Osteomeles schwerinae Extract and Its Major Compounds Inhibit Methylglyoxal-Induced Apoptosis in Human Retinal Pigment Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of the OSSC Extract and Its Major Compounds on the Viability of MG-Treated Cells
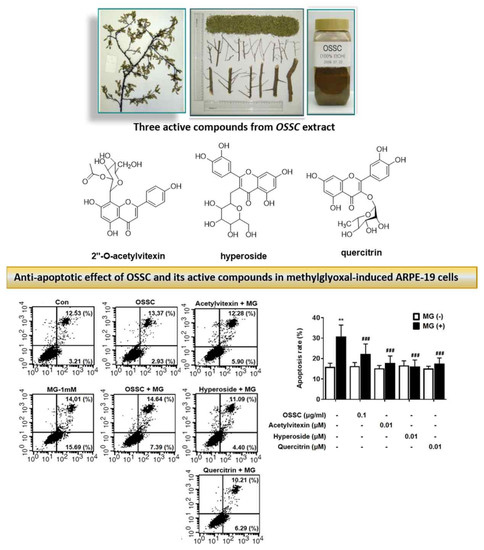
2.2 Effects of the OSSC Extract and Its Maker Compounds on MG-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Preparation of the OSSC Extract and Its Major Compounds
4.2. Cell Culture
4.3. Determination of Apoptosis Using Flow Cytometry
4.4. Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Diabetes 2008, 93, 1143–1152. [Google Scholar]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.R.; Wang, R.X.; Tuo, J.; Chan, C.C. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Xing, Y.; Chen, C.; Chen, Z.; Qian, Z. Advanced glycation end-product (AGE) induces apoptosis in human retinal ARPE-19 cells via promoting mitochondrial dysfunction and activating the Fas-FasL signaling. Biosci. Biotechnol. Biochem. 2016, 80, 250–256. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, Y.S.; Jung, D.H.; Lee, J.; Kim, J.S. Increased glyoxalase I levels inhibit accumulation of oxidative stress and an advanced glycation end product in mouse mesangial cells cultured in high glucose. Exp. Cell Res. 2012, 318, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Pyun, B.J.; Kim, J.S. Osteomeles schwerinae extracts inhibits the binding to receptors of advanced glycation end products and TGF-beta1 expression in mesangial cells under diabetic conditions. Phytomedicine 2016, 23, 388–397. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, C.; Hong, Y.; Sun, Z.; Fang, X.; Wu, B.; Li, S. The role of cPLA2 in Methylglyoxal-induced cell apoptosis of HUVECs. Toxicol. Appl. Pharm. 2017, 323, 44–52. [Google Scholar] [CrossRef]
- Song, M.K.; Roufogalis, B.D.; Huang, T.H.W. Modulation of diabetic retinopathy pathophysiology by natural medicines through PPAR-gamma-related pharmacology. Br. J. Pharm. 2012, 165, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Zhang, Y.; Ranjitkar, S.; Huai, H.; Wang, Y. Traditional knowledge and its transmission of wild edibles used by the Naxi in Baidi Village, northwest Yunnan province. J. Ethnobiol. Ethnomed. 2016, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-F.; Chaw, S.-M. Osteomeles schwerinae CK Schneid. (Rosaceae): A new record for the flora of Taiwan. Bot. Bull. Acad. Sin. 1996, 37, 281–285. [Google Scholar]
- Song, L.; Hu, L.; Hong, X.J.S.S. Chinese Materia Medica; Technology Press: Shanghai, China, 1999; Volume 4, p. 166. [Google Scholar]
- Kim, C.S.; Kim, J.; Kim, Y.S.; Jo, K.; Lee, Y.M.; Jung, D.H.; Lee, I.S.; Kim, J.H.; Kim, J.S. Improvement in Diabetic Retinopathy through Protection against Retinal Apoptosis in Spontaneously Diabetic Torii Rats Mediated by Ethanol Extract of Osteomeles schwerinae C.K. Schneid. Nutrients 2019, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jang, D.S.; Yoo, N.H.; Lee, Y.M.; Kim, J.-H.; Kim, J.S. Single-step separation of bioactive flavonol glucosides from Osteomeles schwerinae by high-speed counter-current chromatography. J. Sep. Sci. 2010, 33, 582–586. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complication. Nature 2001, 14, 813–820. [Google Scholar] [CrossRef]
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose reductase inhibitors of plant origin. Phytother. Res. 2014, 8, 317–33. [Google Scholar] [CrossRef]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–89. [Google Scholar] [CrossRef]
- Rao, B.K.; Kesavulu, M.M.; Giri, R.; Appa Rao, C. Antidiabetic and hypolipidemic effects of Momordica cymbalaria Hook. fruit powder in alloxan-diabetic rats. J. Ethnopharmacol. 1999, 67, 103–109. [Google Scholar] [PubMed]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.J.; Yu, S.Y.; Ku, S.K.; Kim, M.H.; Kim, J.S. Effects of Allium victorialis leaf extracts and its single compounds on aldose reductase, advanced glycation end products and TGF-beta1 expression in mesangial cells. BMC Complement. Altern. Med. 2013, 13, 251. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.Z.; Zhang, X.P.; Xu, X.D.; Zheng, Q.X.; Yang, J.S.; Ding, W.L. Characterization of aromatic glycosides in the extracts of Trollius species by ultra high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013, 75, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside Induces Endogenous Antioxidant System to Alleviate Oxidative Stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.K.; Kang, H.S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Jung, D.H.; Kim, N.H.; Lee, Y.M.; Jang, D.S.; Song, G.Y.; Kim, J.S. KIOM-79 inhibits high glucose or AGEs-induced VEGF expression in human retinal pigment epithelial cells. J. Ethnopharmacol. 2007, 112, 166–172. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyun, B.-J.; Kim, Y.S.; Lee, I.S.; Jung, D.H.; Kim, J.-H.; Kim, J.S. Osteomeles schwerinae Extract and Its Major Compounds Inhibit Methylglyoxal-Induced Apoptosis in Human Retinal Pigment Epithelial Cells. Molecules 2020, 25, 2605. https://doi.org/10.3390/molecules25112605
Pyun B-J, Kim YS, Lee IS, Jung DH, Kim J-H, Kim JS. Osteomeles schwerinae Extract and Its Major Compounds Inhibit Methylglyoxal-Induced Apoptosis in Human Retinal Pigment Epithelial Cells. Molecules. 2020; 25(11):2605. https://doi.org/10.3390/molecules25112605
Chicago/Turabian StylePyun, Bo-Jeong, Young Sook Kim, Ik Soo Lee, Dong Ho Jung, Joo-Hwan Kim, and Jin Sook Kim. 2020. "Osteomeles schwerinae Extract and Its Major Compounds Inhibit Methylglyoxal-Induced Apoptosis in Human Retinal Pigment Epithelial Cells" Molecules 25, no. 11: 2605. https://doi.org/10.3390/molecules25112605