Effect of Blanching Pomegranate Seeds on Physicochemical Attributes, Bioactive Compounds and Antioxidant Activity of Extracted Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oil Yield and Seeds Microstructure Changes
2.2. Refractive and Yellowness Index
2.3. Conjugated Dienes and Trienes
2.4. Total Carotenoids, Total Phenols and Antioxidant Activity
2.5. Phytosterols Composition
2.6. Fatty Acids Composition
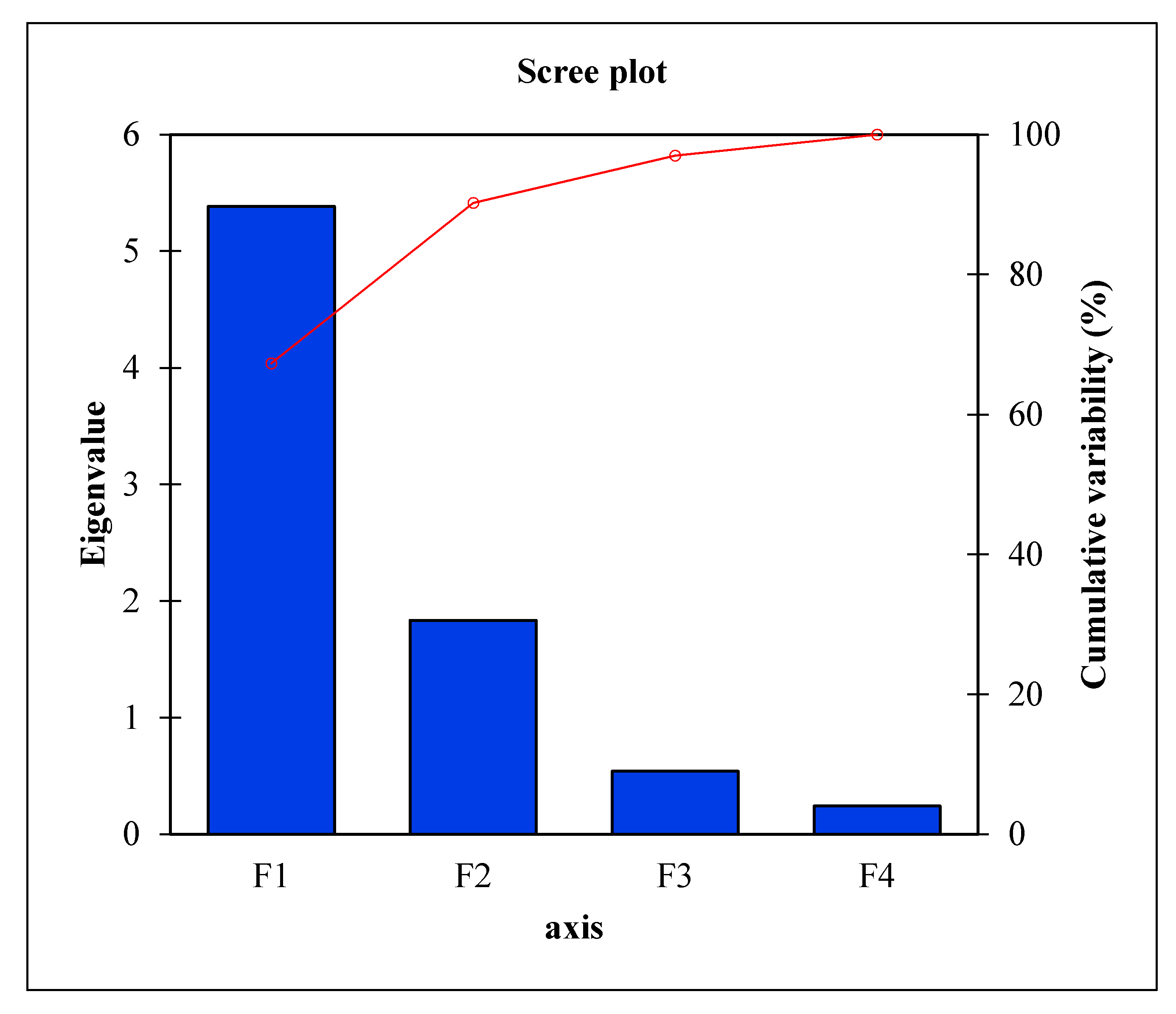
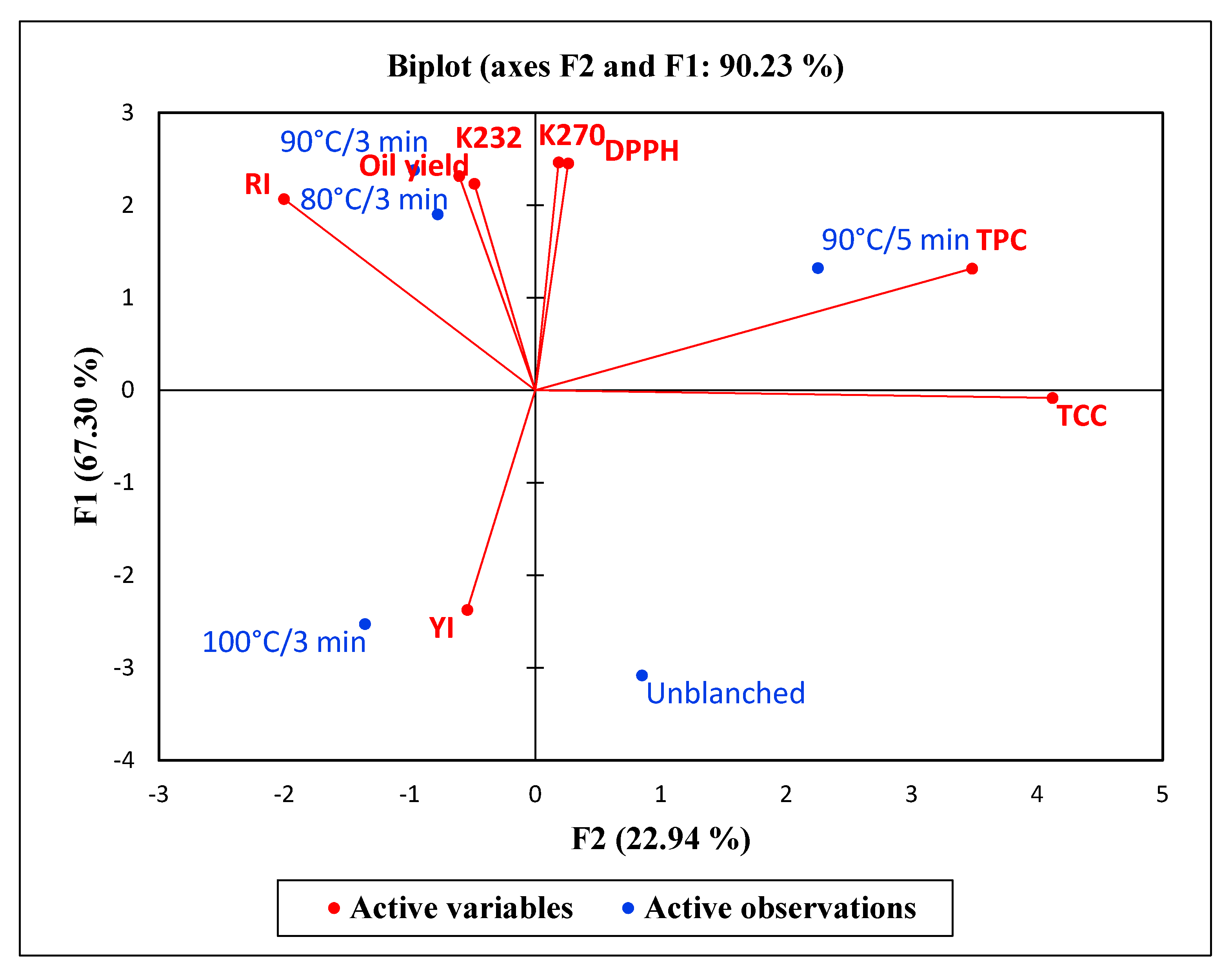
2.7. Principal Component Analysis
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation and Blanching Pretreatments
3.3. Oil Extraction
3.4. Pomegranate Seeds Microstructure Analysis
3.5. Physicochemical Attributes Analysis
3.5.1. Yellowness and Refractive Index
3.5.2. Conjugated Dienes and Trienes
3.6. Bioactive Compounds and Antioxidant Activity Determination
3.6.1. Total Carotenoids
3.6.2. Total Phenolic Compounds
3.6.3. Phytosterol Composition
3.6.4. Antioxidant Activity
3.7. Analysis of Fatty Acid Composition
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Xu, Z.; Zheng, B.; Lo, Y.M. Ultrasonics sonochemistry optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason. Sonochem. 2013, 20, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; Macfarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [PubMed]
- Mallek-ayadi, S.; Bahloul, N.; Kechaou, N. Chemical composition and bioactive compounds of Cucumis melo L. seeds: Potential source for new trends of plant oils. Process. Saf. Environ. 2017, 113, 68–77. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L.L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [PubMed]
- Carolina, A.; Jorge, N. Data Report on bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1–5. [Google Scholar]
- Đurđević, S.; Šavikin, K.; Živković, J.; Böhm, V.; Stanojković, T.; Damjanović, A.; Petrović, S. Antioxidant and cytotoxic activity of fatty oil isolated by supercritical fluid extraction from microwave pretreated seeds of wild growing Punica granatum L. J. Supercrit. Fluids 2018, 133, 225–232. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar]
- Fawole, O.A.; Opara, U.L. Developmental changes in maturity indices of pomegranate fruit: A descriptive review. Sci. Hortic. 2013, 159, 152–161. [Google Scholar] [CrossRef]
- Mohagheghi, M.; Rezaei, K.; Labbafi, M.; Ebrahimzadeh, S.M. Pomegranate seed oil as a functional ingredient in beverages. Eur. J. Lipid Sci. Technol. 2011, 113, 730–736. [Google Scholar]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Abbasi, H.; Rezaei, K.; Emamdjomeh, Z.; Ebrahimzadeh Mousavi, S.M. Effect of various extraction conditions on the phenolic contents of pomegranate seed oil. Eur. J. Lipid Sci. Technol. 2008, 110, 435–440. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A method for pomegranate seed application in food industries: Seed oil encapsulation. Food Bioprod. Process. 2012, 90, 639–652. [Google Scholar] [CrossRef]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R.P. Health benefits of punicic acid: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 16–27. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Singh, R.; Vijayraghavan, R.; Arora, A. From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crop. Prod. 2018, 112, 790–802. [Google Scholar] [CrossRef]
- Mailer, R.J.; Ayton, J. Comparison of olive oil (Olea europaea) quality extracted by stonemill and hammermill. N. Z. J. Crop Hortic. Sci. 2004, 32, 325–330. [Google Scholar] [CrossRef]
- Güneşer, A.B.; Yilmaz, E. Effects of microwave roasting on the yield and composition of cold pressed orange seed oils. Grasas Aceites 2017, 68, 1–10. [Google Scholar]
- Hassini, L.; Bettaieb, E.; Motri, S.; Desmorieux, H. Studies on convective drying of (Ameclyae) Opuntia ficus-indica seeds and its effect on the quality of extracted oil based on its α-tocopherol content. Int. J. Heat Mass Transf. 2018, 54, 393–402. [Google Scholar] [CrossRef]
- Gogolewski, M.; Nogala-Kalucka, M.; Szeliga, M. Changes of the tocopherol and fatty acid contents in rapeseed oil during refining. Eur. J. Lipid Sci. Technol. 2000, 102, 618–623. [Google Scholar] [CrossRef]
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wähälä, K.; Heinonen, M. Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. Eur. Food Res. Technol. 2003, 217, 110–114. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Fetzer, D.L.; Cruz, P.N.; Hamerski, F.; Corazza, M.L. Extraction of baru (Dipteryx alata vogel) seed oil using compressed solvents technology. J. Supercrit. Fluids 2018, 137, 23–33. [Google Scholar] [CrossRef]
- Tir, R.; Dutta, P.C.; Badjah-hadj-ahmed, A.Y. Effect of the extraction solvent polarity on the sesame seeds oil composition. Eur. J. Lipid Sci. Technol. 2012, 114, 1427–1438. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L.; Huang, Q.; Wang, J.; Lin, Q.; Liu, M.; Lee, W.Y.; Song, H. Ultrasonic-assisted extraction of raspberry seed oil and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. PLoS ONE 2016, 11, 1–18. [Google Scholar]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Russo-pro, A.; Eveleigh, A.; Aliev, A.; Kay, A.; Mills-lamptey, B. Influence of solvent selection and extraction temperature on yield and composition of lipids extracted from spent coffee grounds. Ind. Crops Prod. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Saxena, B.D.K.; Sharma, S.K.; Sambi, S.S. Kinetics and thermodynamics of cottonseed oil extraction. Grasas Aceites 2011, 62, 198–205. [Google Scholar] [CrossRef]
- Citeau, M.; Slabi, S.A.; Joffre, F.; Carré, P. Improved rapeseed oil extraction yield and quality via cold separation of ethanol miscella. OCL 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Passos, C.P.; Yilmaz, S.; Silva, C.M.; Coimbra, M.A. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009, 115, 48–53. [Google Scholar] [CrossRef]
- Wroniak, M.; Rekas, A.; Siger, A.; Janowicz, M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT Food Sci. Technol. 2016, 68, 634–641. [Google Scholar] [CrossRef]
- Moradi, N.; Rahimi, M. Effect of simultaneous ultrasound/pulsed electric field pretreatments on the oil extraction from sunflower seeds. Sep. Sci. Technol. 2018, 53, 2088–2099. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Azadmard-damirchi, S.; Zahedi, Y.; Shaddel, R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind. Crops Prod. 2019, 128, 527–533. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Effect of roasting and microwave pretreatments of Nigella sativa L. seeds on lipase activity and the quality of the oil. Food Chem. 2019, 274, 480–486. [Google Scholar]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Subramaniam, S.; Vaughn, K.; Carrier, D.J.; Clausen, E.C. Pretreatment of milk thistle seed to increase the silymarin yield: An alternative to petroleum ether defatting. Bioresour. Technol. 2008, 99, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Mothibe, K.J.; Zhang, M.; Nsor-atindana, J.; Yu-Chuan, W. Use of ultrasound pretreatment in drying of fruits: Drying rates, quality attributes, and shelf life extension. Dry. Technol. 2011, 29, 1611–1621. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Gordon, M.H. Aqueous enzyme assisted oil extraction from oilseeds and emulsion methods: A review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Wen, T.N.; Prasad, K.N.; Yang, B.; Ismail, A. Bioactive substance contents and antioxidant capacity of raw and blanched vegetables. Innov. Food Sci. Emerg. Technol. 2010, 11, 464–469. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Chen, H.; Sun, D.; Deng, B.; Li, J.; Liu, Y.; Wang, F. Inactivation of lipase and lipoxygenase of wheat germ with temperature-controlled short wave infrared radiation and its effect on storage stability and quality of wheat germ oil. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Q.; Chen, S.; Hu, Z.; Xie, H. Effect of hot-water blanching pretreatment on drying characteristics and product qualities for the novel integrated freeze-drying of apple slices. J. Food Qual. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching – A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Uquiche, E.; Jeréz, M.; Ortíz, J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol.). Innov. Food Sci. Emerg. Technol. 2008, 9, 495–500. [Google Scholar]
- Davis, J.P.; Sweigart, D.S.; Price, K.M.; Dean, L.L.; Sanders, T.H. Refractive index and density measurements of peanut oil for determining oleic and linoleic acid contents. J. Am. Oil Chem. Soc. 2013, 90, 199–206. [Google Scholar]
- Costa, A.M.M.; Silva, L.O.; Torres, A.G. Chemical composition of commercial cold-pressed pomegranate (Punica granatum) seed oil from Turkey and Israel, and the use of bioactive compounds for samples’ origin preliminary discrimination. J. Food Compos. Anal. 2019, 75, 8–16. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Khoo, H.; Nagendra Prasad, K.; Kong, K.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Colour pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wei, B.; Ren, X.; Liu, Y.; Jiang, H.; Zhou, C.; Ma, H. Dielectric pretreatment of rapeseed 1: Influence on the drying characteristics of the seeds and physicochemical properties of cold-pressed oil. Food Bioprocess Technol. 2018, 11, 1236–1247. [Google Scholar] [CrossRef]
- Destaillats, F.; Angers, P. Thermally induced formation of conjugated isomers of linoleic acid. Eur. J. Lipid Sci. Technol. 2005, 107, 167–172. [Google Scholar]
- Amri, Z.; Lazreg-Aref, H.; Mekni, M.; El-gharbi, S.; Dabbaghi, O.; Mechri, B.; Hammami, M. Oil characterization and lipids class composition of pomegranate seeds. Biomed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Zhu, M.; Hu, R.; Zhao, J.; Chen, Z.; Li, J.; Ni, Y. Characterisation of seed oils from different grape cultivars grown in China. J. Food Sci. Technol. 2016, 53, 3129–3136. [Google Scholar] [CrossRef]
- Moghimi, M.; Farzaneh, V. The effect of ultrasound pretreatment on some selected physicochemical properties of black cumin (Nigella Sativa). Nutrire 2018, 43, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Cao, A.; Aisikaer, G.; Ying, T. Influence of kernel roasting on bioactive components and oxidative stability of pine nut oil. Eur. J. Lipid Sci. Technol. 2013, 115, 556–563. [Google Scholar] [CrossRef]
- Fiedor, J.; Science, A.C. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Bélliard, T.; Castello, J.; Van Hecke, É.; Lanoisellé, J.L. Grape seed oil extraction: Interest of supercritical fluid extraction and gas-assisted mechanical extraction for enhancing polyphenol co-extraction in oil. Proc. Natl. Acad. Sci. USA 2014, 17, 284–292. [Google Scholar] [CrossRef]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the antiradical properties of phenolic acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Ferdosh, S.; Haque Akanda, M.J.; Ghafoor, K.; Rukshana, A.H.; Ali, M.E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci.Technol. 2018, 53, 2206–2223. [Google Scholar]
- Zhou, Y.; Fan, W.; Chu, F.; Pei, D. Improvement of the flavour and oxidative stability of walnut oil by microwave pretreatment. J. Am. Oil Chem. Soc. 2016, 93, 1563–1572. [Google Scholar] [CrossRef]
- Kittiphoom, S.; Sutasinee, S. Effect of microwaves pretreatments on extraction yield and quality of mango seed kernel oil. Int. Food Res. 2015, 22, 960–964. [Google Scholar]
- Đurđević, S.; Šavikin, K.; Živković, J.; Böhm, V.; Stanojković, T.; Damjanović, A.; Petrović, S. Improvement of supercritical CO2 and n-hexane extraction of wild growing pomegranate seed oil by microwave pretreatment. Ind. Crops Prod. 2017, 104, 21–27. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Seasonal variation in chemical composition, aroma volatiles and antioxidant capacity of pomegranate during fruit development. Afr. J. Biotechnol. 2013, 12, 4006–4019. [Google Scholar]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Functional properties of pomegranate fruit parts: Influence of packaging systems and storage time. J. Food Meas. Charact. 2017, 11, 2233–2246. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Segliņa, D. Lipophilic composition of eleven apple seed oils: A promising source of unconventional oil from industry by-products. Ind. Crops Prod. 2014, 60, 86–91. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Wang, Y.; Chen, H.; Huang, X. Phenolic compounds and the antioxidant activities in litchi pericarp: Difference among cultivars. Sci. Hortic. 2011, 129, 784–789. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M. Ultrasound-assisted extraction (UAE) and solvent extraction of papaya seed oil: Yield, fatty acid composition and triacylglycerol profile. Molecules 2013, 18, 12474–12487. [Google Scholar] [PubMed] [Green Version]
- ISO. Animal and Vegetable Fats and Oils. In: ISO 3656: Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. International Organisation for Standardisation. 2011, pp. 1–8. Available online: https://www.iso.org/standard/51008 (accessed on 27 May 2020).
- Siano, F.; Straccia, M.C.; Paolucci, M.; Fasulo, G.; Boscaino, F.; Volpe, M.G. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agric. 2015, 96, 1730–1735. [Google Scholar] [PubMed]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) hill. Chem. Cent. J. 2012, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mphahlele, R.R.; Fawole, O.A.; Mokwena, L.M.; Opara, U.L. Effect of extraction method on chemical, volatile composition and antioxidant properties of pomegranate juice. S. Afr. J. Bot. 2016, 103, 135–144. [Google Scholar] [CrossRef]
Sample Availability: Samples of the pomegranate seed oil extracts are available from the authors. |



| Blanching Temperature (°C) | Oil Yield (%) | YI | K232 | K270 | RI | TCC | TPC | DPPH |
|---|---|---|---|---|---|---|---|---|
| Unblanched | 13.70 ± 0.10 c | 83.86 ± 0.13 a | 0.14 ± 0.01 c | 0.13 ± 0.02 c | 1.5215 ± 0.00 a | 30.12 ± 1.07 a | 3.41 ± 0.05 a | 4.06 ± 0.11 c |
| 80 | 15.80 ± 0.60 b | 65.47 ± 0.30 c | 0.41 ± 0.02b b | 0.48 ± 0.02 b | 1.5218 ± 0.00 a | 28.74 ± 1.07 a | 3.03 ± 0.17 c | 4.32 ± 0.05 ab |
| 90 | 18.55 ± 0.55 a | 67.04 ± 0.06 b | 0.32 ± 0.04 a | 0.41 ± 0.01 a | 1.5218 ± 0.00 a | 26.79 ± 1.12 a | 4.04 ± 0.08 d | 4.41 ± 0.07 b |
| 100 | 14.20 ± 0.20 bc | 91.52 ± 0.16 d | 0.16 ± 0.00 c | 0.15 ± 0.02 c | 1.5217 ± 0.00 a | 27.74 ± 0.10 a | 2.01 ± 0.07 b | 4.13 ± 0.03 ac |
| Blanching Time (min) | Oil Yield (%) | YI | K232 | K270 | RI | TCC | TPC | DPPH |
|---|---|---|---|---|---|---|---|---|
| Unblanched | 13.70 ± 0.10 b | 83.86 ± 0.13 c | 0.14 ± 0.01 a | 0.13 ± 0.02 a | 1.5215 ± 0.00 a | 30.12 ± 1.07 ab | 3.41 ± 0.05 b | 4.06 ± 0.11 a |
| 3 | 18.55 ± 0.55 c | 67.04 ± 0.06 b | 0.32 ± 0.04 b | 0.41 ± 0.01 b | 1.5218 ± 0.00 a | 26.79 ± 1.12 b | 4.04 ± 0.08 a | 4.44 ± 0.06 b |
| 5 | 16.23 ± 0.13 a | 71.05 ± 0.19 a | 0.28 ± 0.04 b | 0.41 ± 0.01 b | 1.5217 ± 0.00 a | 32.94 ± 2.49 a | 5.58 ± 0.07 c | 4.36 ± 0.05 b |
| Phytosterol | Unblanched | 90 °C/3 min |
|---|---|---|
| Stigmasterol | 4.02 ± 0.57 a | 7.59 ± 1.00 b |
| β-Sitosterol | 949.09 ± 18.87 b | 571.73 ± 23.55 a |
| Brassicasterol | 1.99 ± 0.07 a | 2.22 ± 0.01 a |
| Fatty Acid | Unblanched | 90 °C/3 min |
|---|---|---|
| Palmitic (C16:0) | 6.70 ± 0.015 b | 5.61 ± 0.14 a |
| Stearic (C18:0) | 2.64 ± 0.09 a | 2.37 ± 0.04 a |
| Oleic (C18:1) | 7.18 ± 0.09 a | 6.72 ± 0.15 a |
| Linoleic (C18:2) | 12.59 ± 0.40 b | 10.28 ± 0.35 a |
| Punicic (C18:3) | 68.91 ± 0.77 a | 73.55 ± 0.36 b |
| Arachidic (C20:0) | 0.68 ± 0.08 a | 0.46 ± 0.02 a |
| ƩSFA | 10.02 ± 0.27 b | 8.44 ± 0.16 a |
| ƩMUFA | 7.18 ± 0.09 a | 6.72 ± 0.15 a |
| ƩPUFA | 81.49 ± 0.37 a | 83.83 ± 0.06 b |
| UFA/SFA ratio | 8.86 ± 0.26 a | 10.74 ± 0.22 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaseke, T.; Opara, U.L.; Fawole, O.A. Effect of Blanching Pomegranate Seeds on Physicochemical Attributes, Bioactive Compounds and Antioxidant Activity of Extracted Oil. Molecules 2020, 25, 2554. https://doi.org/10.3390/molecules25112554
Kaseke T, Opara UL, Fawole OA. Effect of Blanching Pomegranate Seeds on Physicochemical Attributes, Bioactive Compounds and Antioxidant Activity of Extracted Oil. Molecules. 2020; 25(11):2554. https://doi.org/10.3390/molecules25112554
Chicago/Turabian StyleKaseke, Tafadzwa, Umezuruike Linus Opara, and Olaniyi Amos Fawole. 2020. "Effect of Blanching Pomegranate Seeds on Physicochemical Attributes, Bioactive Compounds and Antioxidant Activity of Extracted Oil" Molecules 25, no. 11: 2554. https://doi.org/10.3390/molecules25112554








