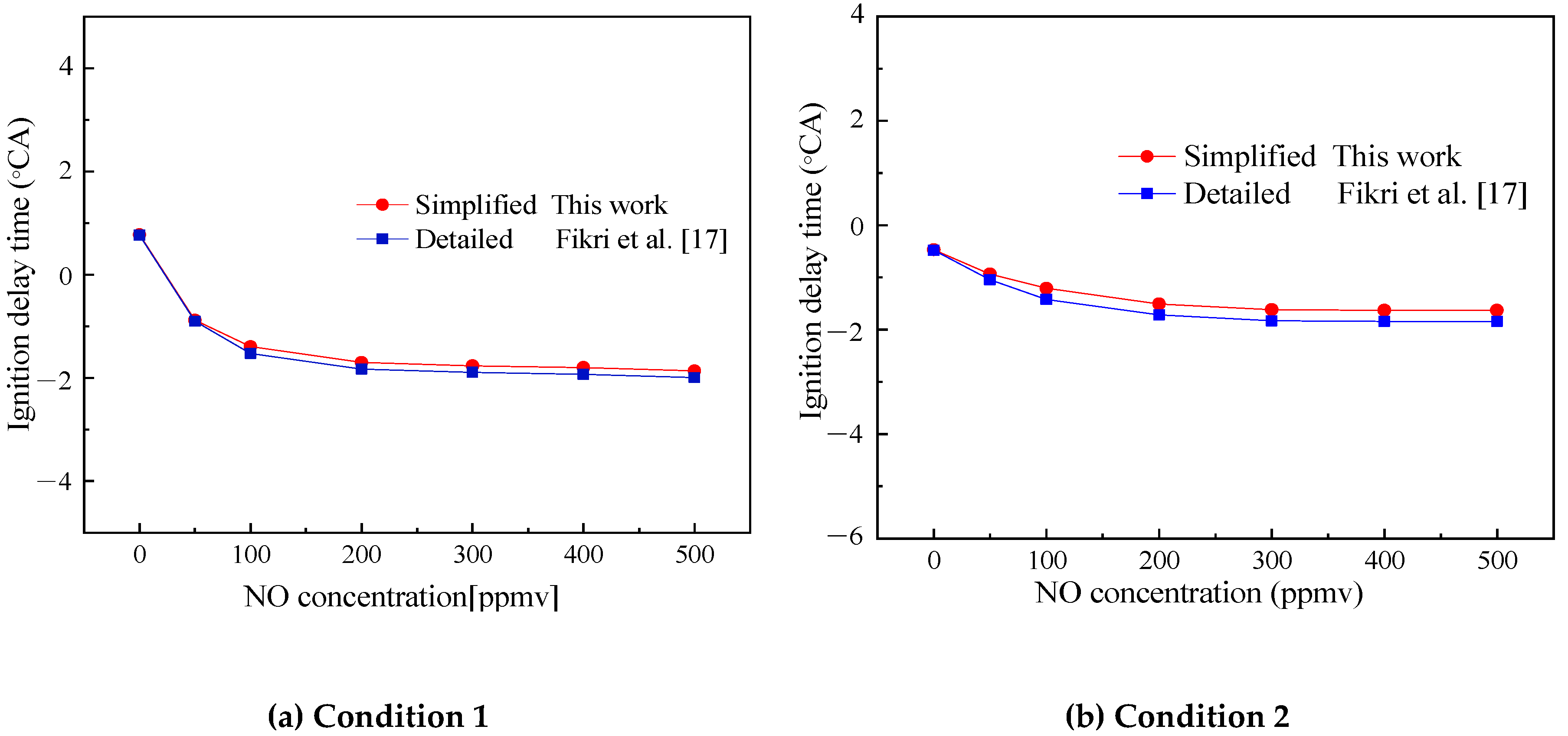
- The TDRF and NO submechanisms were coupled into a TDRF–NO chemical kinetic simplified mechanism. The two fuels were compared with the detailed mechanisms and experimental data. The ignition delay time of the simplified and detailed mechanism simulations increased with increasing NO concentration. The reliability of TDRF–NO simplified mechanism was illustrated.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, K.; Cho, S.; Kim, N.; Min, K. A study on combustion control and operating range expansion of gasoline HCCI. Energy 2015, 91, 1038–1048. [Google Scholar] [CrossRef]
- Gowthaman, S.; Sathiyagnanam, A.P. Analysis the optimum inlet air temperature for controlling homogeneous charge compression ignition (HCCI) engine. Alex. Eng. J. 2018, 57, 2209–2214. [Google Scholar] [CrossRef]
- Calam, A.; Solmaz, H.; Yılmaz, E.; Içingür, Y. Investigation of effect of compression ratio on combustion and exhaust emissions in a HCCI engine. Energy 2019, 168, 1208–1216. [Google Scholar] [CrossRef]
- Polat, S. An experimental study on combustion, engine performance and exhaust emissions in a HCCI engine fuelled with diethyl ether–ethanol fuel blends. Fuel Process. Technol. 2016, 143, 140–150. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Hariharan, D.; Yang, R.N.; Mamalis, S.; Lawler, B. A predictive 0-D HCCI combustion model for ethanol, natural gas, gasoline, and primary reference fuel blends. Fuel 2019, 237, 658–675. [Google Scholar] [CrossRef]
- Andrae, J.; Johansson, D.; Björnbom, P.; Risberg, P.; Kalghatgi, G. Co-oxidation in the auto-ignition of primary reference fuels and n-heptane/toluene blends. Combust. Flame 2005, 140, 267–286. [Google Scholar] [CrossRef]
- Aleiferis, P.G.; van Romunde, Z.R. An analysis of spray development with iso-octane, n-pentane, gasoline, ethanol and n-butanol from a multi-hole injector under hot fuel conditions. Fuel 2013, 105, 143–168. [Google Scholar] [CrossRef] [Green Version]
- Galmiche, B.; Halter, F.; Foucher, F. Effects of high pressure, high temperature and dilution on laminar burning velocities and Markstein lengths of iso-octane/air mixtures. Combust. Flame 2012, 159, 3286–3299. [Google Scholar] [CrossRef]
- Liu, Y.D.; Jia, M.; Xie, M.Z.; Pang, B. Improvement on a skeletal chemical kinetic model of iso-octane for internal combustion engine by using a practical methodology. Fuel 2013, 103, 884–891. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Pepiot, P.; Pitsch, H. A chemical mechanism for low to high temperature oxidation of n-dodecane as a component of transportation fuel surrogates. Combust. Flame 2014, 161, 866–884. [Google Scholar] [CrossRef]
- Vuilleumier, D.; Kozarac, D.; Mehl, M.; Saxena, S.; William, J.P.; Robert, W.D.; Chen, Y.J.; Sarathy, S.M. Intermediate temperature heat release in an HCCI engine fueled by ethanol/n-heptane mixtures: An experimental and modeling study. Combust. Flame 2014, 161, 680–695. [Google Scholar] [CrossRef]
- Su, W.H.; Huang, H.Z. Development and calibration of a reduced chemical kinetic model of n-heptane for HCCI engine combustion. Fuel 2005, 84, 1029–1040. [Google Scholar] [CrossRef]
- Risberg, P.; Johansson, D.; Andrae, J.; Kalghatgi, G.; Björnbom, P.; Ångström, H.E. The influence of NO on the combustion phasing in an HCCI engine. Sae Tech. Pap. 2006, 2, 209–213. [Google Scholar] [CrossRef]
- Andrae, J.C.G. Development of a detailed kinetic model for gasoline surrogate fuels. Fuel 2008, 87, 2013–2022. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Zheng, Z.L.; He, Z.W.; Wang, Y. Reduced Chemical Kinetic Model of Toluene Reference Fuels for HCCI Combustion. Acta Phys. Chim. Sin. 2011, 27, 530–538. [Google Scholar] [CrossRef]
- Coskun, G.; Demir, U.; Yilmaz, N.; Soyhan, H.S. Computational investigation of combustion and emission characteristics of toluene reference fuel (TRF) mixtures in an HCCI engine using stochastic reactor model. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 2935–2943. [Google Scholar] [CrossRef]
- Fikri, M.; Herzler, J.; Starke, R.; Schulz, C.; Roth, P.; Kalghatgi, G.T. Autoignition of gasoline surrogates mixtures at intermediate temperatures and high pressures. Combust. Flame 2008, 152, 276–281. [Google Scholar] [CrossRef]
- Griffiths, J.F. Reduced kinetic models and their application to practical combustion systems. Prog. Energy Combust. Sci. 1995, 21, 25–107. [Google Scholar] [CrossRef]
- Frassoldati, A.; Faravelli, T.; Ranzi, E. Kinetic modeling of the interactions between NO and hydrocarbons at high temperature. Combust. Flame 2003, 135, 97–112. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Z.; He, Z.; Wang, F. Kinetic Modeling of Nitric Oxide Sensitization of n-heptane Auto-ignition and Combustion. Energy Sources 2015, 37, 997–1004. [Google Scholar] [CrossRef]
- Glaude, P.A.; Marinov, N.; Koshiishi, Y.; Matsungaga, N.; Hori, M. Kinetic modeling of the mutual oxidation of NO and larger alkanes at low temperature. Energy Fuels 2005, 19, 1839–1849. [Google Scholar] [CrossRef]
- Dayma, G.; Dagaut, P. Experimental and Kinetic Modeling Study of the Impact of NO and NO2 on the Oxidation of a Primary Reference Fuels Mixture. In Proceedings of the Combustion Institute, Vienna, Austria, 14–17 April 2009. [Google Scholar]
- Contino, F.; Foucher, F.; Dagaut, P.; Lucchini, T.; D’Errico, G.; Rousselle, C.M. Experimental and numerical analysis of nitric oxide effect on the ignition of iso-octane in a single cylinder HCCI engine. Combust. Flame 2013, 160, 1476–1483. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Lv, Z.M. A new skeletal chemical kinetic model of gasoline surrogate fuel with nitric oxide in HCCI combustion. Appl. Energy 2015, 147, 59–66. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Liang, Z.L. Reduced Chemical Kinetic Model of a Gasoline Surrogate Fuel for HCCI Combustion. Acta Phys. Chim. Sin. 2015, 31, 1265–1274. [Google Scholar] [CrossRef]
- Zhong, B.J.; Zheng, D. A chemical mechanism for ignition and oxidation of multi-component gasoline surrogate fuels. Fuel 2014, 128, 458–466. [Google Scholar] [CrossRef]
- Metcalfe, W.K.; Pitz, W.J.; Curran, H.J.; Simmie, J.M.; Westbrook, C.K. The development of a detailed chemical kinetic mechanism for diisobutylene and comparison to shock tube ignition times. Proc. Combust. Inst. 2007, 31, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, B.M.; Davidson, D.F.; Hanson, R.K. Shock tube determination of ignition delay times in full-blend and surrogate fuel mixtures. Combust. Flame 2004, 139, 300–311. [Google Scholar] [CrossRef]
- Pera, C.; Knop, V. Methodology to define gasoline surrogates dedicated to auto-ignition in engines. Fuel 2012, 96, 59–69. [Google Scholar] [CrossRef]
- Cancino, L.R.; Fikri, M.; Oliveira, A.M.; Schulz, C. Ignition delay times of ethanol-containing multi-component gasoline surrogates: Shock-tube experiments and detailed modeling. Fuel 2011, 90, 1238–1244. [Google Scholar] [CrossRef]
- Andrae, J.C.G.; Head, R.A. HCCI experiments with gasoline surrogate fuels modeled by a semidetailed chemical kinetic model. Combust. Flame 2008, 156, 842–851. [Google Scholar] [CrossRef]
Sample Availability: Simplified mechanism of the TDRF–NO are available from the authors. |





| Index Item | Value |
|---|---|
| Compression ratio | 17.5 |
| Bore (mm) | 112 |
| Stroke (mm) | 132 |
| Displacement (cm3) | 1300 |
| Speed (rpm) | 1400 |
| Crankshaft radius ratio | 3.714 |
| Temperature (K) | 400 |
| Pressure (atm) | 1.5 |
| Intake valve closing time (°CA) | 38.5 |
| Fuel | Isooctane | Toluene | n-heptane | Diisobutylene | Ref. |
|---|---|---|---|---|---|
| Surrogate A | 63% | 20% | 17% | - | [28] |
| Surrogate B | 69% | 14% | 17% | - | [28] |
| Surrogate C | 13.7% | 34.8% | 51.5% | - | [29] |
| Surrogate D | 20% | 45% | 25% | 10% | [17] |
| Fuel | JC8H18 | IC8H16 | O2 | Ar |
|---|---|---|---|---|
| Fuel1 | - | 0.75% | 18% | 81.25% |
| Fuel2 | 0.75% | - | 18% | 81.25% |
| Fuel3 | 0.56% | 0.19% | 18% | 81.25% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zheng, Z. Chemical Kinetic Model of Multicomponent Gasoline Surrogate Fuel with Nitric Oxide in HCCI Combustion. Molecules 2020, 25, 2273. https://doi.org/10.3390/molecules25102273
Yang C, Zheng Z. Chemical Kinetic Model of Multicomponent Gasoline Surrogate Fuel with Nitric Oxide in HCCI Combustion. Molecules. 2020; 25(10):2273. https://doi.org/10.3390/molecules25102273
Chicago/Turabian StyleYang, Chao, and Zhaolei Zheng. 2020. "Chemical Kinetic Model of Multicomponent Gasoline Surrogate Fuel with Nitric Oxide in HCCI Combustion" Molecules 25, no. 10: 2273. https://doi.org/10.3390/molecules25102273





