Reliable Ultra Trace Analysis of Cd, U and Zn Concentrations in Greenland Snow and Ice by Using Ultraclean Methods for Contamination Control
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Procedural Blank Contribution
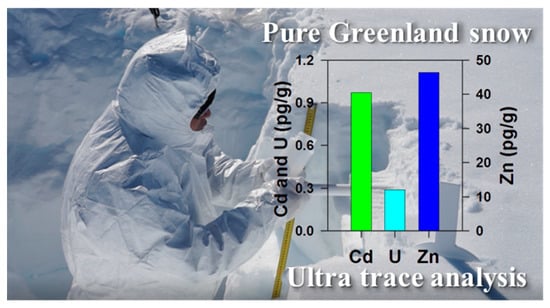
2.2. Cd, U and Zn Concentrations in the NEEM Snow and Ice Samples
2.3. Crustal Enrichment Factor (EF)
2.4. Comparison with Published Data
2.5. Seasonal Variations in Concentrations and EFs
3. Experimental
3.1. Clean Facilities
3.2. Ultrapure Water
3.3. Laboratory Materials
3.4. Cleaning Procedures
3.5. Sample Description and Dating
3.6. Total Procedural Blank (TPB)
3.7. Analytical Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murozumi, M.; Chow, T.J.; Patterson, C.C. Chemical concentrations of pollutant lead aerosols, terrestrial dusts and sea salts in Greenland and Antarctic snow strata. Geochim. Cosmochim. Acta 1969, 33, 1247–1294. [Google Scholar] [CrossRef]
- Boutron, C.F.; Görlach, U.; Candelone, J.-P.; Bolshov, M.A.; Delmas, R.J. Decrease in anthropogenioc lead, cadmium, and zinc in Greenland snows since the late 1960s. Nature 1991, 353, 153–156. [Google Scholar] [CrossRef]
- Boutron, C.F.; Vandal, G.M.; Fitzgerald, W.F.; Ferrari, C.P. A forty year record of mercury in central Greenland snow. Geophys. Res. Lett. 1998, 25, 3315–3318. [Google Scholar] [CrossRef]
- Hong, S.; Candelone, J.-P.; Patterson, C.C.; Boutron, C.F. Greenland ice evidence of hemispheric lead pollution two millennia ago by Greek and Roman civilization. Science 1994, 265, 1841–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Candelone, J.-P.; Patterson, C.C.; Boutron, C.F. History of ancient copper smelting pollution during Roman and Medieval times recorded in Greenland ice. Science 1996, 272, 246–249. [Google Scholar] [CrossRef]
- Candelone, J.-P.; Hong, S.; Pellone, C.; Boutron, C.F. Post-Industrial Revolution changes in large-scale atmospheric pollution of the northern hemisphere by heavy metals as documented in central Greenland snow and ice. J. Geophys. Res. 1995, 100, 16605–16616. [Google Scholar] [CrossRef]
- McConnell, J.R.; Lamorey, G.W.; Hutterli, M.A. A 250-year high-resolution record of Pb flux and crustal enrichment in central Greenland. Geophy. Res. Lett. 2002, 29, 2130. [Google Scholar] [CrossRef]
- McConnell, J.R.; Edwards, R. Coal burning leaves toxic heavy metal legacy in the Arctic. Proc. Natl. Acad. Sci. USA. 2008, 105, 12140–12144. [Google Scholar] [CrossRef] [Green Version]
- McConnell, J.R.; Wilson, A.I.; Stohl, A.; Arienzo, M.M.; Chellman, N.J.; Eckhardt, S.; Thompson, E.M.; Pollard, M.; Steffensen, J.P. Lead pollution recorded in Greenland ice indicates European emissions tracked plagues, wars, and imperial expansion during antiquity. Proc. Natl. Acad. Sci. USA. 2018, 115, 5726–5731. [Google Scholar] [CrossRef] [Green Version]
- Boutron, C.F. A clean laboratory for ultralow concentration heavy metal analysis. Fresenius J. Anal. Chem. 1990, 337, 482–491. [Google Scholar] [CrossRef]
- Howard, A.G.; Statham, P.J. Inorganic Trace Analysis: Philosophy and Practice; John Wiley & Sons, Inc.: Chichester, UK, 1993; p. 183. [Google Scholar]
- Candelone, J.-P.; Hong, S.; Boutron, C.F. An improved method for decontaminating polar snow and ice cores for heavy metal analysis. Anal. Chim. Acta 1994, 299, 9–46. [Google Scholar] [CrossRef]
- Scarponi, G.; Barbante, C.; Cescon, P. Differential pulse anodic stripping voltametry for ultratrace determination of cadmium and lead in Antarctic snow. Analusis 1994, 22, M47–M50. [Google Scholar]
- Barbante, C.; Bellomi, T.; Mezzadri, G.; Cescon, P.; Scarponi, G.; Morel, C.; Jay, P.; Van de Velde, K.; Ferrari, C.; Boutron, C.F. Direct determination of heavy metals at pictogram per gram levels in Greenland and Antarctic snow by double focusing inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1997, 12, 925–931. [Google Scholar] [CrossRef]
- Scarponi, G.; Barbante, C.; Turetta, C.; Gambaro, A.; Cescon, P. Chemical contamination of Antarctic snow: The case of lead. Microchem. J. 1997, 55, 24–32. [Google Scholar] [CrossRef]
- Barbante, C.; Turetta, C.; Gambaro, A.; Capodaglio, G.; Scarponi, G. Sources and origins of aerosols reaching Antarctica as revealed by lead concentration profiles in shallow snow. Ann. Glaciol. 1998, 27, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lluberas, A.; Rodriguez, F. A clean protocol for determining ultralow heavy metal concentrations: Its application to the analysis of Pb, Cd, Cu, Zn and Mn in Antarctic snow. Korean J. Polar Res. 2000, 11, 35–47. [Google Scholar]
- Han, C.; Burn-Nunes, L.J.; Lee, K.; Chang, C.; Kang, J.-H.; Han, Y.; Hur, S.D.; Hong, S. Determination of lead isotopes in a new Greenland deep ice core at the sub-picogram per gram level by thermal ionization mass spectrometry using an improved decontamination method. Talanta 2015, 140, 20–28. [Google Scholar] [CrossRef]
- Weiss, H.V.; Koide, M.; Goldberg, E.D. Mercury in a Greenland Ice Sheet: Evidence of recent input by man. Science 1971, 174, 692–694. [Google Scholar] [CrossRef]
- Weiss, H.V.; Bertine, K.; Koide, M.; Goldberg, E.D. The chemical composition of a Greenland glacier. Geochim. Cosmochim. Acta 1975, 39, 1–10. [Google Scholar] [CrossRef]
- Carr, R.A.; Wilkniss, P.E. Mercury in the Greenland ice sheet: Further data. Science 1973, 181, 843–844. [Google Scholar] [CrossRef]
- Appelquist, H.; Jensen, K.O.; Sevel, T.; Hammer, C. Mercury in the Greenland ice sheet. Nature 1978, 273, 657–659. [Google Scholar] [CrossRef]
- Patterson, C.C.; Settle, D.M. The reduction of orders of magnitude errors in lead analysis of biological materials and natural waters by evaluating and controlling the extent and sources of industrial lead contamination introduced during sample collection and analysis. Nat. Bur. Stand. Special Publ 1976, 422, 321–351. [Google Scholar]
- Ng, A.; Patterson, C.C. Natural concentrations of lead in ancient Arctic and Antarctic ice. Geochim. Cosmochim. Acta 1981, 45, 2109–2121. [Google Scholar] [CrossRef]
- Boutron, C.F.; Patterson, C.C. The occurrence of lead in Antarctic recent snow, firn deposited over the last two centuries and prehistoric ice. Geochim. Cosmochim. Acta 1983, 47, 1355–1368. [Google Scholar] [CrossRef]
- Lee, K.; Han, C.; Hong, S.-B.; Jun, S.-J.; Han, Y.; Xiao, C.; Du, Z.; Hur, S.D.; Lee, J.I.; Boutron, C.F.; et al. A 300-year high-resolution Greenland ice record of large-scale atmospheric pollution by arsenic in the Northern Hemisphere. Environ. Sci. Technol. 2019, 53, 12999–13008. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Hwang, H.; Han, C.; Hur, S.D.; Kim, S.-J.; Hong, S. Pb concentrations and isotopic record preserved in northwest Greenland snow. Chemosphere 2017, 187, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.M.; Shafer, M.M.; Dibb, J.E.; Polashenski, C.M.; Schauer, J.J. Elements and inorganic ions as source tracers in recent Greenland snow. Atmos. Environ. 2017, 164, 205–215. [Google Scholar] [CrossRef]
- Barbante, C.; Boutron, C.; Morel, C.; Ferrari, C.; Jaffrezo, J.-L.; Cozzi, G.; Gaspari, V.; Cescon, P. Seasonal variations of heavy metals in central Greenland snow deposited from 1991 to 1995. J. Environ. Monit. 2003, 5, 328–335. [Google Scholar] [CrossRef]
- Candelone, J.-P.; Jaffrezo, J.-L.; Hong, S.; Davidson, C.I.; Boutron, C.F. Seasonal variations in heavy metals concentrations in present day Greenland snow. Sci. Total Environ. 1996, 193, 101–110. [Google Scholar] [CrossRef]
- Sherrell, R.M.; Boyle, E.A.; Harris, N.R.; Falkner, K.J. Temporal variability of Cd, Pb, and Pb isotope deposition in central Greenland snow. Geochem. Geophys. Geosys. 2000, 1, 1002. [Google Scholar] [CrossRef] [Green Version]
- Boyle, E.A.; Sherrell, R.M.; Bacon, M.P. Lead variability in the western North Atlantic ocean and central Greenland ice: Implications for the search for decadal trends in anthropogenic emissions. Geochim. Cosmochim. Acta 1994, 58, 3227–3238. [Google Scholar] [CrossRef]
- Savarino, J.; Boutron, C.F.; Jaffrezo, J.L. Short-term variations of Pb, Cd, Zn and Cu in recent Greenland snow. Atmos. Environ. 1994, 28, 1731–1737. [Google Scholar] [CrossRef]
- Wolff, E.W.; Peel, D.A. Concentration of cadmium, copper, lead and zinc in snow from near Dye 3 in South Greenland. Ann. Glaciol. 1988, 10, 193–197. [Google Scholar] [CrossRef]
- Hong, S.; Candelone, J.-P.; Turetta, C.; Boutron, C.F. Changes in natural lead, copper, zinc and cadmium concentrations in central Greenland ice from 8250 to 149,100 years ago: Their association with climatic changes and resultant variations of dominant source contributions. Earth Planet. Sci. Lett. 1996, 143, 233–244. [Google Scholar] [CrossRef]
- Kang, J.-H.; Hwang, H.; Hong, S.B.; Hur, S.D.; Choi, S.-D.; Lee, J.; Hong, S. Mineral dust and major ion concentrations in snowpit samples from the NEEM site, Greenland. Atmos. Environ. 2015, 120, 137–143. [Google Scholar] [CrossRef]
- Lee, K.; Hong, S.-B.; Lee, J.; Chung, J.; Hur, S.D.; Hong, S. Seasonal variation in the input of atmospheric selenium to northwestern Greenland snow. Sci. Total Environ. 2015, 526, 49–57. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Distinguishing between natural and anthropogenic sources for elements in the environment: Regional geochemical survey versus enrichment factors. Sci. Total Environ. 2005, 337, 91–107. [Google Scholar] [CrossRef]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Gabrielli, P.; Planchon, F.A.M.; Hong, S.; Lee, K.; Hur, S.D.; Barbante, C.; Ferrari, C.P.; Petit, J.R.; Lipenkov, V.Y.; Cescon, P.; et al. Trace elements in Vostok ice during the last four climatic cycles. Earth Planet. Sci. Lett. 2005, 234, 249–259. [Google Scholar] [CrossRef]
- Hong, S.; Boutron, C.F.; Barbante, C.; Hur, S.D.; Lee, K.; Gabrielli, P.; Capodaglio, G.; Ferrari, C.P.; Turetta, C.; Petit, J.R.; et al. Glacial-interglacial changes in the occurrence of Pb, Cd, Cu and Zn in Vostok Antarctic ice from 240,000 to 410,000 years BP. J. Environ. Monit. 2005, 7, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- VanCuren, R.A.; Cahill, T.; Burkhart, J.; Barnes, D.; Zhao, Y.; Perry, K.; Cliff, S.; McConnell, J. Aerosols and their sources at Summit Greenland-Fist results of continuous size- and time-resolved sampling. Atmos. Environ. 2012, 52, 82–97. [Google Scholar] [CrossRef]
- Bory, A.J.M.; Abouchami, W.; Galer, S.J.G.; Svensson, A.; Christensen, J.N.; Biscaye, P.E. A Chinese imprint in insoluble pollutants recently deposited in central Greenland as indicated by lead isotopes. Environ. Sci. Technol. 2014, 48, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Uno, I.; Eguchi, K.; Yumimoto, K.; Takemura, T.; Shimizu, A.; Uematsu, M.; Liu, Z.; Wang, Z.; Hara, Y.; Sugimoto, N. Asian dust transported one full circuit around the globe. Nat. Geosci. 2009, 2, 557–560. [Google Scholar] [CrossRef]
- Kahl, J.D.W.; Martinez, D.A.; Kuhns, H.; Davidson, C.I.; Jaffrezo, J.L.; Harris, J.M. Air mass trajectories to Summit, Greenland: A 44-year climatology and some episodic events. J. Geophys. Res. 1997, 102, 26861–26875. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Fudala, J.; Strzelecka-Jastrzab, E.; Hlawiczka, S.; Panasiuk, D.; Nitter, S.; Pregger, T.; Pfeiffer, H.; Friedrich, R. Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmos. Environ. 2007, 41, 8557–8566. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, Y.; Tian, H.; Gao, X.; Zhang, Y.; Wu, X.; Zhu, C.; Gao, J. Atmospheric emission characteristics and control policies of five precedent-controlled toxic heavy metals from anthropogenic sources in China. Environ. Sci. Technol. 2015, 49, 1206–1214. [Google Scholar] [CrossRef]
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Nao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 2015, 15, 10127–10147. [Google Scholar] [CrossRef] [Green Version]
- Barbante, C.; Van de Velde, K.; Cozzi, G.; Capodaglio, G.; Cescon, P.; Planchon, F.; Hong, S.; Ferrari, C.; Boutron, C.F. Post-World War II uranium changes in dated Mont Blanc ice and snow. Environ. Sci. Technol. 2001, 35, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, S.; Mayewski, P.A.; Handley, M.; Osterberg, E.; Kang, S.; Sneed, S.; Hou, S.; Qin, D. Recent increases in atmospheric concentrations of Bi, U, Cs, S and Ca from a 350-year Mount Everest ice core record. J. Geophys. Res. 2009, 114, D04302. [Google Scholar] [CrossRef] [Green Version]
- Planchon, F.A.M.; Boutron, C.F.; Barbante, C.; Cozzi, G.; Gaspari, V.; Wolff, E.W.; Ferrari, C.P.; Cescon, P. Changes in heavy metals in Antarctic snow from Coats Land since the mid-19th to the late-20th century. Earth Planet. Sci. Lett. 2002, 200, 207–222. [Google Scholar] [CrossRef]
- Potocki, M.; Mayewski, P.; Kurbatov, A.V.; Simões, J.C.; Dixon, D.A.; Goodwin, I.; Carleton, A.M.; Handley, M.J.; Jaña, R.; Korotkikh, E.V. Recent increase in Antarctic Peninsula ice core uranium concentrations. Atmos. Environ. 2016, 140, 381–385. [Google Scholar] [CrossRef]
- Görlach, U.; Boutron, C.F. Preconcentration of lead, cadmium, copper and zinc in water at the pg g–1 level by non-boiling evaporation. Anal. Chim. Acta 1990, 236, 391–398. [Google Scholar] [CrossRef]
- Moody, J.R.; Lindstrom, R.M. Selection and cleaning of plastic containers for storage of trace element samples. Anal. Chem. 1997, 49, 2264–2267. [Google Scholar] [CrossRef]
- NEEM Community Members. Eemian interglacial reconstructed from a Greenland folded ice core. Nature 2013, 493, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-B.; Lee, K.; Hur, S.D.; Hong, S.; Soyol-Erdene, T.-O.; Kim, S.M.; Chung, J.W.; Jun, S.-J.; Kang, C.H. Development of melting system for measurement of trace elements and ions in ice core. Bull. Korean Chem. Soc. 2015, 36, 1069–1081. [Google Scholar] [CrossRef]
Sample Availability: Samples of Greenland snow and ice analyzed in this study are available from the authors. |



| Element | Detection Limit a | MQW | SDW | TPB | |
|---|---|---|---|---|---|
| Snow | AICs b | ||||
| As | 0.292 | 0.029 (0.039) c | 0.045 (0.036) d | 0.003 (0.002) e | 0.108 (0.020) f |
| Ba | 0.056 | 0.077 (0.031) | 0.036 (0.015) | 0.005 (0.005) | <LOQ g |
| Cd | 0.009 | 0.008 (0.009) | 0.003 (0.003) | 0.0002 (0.0001) | 0.016 (0.019) |
| Pb | 0.026 | 0.286 (0.278) | 0.150 (0.050) | 0.005 (0.003) | 0.061 (0.037) |
| U | 0.005 | 0.001 (0.001) | 0.001 (0.001) | 0.0001 (0.0001) | 0.009 (0.0003) |
| Zn | 0.118 | 0.260 (0.171) | 0.234 (0.122) | 0.052 (0.020) | 0.632 (0.198) |
| Site | Altitude (m a.s.l.) | Sampling Method | Period | Accumulation Rate (g H2O/cm2/yr) | Concentration (pg/g) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Pb | U | Zn | ||||||
| NEEM (77°26′N, 51°03′W) | 2461 | Snow pit | 2003–2009 | 22.5 | 2.63 ± 2.75 a (0.50–15.8) | 0.97 ± 0.89 (0.07–5.57) | 21.7 ± 22.7 b (2.7–97.3) | 0.29 ± 0.37 (0.04–1.59) | 46.4 ± 33.5 (6.97–156) | This study |
| max/min | 32 | 80 | 36 | 40 | 22 | [26] | ||||
| Enrichment factor | 54 ± 37 (12–163) | 430 ± 376 (104–2196) | 43 ± 20 (15–105) | 3.5 ± 1.8 (1.4–13) | 44 ± 37 (9–173) | [27] | ||||
| Northwest and central Greenland | coast ~ 3500 m | Snow pit | spring 2012–2013, summer 2013–2014 | 22 ± 29 | 43 ± 50 | 200 ± 690 | 0.7 ± 1.1 | 4,120 ± 4,830 | [28] | |
| Enrichment factor | 124 ± 101 | 5595 ± 1850 | 118 ± 147 | 2.8 ± 1.5 | 971 ± 156 | |||||
| Summit (72°20′N, 38°45′W) | 3270 | Snow pit | 1991–1995 | 23 | 1.15 ± 1.7 (0.1–11.6) | 17.3 ± 17.7 (2.0–108) | 1.75 ± 2.6 (0.2–15) | 50.6 ± 49.4 (2–285) | [29] | |
| Enrichment factor | 326 ± 1066 (20–8731) | 30 ± 59 (1.5–456) | 15 ± 23 (1.1–132) | 24 ± 32 (1.2–177) | ||||||
| Summit (72°20′N, 38°45′W) | 3270 | Snow pit | 1990–1992 | 23 | 0.67 (0.08–2.5) | 15 (0.6–44) | 51 (9–194) | [30] | ||
| Summit (72°20′N, 38°45′W) | 3190 | Snow pit | 1981–1990 | 23 | 1.3 ± 0.91 (0.09–5.72) | 43.6 ± 31.4 (4.37–158) | [31,32] | |||
| Summit (72°20′N, 38°45′W) | 3270 | Tube sampling | 1989–1990 | 23 | 1.3 (0.3–3.7) | 21.6 (3–50) | 42 (2–144) | [33] | ||
| South Greenland (Dye 3) (65°11′N, 43°50′W) | 2479 | Tube sampling | 1983–1984 | 50 | 0.74 (0.2–1.3) | 28 (5–90) | 27 (10–50) | [34] | ||
| NEEM (77°26′N, 51°03′W) | 2461 | Deep ice core | 8266–9166 yr BP | 22.5 | 2.15 ± 0.64 (1.44–2.69) | 0.32 ± 0.11 (0.20–0.40) | 1.60 ± 0.07 (1.55–1.67) | 0.34 ± 0.14 (0.21–0.50) | 33.8 ± 7.7 (27.1–42.2) | This study |
| Enrichment factor | 19 ± 7 (14–27) | 58 ± 27 (39–88) | 1.8 ± 0.7 (1.0–2.2) | 2.6 ± 1.8 (1.3–4.7) | 12 ± 2.6 (8.7–13.7) | |||||
| Deep ice core | 8056 yr BP | 1.47 | 2.03 | 24.4 | 0.06 | 2,880 | ||||
| Summit (72°34′N, 37°37′W) | 3238 | GRIP deep ice core | 8250–9310 yr BP | 23 | 0.59 ± 0.10 (0.50–0.69) | 0.90 ± 0.17 (0.79–1.10) | 26 ± 6.08 (19–30) | [35] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Hwang, H.; Kang, J.-H.; Hong, S.-B.; Han, Y.; Lee, K.; Hur, S.D.; Hong, S. Reliable Ultra Trace Analysis of Cd, U and Zn Concentrations in Greenland Snow and Ice by Using Ultraclean Methods for Contamination Control. Molecules 2020, 25, 2519. https://doi.org/10.3390/molecules25112519
Han C, Hwang H, Kang J-H, Hong S-B, Han Y, Lee K, Hur SD, Hong S. Reliable Ultra Trace Analysis of Cd, U and Zn Concentrations in Greenland Snow and Ice by Using Ultraclean Methods for Contamination Control. Molecules. 2020; 25(11):2519. https://doi.org/10.3390/molecules25112519
Chicago/Turabian StyleHan, Changhee, Heejin Hwang, Jung-Ho Kang, Sang-Bum Hong, Yeongcheol Han, Khanghyun Lee, Soon Do Hur, and Sungmin Hong. 2020. "Reliable Ultra Trace Analysis of Cd, U and Zn Concentrations in Greenland Snow and Ice by Using Ultraclean Methods for Contamination Control" Molecules 25, no. 11: 2519. https://doi.org/10.3390/molecules25112519