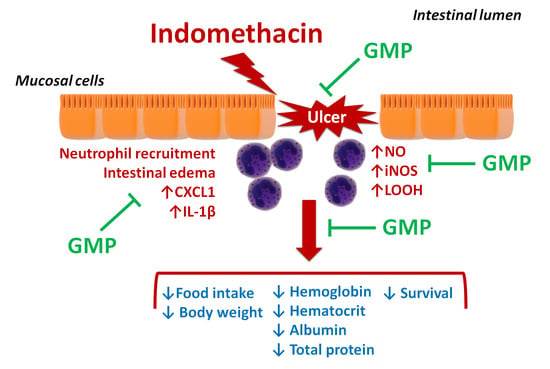
Glycomacropeptide Ameliorates Indomethacin-Induced Enteropathy in Rats by Modifying Intestinal Inflammation and Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Effect of GMP on Food Intake and Body Weight Gain
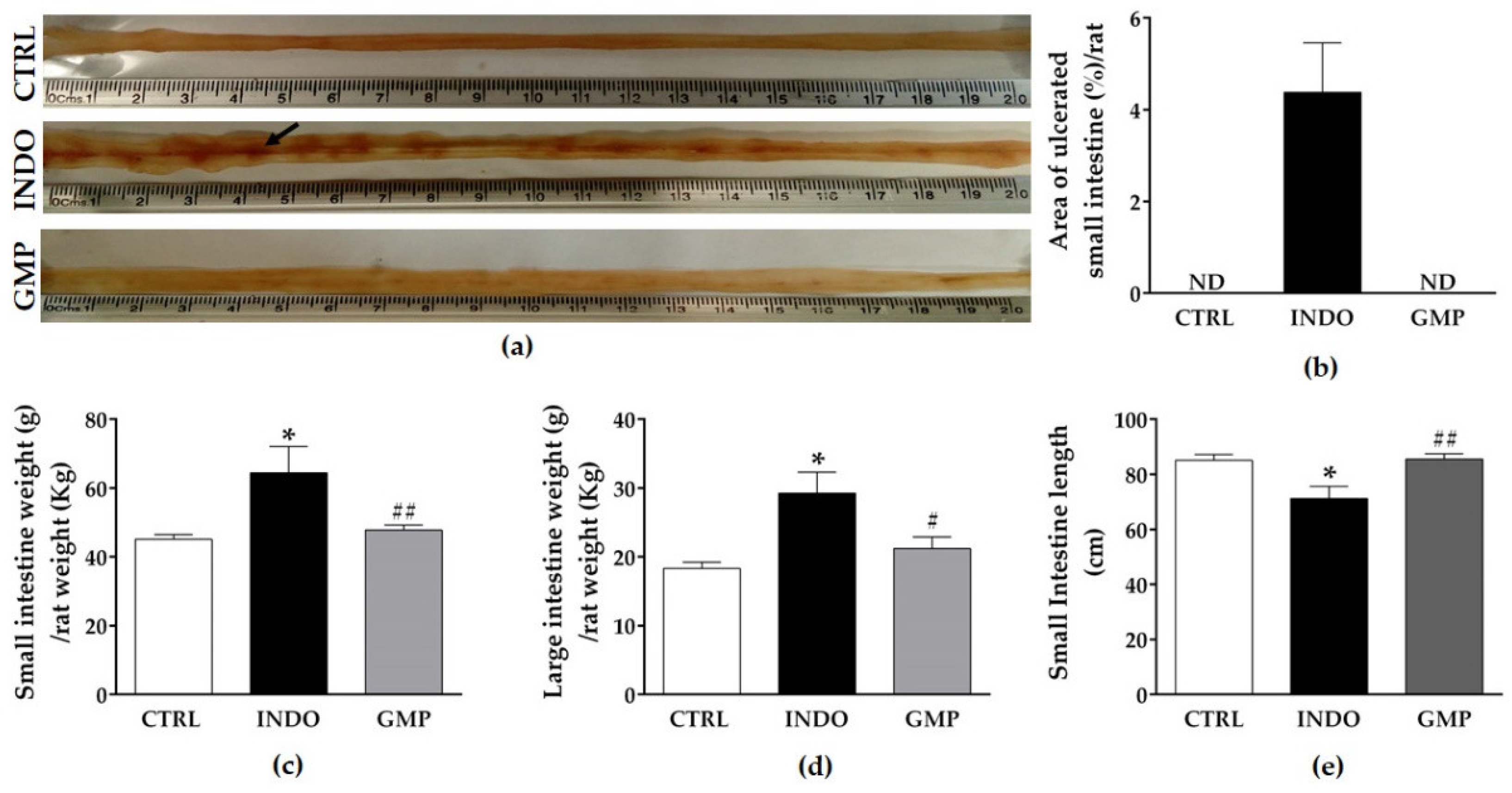
2.2. Effect of GMP on Intestinal Damage, and Weight and Length
2.3. Effect of GMP on Hematological and Biochemical Parameters
2.4. Effect of GMP on Intestinal Inflammation
2.5. Effect of GMP on Intestinal Oxidative Damage
2.6. Effect of GMP on Mucosal Barrier Integrity
2.7. Effect of GMP on Survival Rate
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Indomethacin-Induced Enteropathy Model and Sample Collection
4.3. Changes in Food Intake and Body Weight
4.4. Macroscopic Assessment of Small Intestine Damage
4.5. Evaluation of Hematological and Biochemical Parameters
4.6. Myeloperoxidase Activity
4.7. Immunofluorescence Assay
4.8. Determination of Nitric oxide and Lipids Hydroperoxides
4.9. RNA Extraction, Retrotranscription and qPCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinha, M.; Gautam, L.; Shulka, P.K.; Kaur, P.; Sharma, S.; Singh, T.P. Current perspectives in NSAID-induced gastrophaty. Mediators Inflam. 2013, 2013, 258209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamil, R.; Geier, M.S.; Butler, R.N.; Howarth, G.S. Lactobacillus rhamnosus GG exacerbates intestinal ulceration in a model of indomethacin-induced enteropathy. Dig. Dis. Sci. 2007, 52, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2012, 32, 1491–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef] [Green Version]
- Haworth, R.; Oakley, K.; McCormack, N.; Pilling, A. Differential expression of COX-1 and COX-2 in the gastrointestinal tract of the rat. Toxicol. Pathol. 2005, 33, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Sigthorsson, G.; Simpson, R.J.; Walley, M.; Anthony, A.; Foster, R.; Hotz-Behoftsitz, C.; Palizban, A.; Pombo, J.; Watts, J.; Morham, S.G.; et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 2002, 122, 1913–1923. [Google Scholar] [CrossRef]
- Park, S.C.; Chun, H.J.; Kang, C.D.; Sul, D. Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J. Gastroenterol. 2011, 17, 4647–4653. [Google Scholar] [CrossRef]
- Shin, S.J.; Noh, C.K.; Lim, S.G.; Lee, K.M.; Lee, K.J. Non-steroidal anti-inflammatory drug-induced enteropathy. Intest. Res. 2017, 15, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Bali, R.S.; Verma, S.; Agarwal, P.N.; Singh, R.; Talwar, N. Perforation peritonitis and the developing world. ISRN Surg. 2014, 2014, 105492. [Google Scholar] [CrossRef] [Green Version]
- Scarpignato, C.; Hunt, R.H. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. North. Am. 2010, 39, 433–464. [Google Scholar] [CrossRef]
- Tai, F.W.D.; McAlindon, M.E. NSAIDs and the small bowel. Curr. Opin. Gastroenterol. 2018, 34, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, D.; Bjarnason, I. Is non-steroidal anti-inflammaory drug (NSAID) enteropathy clinically more important than NSAID gastropathy? Postgrad. Med. J. 2006, 82, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.; Sigthorsson, G.; Simpson, R.J.; Watts, J.; Jacob, M.; Tavares, I.A.; Rafi, S.; Roseth, A.; Foster, R.; Price, A.B.; et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol. Ther. 2000, 14, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Basivireddy, J.; Vasudevan, A.; Jacob, M.; Balasubramanian, K.A. Indomethacin-induced mitochondrial dysfunction and oxidative stress in villus enterocytes. Biochem. Pharmacol. 2002, 64, 339–349. [Google Scholar] [CrossRef]
- Tanaka, A.; Hase, S.; Miyazawa, T.; Ohno, R.; Takeuchi, K. Role of cyclooxygenase (COX)-1 and COX-2 inhibition in nonsteroidal anti-inflammatory drug-induced intestinal damage in rats: Relation to various pathogenic events. J. Pharmacol. Exp. Ther. 2002, 303, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Mion, F.; Cuber, J.C.; Minaire, Y.; Chayvialle, J.A. Short term effects of indomethacin on rat small intestinal permeability. Role of eicosanoids and platelet activating factor. Gut 1994, 35, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Kunikata, T.; Araki, H.; Takeeda, M.; Kato, S.; Takeuchi, K. Prostaglandin E prevents indomethacin-induced gastric and intestinal damage through different EP receptor subtypes. J. Physiol. Paris. 2001, 95, 157–163. [Google Scholar] [CrossRef]
- Wallace, J.L. Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J. Gastroenterol. 2013, 19, 1861–1876. [Google Scholar] [CrossRef]
- Watanabe, T.; Higuchi, K.; Kobata, A.; Nishio, H.; Tanigawa, T.; Shiba, M.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 2008, 57, 181–187. [Google Scholar] [CrossRef]
- Konaka, A.; Kato, S.; Tanaka, A.; Kunikata, T.; Korolkiewicz, R.; Takeuchi, K. Roles of enterobacteria, nitric oxide and neutrophil in pathogenesis of indomethacin-induced small intestinal lesions in rats. Pharmacol. Res. 1999, 40, 517–524. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. The scientific evidence for the role of milk protein-derived bioactive peptides in humans: A Review. J. Funct. Foods. 2015, 17, 640–656. [Google Scholar] [CrossRef] [Green Version]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk--sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [Green Version]
- Yvon, M.; Beucher, S.; Guilloteau, P.; Le Huerou-Luron, I.; Corring, T. Effects of caseinomacropeptide (CMP) on digestion regulation. Reprod. Nutr. Dev. 1994, 34, 527–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomä-Worringer, C.; Sørensen, J.; López-Fandiño, R. Health effects and technological features of caseinomacropeptide. Int. Dairy J. 2006, 16, 1324–1333. [Google Scholar] [CrossRef]
- Mikkelsen, T.L.; Rasmussen, E.; Olsen, A.; Barkholt, V.; Frøkiaer, H. Immunogenicity of kappa-casein and glycomacropeptide. J. Dairy Sci. 2006, 89, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Hvas, C.L.; Dige, A.; Bendix, M.; Wernlund, P.G.; Christensen, L.A.; Dahlerup, J.F.; Agnholt, J. Casein glycomacropeptide for active distal ulcerative colitis: A randomized pilot study. Eur. J. Clin. Invest. 2016, 46, 555–563. [Google Scholar] [CrossRef]
- Pena, M.J.; Pinto, A.; Daly, A.; MacDonald, A.; Azevedo, L.; Rocha, J.; Borges, N. The Use of Glycomacropeptide in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1794. [Google Scholar] [CrossRef] [Green Version]
- Roldán, N.R.; Jiménez, M.; Cervantes-García, D.; Marín, E.; Salinas, E. Glycomacropeptide administration attenuates airway inflammation and remodeling associated to allergic asthma in rat. Inflamm. Res. 2016, 65, 273–283. [Google Scholar] [CrossRef]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-García, D.; Jiménez, M.; Ventura-Juárez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J. Nutr. 2005, 135, 1164–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, P.; Daddaoua, A.; Martínez-Plata, E.; González, M.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregulation of interleukin 17. Br. J. Pharmacol. 2008, 154, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Posadas, R.; Requena, P.; González, R.; Suárez, M.D.; Zarzuelo, A.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine glycomacropeptide has intestinal antiinflammatory effects in rats with dextran sulfate-induced colitis. J. Nutr. 2010, 140, 2014–2019. [Google Scholar] [CrossRef]
- Mikkelsen, T.L.; Bakman, S.; Sørensen, E.S.; Barkholt, V.; Frøkiaer, H. Sialic acid-containing milk proteins show differential immunomodulatory activities independent of sialic acid. J. Agric. Food Chem. 2005, 53, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, D.; Chen, B.; Mao, X. Endotoxin-Binding Peptides Derived from Casein Glycomacropeptide Inhibit Lipopolysaccharide-Stimulated Inflammatory Responses via Blockade of NF-κB activation in macrophages. Nutrients 2015, 7, 3119–3137. [Google Scholar] [CrossRef] [Green Version]
- Abimosleh, S.M.; Tran, C.D.; Howarth, G.S. Emu oil reduces small intestinal inflammation in the absence of clinical improvement in a rat model of indomethacin-induced enteropathy. Evid. Based. Complement. Alternat. Med. 2013, 2013, 429706. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.P.; Borse, S.P.; Nivsarkar, M. Co-administration of quercetin with pantoprazole sodium prevents NSAID-induced severe gastroenteropathic damage efficiently: Evidence from a preclinical study in rats. Exp. Toxicol. Pathol. 2017, 69, 17–26. [Google Scholar] [CrossRef]
- Davies, N.M.; Wallace, J.L. Nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: New insights into an old problem. J. Gastroenterol. 1997, 32, 127–133. [Google Scholar] [CrossRef]
- Otani, K.; Tanigawa, T.; Watanabe, T.; Shimada, S.; Nadatani, Y.; Nagami, Y.; Tanaka, F.; Kamata, N.; Yamagami, H.; Shiba, M.; et al. Microbiota Plays a Key Role in Non-Steroidal Anti-Inflammatory Drug-Induced Small Intestinal Damage. Digestion 2017, 95, 22–28. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Hol, J.; Wilhelmsen, L.; Haraldsen, G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J. Leukoc. Biol. 2010, 87, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Higashimori, A.; Watanabe, T.; Nadatani, Y.; Takeda, S.; Otani, K.; Tanigawa, T.; Yamagami, H.; Shiba, M.; Tominaga, K.; Fujiwara, Y.; et al. Mechanisms of NLRP3 inflammasome activation and its role in NSAID-induced enteropathy. Mucosal Immunol. 2016, 9, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.P.; Borse, S.P.; Nivsarkar, M. A novel model for NSAID induced gastroenteropathy in rats. J. Pharmacol. Toxicol. Methods. 2016, 78, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Syer, S.; Denou, E.; de Palma, G.; Vong, L.; McKnight, W.; Jury, J.; Bolla, M.; Bercik, P.; Collins, S.M.; et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 2011, 141, 1314–1322. [Google Scholar] [CrossRef]
- Satoh, H.; Amagase, K.; Takeuchi, K. Exacerbation of nonsteroidal anti-inflammatory drug-induced small intestinal lesions by antisecretory drugs in rats: The role of intestinal motility. J. Pharmacol. Exp. Ther. 2012, 343, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Lanas, A.; García-Rodríguez, L.A.; Polo-Tomás, M.; Ponce, M.; Alonso-Abreu, I.; Perez-Aisa, M.A.; Perez-Gisbert, J.; Bujanda, L.; Castro, M.; Muñoz, M.; et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am. J. Gastroenterol. 2009, 104, 1633–1641. [Google Scholar] [CrossRef]
- Maiden, L.; Thjodleifsson, B.; Theodors, A.; Gonzalez, J.; Bjarnason, I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005, 128, 1172–1178. [Google Scholar] [CrossRef]
- Bolten, W.W. Unterschåtzte NSA-Nebenwirkungen im distalen Intestinum. Z. Rheumatol. 2000, 59, 370–372. [Google Scholar] [CrossRef]
- Abrahamian, G.A.; Polhamus, C.D.; Muskat, P.; Karulf, R.E. Diaphragm-like strictures of the ileum associated with NSAID use: A rare complication. South Med. J. 1998, 91, 395–397. [Google Scholar] [CrossRef]
- Yamamoto, A.; Itoh, T.; Nasu, R.; Nishida, R. Sodium alginate ameliorates indomethacin-induced gastrointestinal mucosal injury via inhibiting translocation in rats. World J. Gastroenterol. 2014, 20, 2641–2652. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, C.; Ming, Z.; Cao, J.; Yan, Y.; Zhao, P.; Pang, G.; Deng, Z.; Yao, Y.; Chen, Q. Molecular mechanisms by which casein glycomacropeptide maintains internal homeostasis in mice with experimental ulcerative colitis. PLoS ONE 2017, 12, e0181075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerabagu, M.P.; Opara, E.I.; Meguid, M.M.; Nandi, J.; Oler, A.; Holtzapple, P.G.; Levine, R.A. Mode of food intake reduction in Lewis rats with indomethacin-induced ulcerative ileitis. Physiol. Behav. 1996, 60, 381–387. [Google Scholar] [CrossRef]
- Solverson, P.; Murali, S.G.; Brinkman, A.S.; Nelson, D.W.; Clayton, M.K.; Yen, C.L.; Ney, D.M. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E885–E895. [Google Scholar] [CrossRef]
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontol. 2000, 63, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Filaretova, L.P.; Bagaeva, T.R.; Morozova, O.Y.; Zelena, D. The healing of NSAID-induced gastric lesion may be followed by small intestinal and cardiovascular side effects. J. Physiol. Pharmacol. 2011, 62, 619–625. [Google Scholar]
- Hayllar, J.; Smith, T.; Macpherson, A.; Price, A.B.; Gumpel, M.; Bjarnason, I. Nonsteroidal antiinflammatory drug-induced small intestinal inflammation and blood loss. Effects of sulfasalazine and other disease-modifying antirheumatic drugs. Arthritis Rheum. 1994, 37, 1146–1150. [Google Scholar] [CrossRef]
- Morris, A.J.; Wasson, L.A.; MacKenzie, J.F. Small bowel enteroscopy in undiagnosed gastrointestinal blood loss. Gut 1992, 33, 887–889. [Google Scholar] [CrossRef] [Green Version]
- Bjarnason, I.; Macpherson, A.J. Intestinal toxicity of non-steroidal anti-inflammatory drugs. Pharmacol. Ther. 1994, 62, 145–157. [Google Scholar] [CrossRef]
- Bjarnason, I.; Zanelli, G.; Prouse, P.; Smethurst, P.; Smith, T.; Levi, S.; Gumpel, M.J.; Levi, A.J. Blood and protein loss via small-intestinal inflammation induced by non-steroidal anti-inflammatory drugs. Lancet 1987, 2, 711–714. [Google Scholar] [CrossRef]
- Atchison, C.R.; West, A.B.; Balakumaran, A.; Hargus, S.J.; Pohl, L.R.; Daiker, D.H.; Aronson, J.F.; Hoffmann, W.E.; Shipp, B.K.; Treinen-Moslen, M. Drug enterocyte adducts: Possible causal factor for diclofenac enteropathy in rats. Gastroenterology 2000, 119, 1537–1547. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeon, W.K.; Yun, J.W.; Park, D.I.; Cho, Y.K.; Sung, I.K.; Sohn, C.I.; Kim, B.I.; Yeom, J.S.; Park, H.S.; et al. Protective effects of bovine colostrum on non-steroidal anti-inflammatory drug induced intestinal damage in rats. Asia Pac. J. Clin. Nutr. 2005, 14, 103–107. [Google Scholar]
- Schneeberger, E.E.; Lynch, R.D. Structure, function, and regulation of cellular tight junctions. Am. J. Physiol. 1992, 262, L647–L661. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Zhang, Z.; Lin, J.; Zou, W.; Li, C. L-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol. Biol. Rep. 2019, 46, 6435–6451. [Google Scholar] [CrossRef] [Green Version]
- Bjarnason, I.; Smethurst, P.; Macpherson, A.; Walker, F.; McElnay, J.C.; Passmore, A.P.; Menzies, I.S. Glucose and citrate reduce the permeability changes caused by indomethacin in humans. Gastroenterology 1992, 102, 1546–1550. [Google Scholar] [CrossRef]
- Shi, S.; Wang, H.; Gao, H.; Li, Z.; Chen, F.X.; Zuo, X.L.; Li, Y.Q. Increased gap density predicts weakness of the epithelial barrier in vivo by confocal laser endomicroscopy in indomethacin-induced enteropathy. Dig. Dis. Sci. 2014, 59, 1398–1405. [Google Scholar] [CrossRef]
- Thakre-Nighot, M.; Blikslager, A.T. Indomethacin induces increase in gastric epithelial tight junction permeability via redistribution of occludin and activation of p38 MAPK in MKN-28 Cells. Tissue Barriers 2016, 4, e1187325. [Google Scholar] [CrossRef] [Green Version]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef]
- Tibble, J.A.; Sigthorsson, G.; Foster, R.; Scott, D.; Fagerhol, M.K.; Roseth, A.; Bjarnason, I. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut 1999, 45, 362–366. [Google Scholar] [CrossRef]
- da Silva Pantoja, P.; Assreuy, A.M.S.; Silva, R.O.; Damasceno, S.R.B.; Mendonça, V.A.; Mendes, T.S.; Morais, J.A.V.; de Almeida, S.L.; Teixeira, A.É.E.A.; de Souza, M.H.L.P.; et al. The polysaccharide-rich tea of Ximenia americana barks prevents indomethacin-induced gastrointestinal damage via neutrophil inhibition. J. Ethnopharmacol. 2018, 224, 195–201. [Google Scholar] [CrossRef]
- Antoon, J.S.; Perry, M.A.J. Role of neutrophils and mast cells in acute indomethacin-induced small bowel injury in the rat. Gastroenterol. 1997, 32, 747–757. [Google Scholar] [CrossRef]
- Uejima, M.; Kinouchi, T.; Kataoka, K.; Hiraoka, I.; Ohnishi, Y. Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol. Immunol. 1996, 40, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Okayama, T.; Yoshida, N.; Uchiyama, K.; Takagi, T.; Ichikawa, H.; Yoshikawa, T. Mast cells are involved in the pathogenesis of indomethacin-induced rat enteritis. J. Gastroenterol. 2009, 44, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, J.; Jia, Y.; Liu, X.; Yan, Y.; Pang, G. Modulation of mice fecal microbiota by administration of casein glycomacropeptide. Microbial. Res. 2012, 3, e3. [Google Scholar] [CrossRef]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of intestinal infection by glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Muñoz, Y.H.; García, A.; Haro, L.M.; Salinas, E. Novel Mechanisms Underlying the Therapeutic Effect of Glycomacropeptide on Allergy: Change in Gut Microbiota, Upregulation of TGF-β, and Inhibition of Mast Cells. Int. Arch. Allergy. Immunol. 2016, 171, 217–226. [Google Scholar] [CrossRef]
- Córdova-Dávalos, L.E.; Jiménez, M.; Salinas, E. Glycomacropeptide Bioactivity and Health: A Review Highlighting Action Mechanisms and Signaling Pathways. Nutrients 2019, 11, 598. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chen, B.; Du, M.; Song, J.; Cheng, X.; Wang, X.; Mao, X. Casein Glycomacropeptide Hydrolysates Exert Cytoprotective Effect against Cellular Oxidative Stress by Up-Regulating HO-1 Expression in HepG2 Cells. Nutrients 2017, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Gao, D.X.; Song, J.J.; Ren, F.Z.; Mao, X.Y. Casein glycomacropeptide hydrolysate exerts cytoprotection against H2O2-induced oxidative stress in RAW 264.7 macrophages via ROS-dependent heme oxygenase-1 expression. R.S.C. Adv. 2015, 5, 4511–4523. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Castillo, R.L.; Beltrán, C.; Miranda, A.; Fuentes, J.; Gotteland, M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: Role of NF-κB and Nrf2. J. Nutr. Biochem. 2016, 27, 289–298. [Google Scholar] [CrossRef]
- Weruaga, E.; Balkan, B.; Koylu, E.O.; Pogun, S.; Alonso, J.R. Effects of chronic nicotine administration on nitric oxide synthase expression and activity in rat brain. J. Neurosci. Res. 2002, 67, 689–697. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Ling, K.L.; Wolff, S.P. Low-density lipoprotein is the major carrier of lipid hydroperoxides in plasma. Relevance to determination of total plasma lipid hydroperoxide concentrations. Biochem. J. 1996, 313, 781–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid. Res. 1998, 39, 1529–1542. [Google Scholar] [PubMed]
- Gay, C.A.; Gebicki, J.M. Measurement of protein and lipid hydroperoxides in biological systems by the ferric-xylenol orange method. Anal. Biochem. 2003, 315, 29–35. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |







| Target | Oligonucleotide | Accession Number (NCBI) |
|---|---|---|
| Claudin-1 | Fw: AACCTCTTACCCAACACCACG Rv: GCCAAGACCCTCATAGCCAT | NM_031699.2 |
| Occludin | Fw: AGGACAGACCCAGACCACTA Rv: ACTCTTCGCTCTCCTCTCTG | NM_031329.2 |
| MUC-2 | Fw: GTATGTGCTCGCCTGTATGC Rv: TGACCTCCAGATGTGAGCAG | XM_017604244.1 |
| CXCL1 | Fw: GCACCCAAACCGAAGTCATAG Rv: TGTTGTCAGAAGCCAGCGTT | NM_030845.1 |
| IL-1β | Fw: AAATCTCACAGCAGCATCTC Rv: ACTAGCAGGTCGTCATCATC | NM_031512.2 |
| iNOS | Fw: GATGTGCTGCCTCTGGTCCT Rv: ACTCCAATCTCGGTGCCCAT | NM_012611.3 |
| β-Actin | Fw: GTCGTACCACTGGCATTGTG Rv: GCTGTGGTGGTGAAGCTGTA | NM_031144.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-García, D.; Bahena-Delgado, A.I.; Jiménez, M.; Córdova-Dávalos, L.E.; Ruiz-Esparza Palacios, V.; Sánchez-Alemán, E.; Martínez-Saldaña, M.C.; Salinas, E. Glycomacropeptide Ameliorates Indomethacin-Induced Enteropathy in Rats by Modifying Intestinal Inflammation and Oxidative Stress. Molecules 2020, 25, 2351. https://doi.org/10.3390/molecules25102351
Cervantes-García D, Bahena-Delgado AI, Jiménez M, Córdova-Dávalos LE, Ruiz-Esparza Palacios V, Sánchez-Alemán E, Martínez-Saldaña MC, Salinas E. Glycomacropeptide Ameliorates Indomethacin-Induced Enteropathy in Rats by Modifying Intestinal Inflammation and Oxidative Stress. Molecules. 2020; 25(10):2351. https://doi.org/10.3390/molecules25102351
Chicago/Turabian StyleCervantes-García, Daniel, Armida I. Bahena-Delgado, Mariela Jiménez, Laura E. Córdova-Dávalos, Vanessa Ruiz-Esparza Palacios, Esperanza Sánchez-Alemán, María C. Martínez-Saldaña, and Eva Salinas. 2020. "Glycomacropeptide Ameliorates Indomethacin-Induced Enteropathy in Rats by Modifying Intestinal Inflammation and Oxidative Stress" Molecules 25, no. 10: 2351. https://doi.org/10.3390/molecules25102351