Photoexcitation of Ge9− Clusters in THF: New Insights into the Ultrafast Relaxation Dynamics and the Influence of the Cation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Steady State Spectroscopy in Solution
2.2. Transient Absorption Spectroscopy in Solution
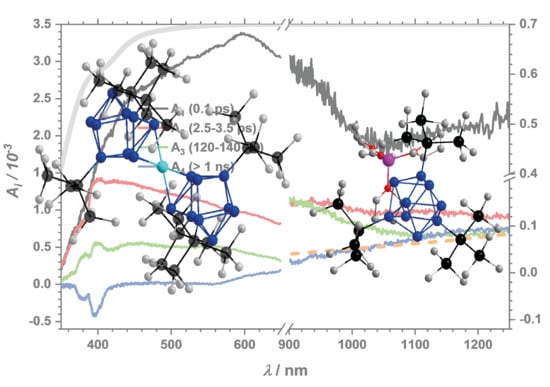
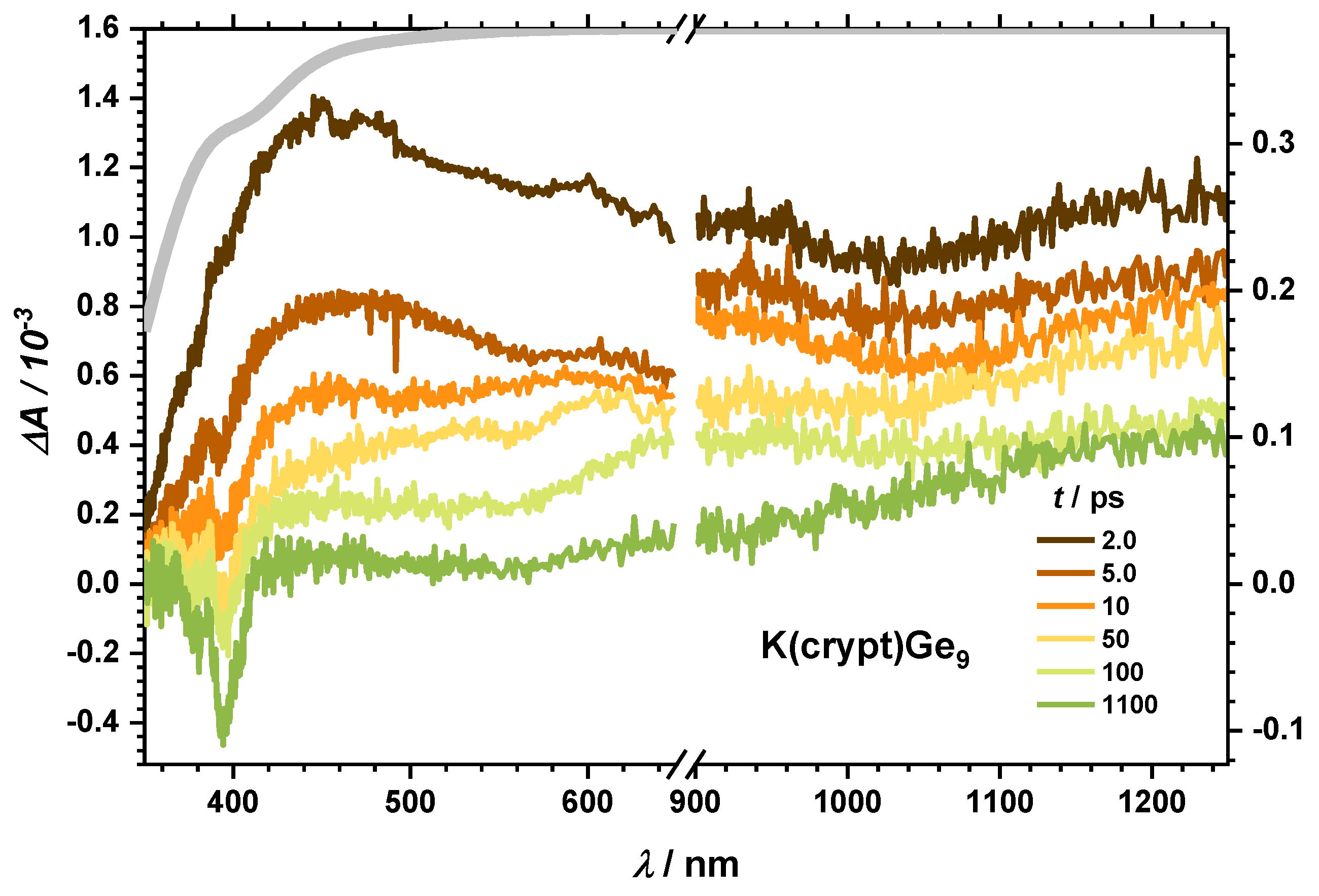
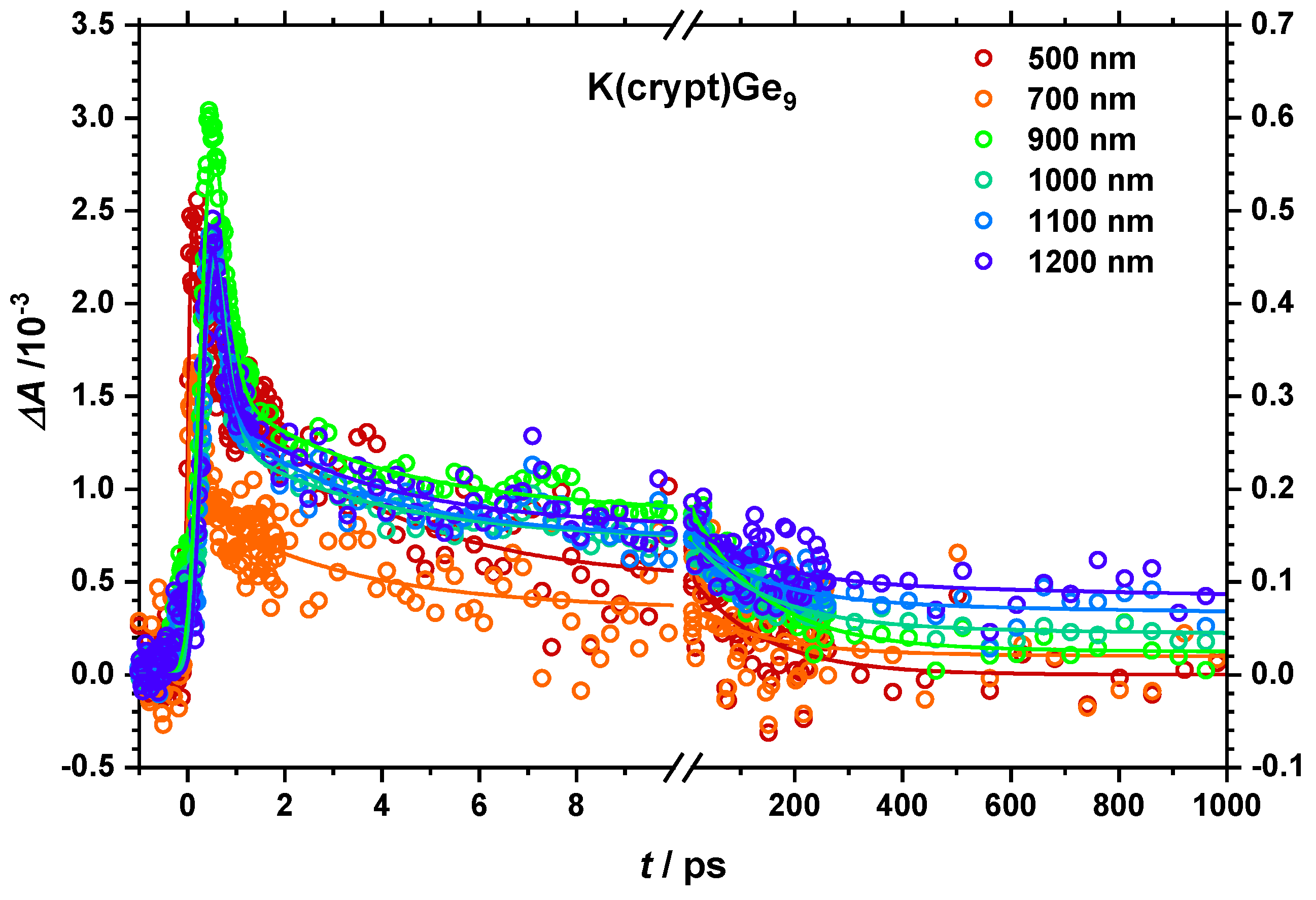
2.2.1. Dynamics of the Monomer
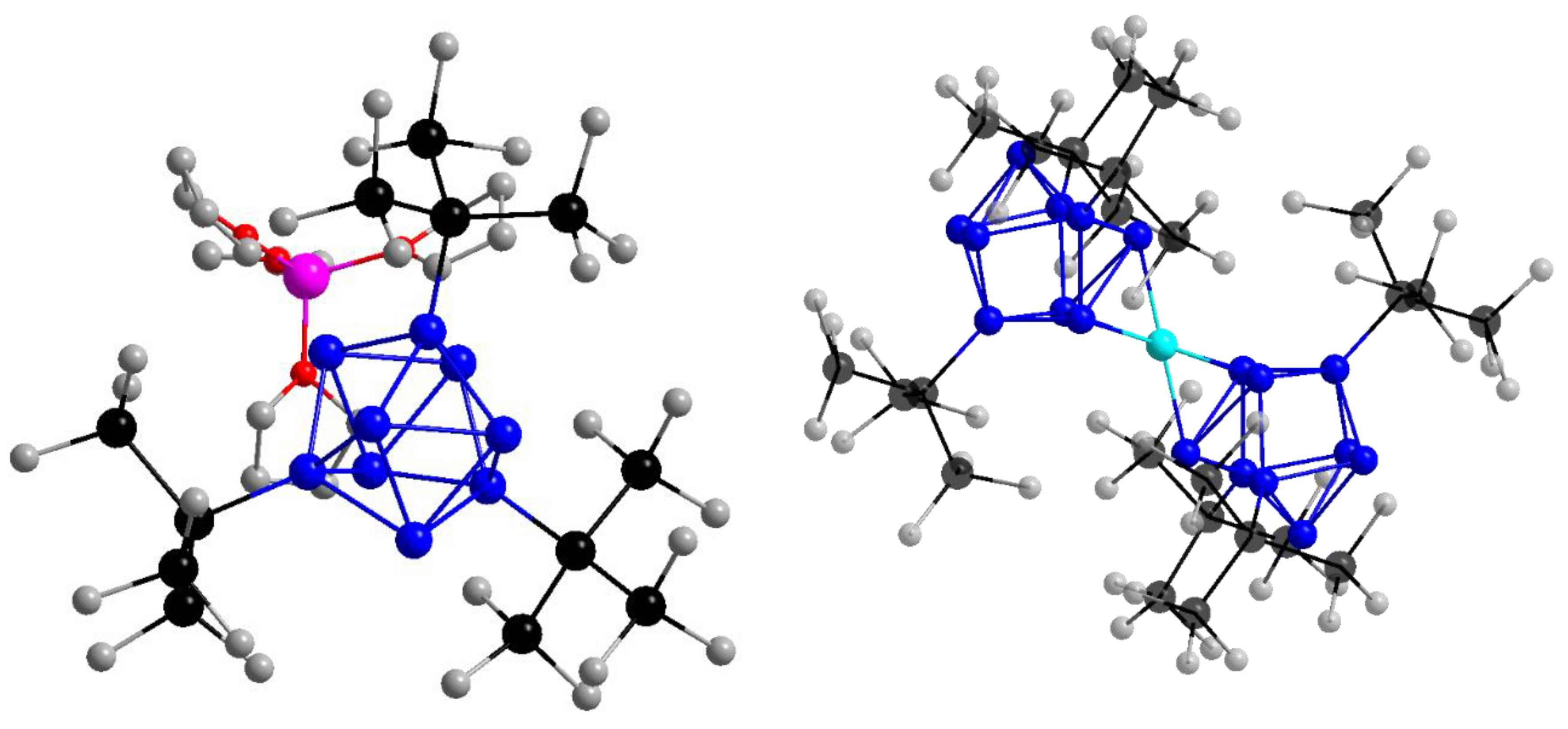
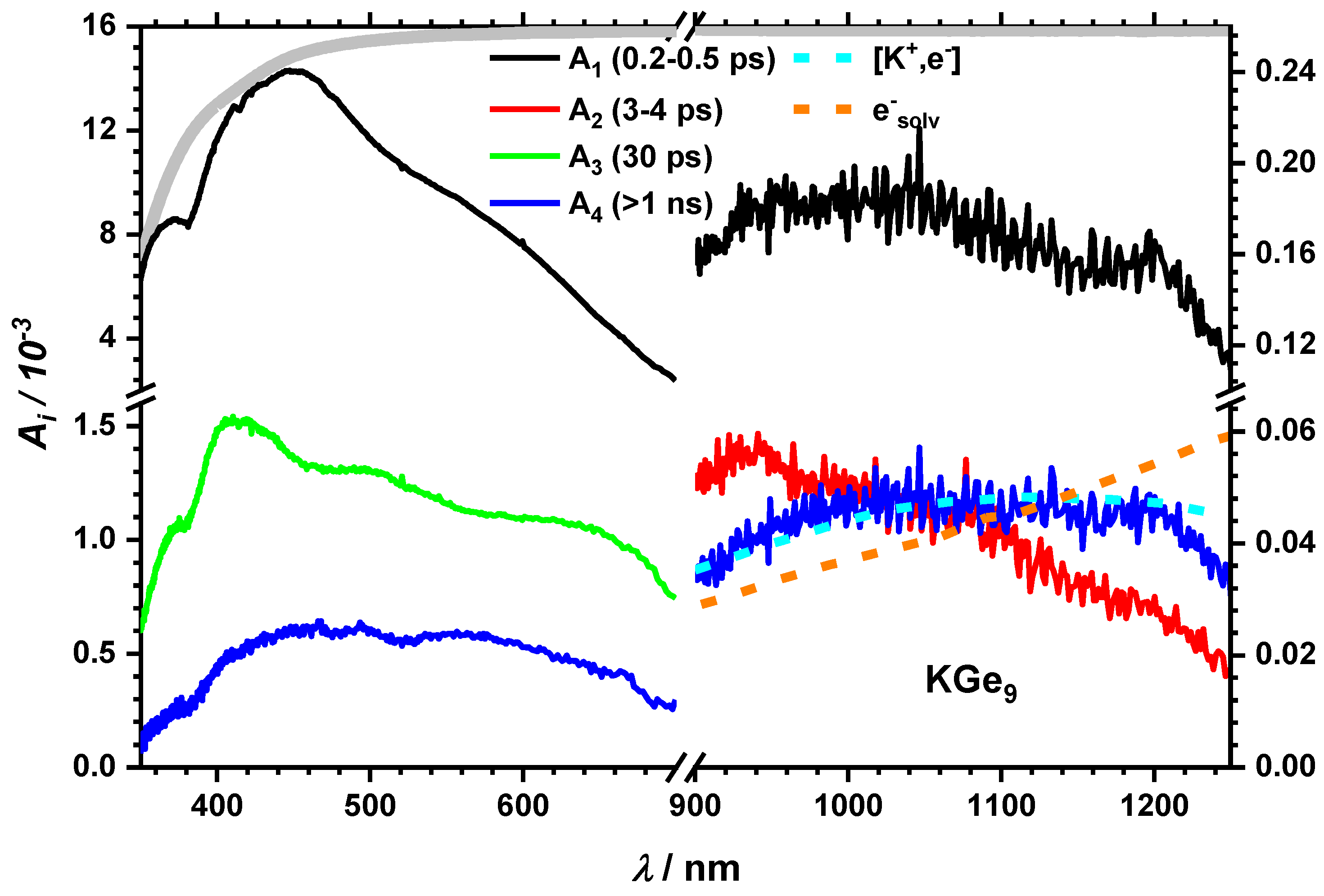
2.2.2. Impact of the Counter Ion
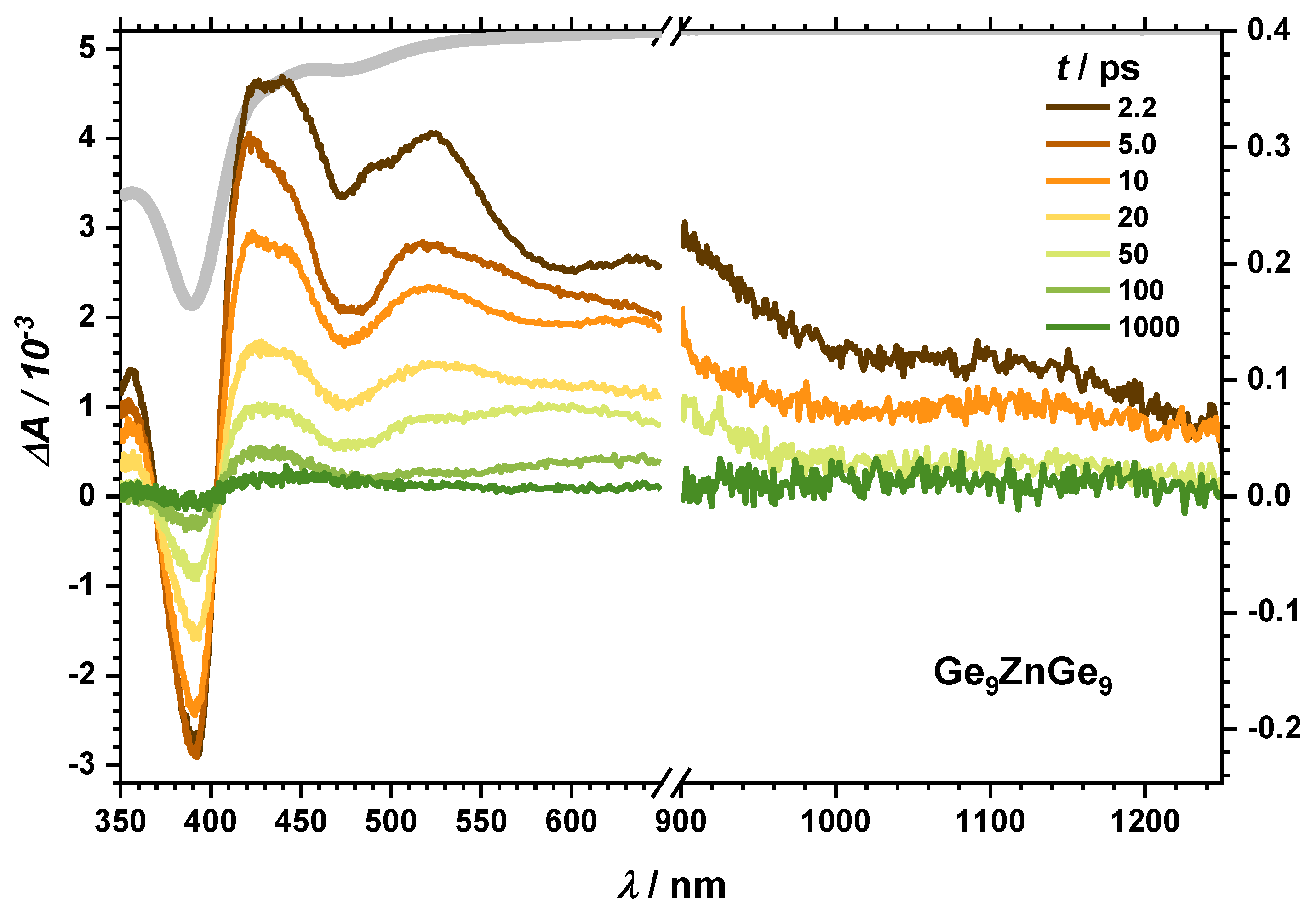
2.2.3. Dynamics of the Dimer
3. Materials and Methods
3.1. Sample Preparation
3.1.1. K4Ge9
3.1.2. KGe9Hyp3
3.1.3. K(crypt-2,2,2)Ge9Hyp3
3.1.4. LiGe9Hyp3
3.1.5. ZnGe18Hyp6
3.2. Steady-State Spectroscopy in Solution
3.3. Transient Absorption Spectroscopy in Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bragg, A.E.; Schwartz, B.J. Ultrafast Charge-Transfer-to-Solvent Dynamics of Iodide in Tetrahydrofuran. 2. Photoinduced Electron Transfer to Counterions in Solution. J. Phys. Chem. A 2008, 112, 3530–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinger, M.; Schenk, C.; Henke, F.; Clayborne, A.; Schnepf, A.; Unterreiner, A.N. UV photoexcitation of a dissolved metalloid Ge9 cluster compound and its extensive ultrafast response. Chem. Commun. 2015, 51, 12278–12281. [Google Scholar] [CrossRef] [PubMed]
- Dotti, N.; Heintze, E.; Slota, M.; Hübner, R.; Wang, F.; Nuss, J.; Dressel, M.; Bogani, L. Conduction mechanism of nitronyl-nitroxide molecular magnetic compounds. Phys. Rev. B 2016, 93, 165201. [Google Scholar] [CrossRef]
- Chen, R.; Hong, Z.-F.; Zhao, Y.-R.; Zheng, H.; Li, G.-J.; Zhang, Q.-C.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. Ligand-Dependent Luminescence Properties of Lanthanide–Titanium Oxo Clusters. Inorg. Chem. 2019, 58, 15008–15012. [Google Scholar] [CrossRef] [PubMed]
- Michenfelder, N.C.; Gienger, C.; Schnepf, A.; Unterreiner, A.-N. The influence of the FeCp(CO)2+ moiety on the dynamics of the metalloid [Ge9(Si(SiMe3)3)3]− cluster in thf: Synthesis and characterization by time-resolved absorption spectroscopy. Dalton Trans. 2019, 48, 15577–15582. [Google Scholar] [CrossRef] [Green Version]
- Liedy, F.; Eng, J.; McNab, R.; Inglis, R.; Penfold, T.J.; Brechin, E.K.; Johansson, J.O. Vibrational coherences in manganese single-molecule magnets after ultrafast photoexcitation. Nat. Chem. 2020, 12, 452–458. [Google Scholar] [CrossRef]
- Vogler, T.; Vöhringer, P. Probing the band gap of liquid ammonia with femtosecond multiphoton ionization spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 25657–25665. [Google Scholar] [CrossRef]
- Baniodeh, A.; Liang, Y.; Anson, C.E.; Magnani, N.; Powell, A.K.; Unterreiner, A.-N.; Seyfferle, S.; Slota, M.; Dressel, M.; Bogani, L.; et al. Unraveling the Influence of Lanthanide Ions on Intra- and Inter-Molecular Electronic Processes in Fe10Ln10 Nano-Toruses. Adv. Funct. Mater. 2014, 24, 6280–6290. [Google Scholar] [CrossRef]
- Tungulin, D.; Leier, J.; Carter, A.B.; Powell, A.K.; Albuquerque, R.Q.; Unterreiner, A.N.; Bizzarri, C. Chasing BODIPY: Enhancement of Luminescence in Homoleptic Bis(dipyrrinato) ZnII Complexes Utilizing Symmetric and Unsymmetrical Dipyrrins. Chem. Eur. J. 2019, 25, 3816–3827. [Google Scholar] [CrossRef]
- Kloepfer, J.A.; Vilchiz, V.H.; Lenchenkov, V.A.; Bradforth, S.E. Femtosecond dynamics of photodetachment of the iodide anion in solution: Resonant excitation into the charge-transfer-to-solvent state. Chem. Phys. Lett. 1998, 298, 120–128. [Google Scholar] [CrossRef]
- Bakker, M.J.; Hartl, F.; Stufkens, D.J.; Jina, O.S.; Sun, X.Z.; George, M.W. Alkene-Stabilized Biradical and Zwitterionic Photoproducts of the Clusters [Os3(CO)10(α-diimine)]: A Time-Resolved Transient Absorption and Infrared Study. Organometallics 2000, 19, 4310–4319. [Google Scholar] [CrossRef]
- Kahnt, A.; Heiniger, L.-P.; Liu, S.-X.; Tu, X.; Zheng, Z.; Hauser, A.; Decurtins, S.; Guldi, D.M. An Electrochemical and Photophysical Study of a Covalently Linked Inorganic–Organic Dyad. ChemPhysChem 2010, 11, 651–658. [Google Scholar] [CrossRef]
- Maverick, A.W.; Gray, H.B. Luminescence and redox photochemistry of the molybdenum(II) cluster Mo6Cl142. J. Am. Chem. Soc. 1981, 103, 1298–1300. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.-S.; Xu, H.-Q.; Su, J.; Rossi, D.; Chen, Y.; Zhang, L.; Lollar, C.; Wang, Q.; Jiang, H.-L.; et al. [Ti8Zr2O12(COO)16] Cluster: An Ideal Inorganic Building Unit for Photoactive Metal–Organic Frameworks. ACS Cent. Sci. 2018, 4, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michenfelder, N.C.; Ernst, H.A.; Schweigert, C.; Olzmann, M.; Unterreiner, A.N. Ultrafast stimulated emission of nitrophenolates in organic and aqueous solutions. Phys. Chem. Chem. Phys. 2018, 20, 10713–10720. [Google Scholar] [CrossRef]
- Barthel, E.R.; Martini, I.B.; Schwartz, B.J. Direct observation of charge-transfer-to-solvent (CTTS) reactions: Ultrafast dynamics of the photoexcited alkali metal anion sodide (Na−). J. Chem. Phys. 2000, 112, 9433–9444. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.M.; You, G.J.; Li, Y.; Zheng, Z.; Tan, H.R.; Shen, Z.X.; Tang, S.H.; Feng, Y.P. Shape-Controlled Synthesis of Single-Crystalline Fe2O3 Hollow Nanocrystals and Their Tunable Optical Properties. J. Phys. Chem. C 2009, 113, 9928–9935. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Lo, K.K.-W.; Wang, C.-R.; Cheung, K.-K. Synthesis, Photophysics, and Transient Absorption Spectroscopic Studies of Luminescent Copper(I) Chalcogenide Complexes. Crystal Structure of [Cu4(μ-dtpm)4(μ4-S)](PF6)2 {dtpm = Bis[bis(4-methylphenyl)phosphino]methane}. J. Phys. Chem. A 1997, 101, 4666–4672. [Google Scholar] [CrossRef]
- Zhou, M.; Long, S.; Wan, X.; Li, Y.; Niu, Y.; Guo, Q.; Wang, Q.-M.; Xia, A. Ultrafast relaxation dynamics of phosphine-protected, rod-shaped Au20 clusters: Interplay between solvation and surface trapping. Phys. Chem. Chem. Phys. 2014, 16, 18288–18293. [Google Scholar] [CrossRef]
- Rothfuss, H.; Knöfel, N.D.; Tzvetkova, P.; Michenfelder, N.C.; Baraban, S.; Unterreiner, A.-N.; Roesky, P.W.; Barner-Kowollik, C. Phenanthroline—A Versatile Ligand for Advanced Functional Polymeric Materials. Chem. Eur. J. 2018, 24, 17475–17486. [Google Scholar] [CrossRef]
- Ruan, L.; Gao, X.; Zhao, J.; Xu, C.; Liang, D. Preparation and characteristics of Eu(DBM)3phen: Synthesis, single-crystal structure and spectroscopic analysis. J. Mol. Struct. 2017, 1149, 265–272. [Google Scholar] [CrossRef]
- Yuetao, Y.; Qingde, S.; Guiwen, Z. Photoacoustic spectroscopy study on lanthanide ternary complexes with dibenzoylmethide and phenanthroline. Spectrochim. Acta A 1999, 55, 1527–1533. [Google Scholar] [CrossRef]
- Thielemann, D.T.; Klinger, M.; Wolf, T.J.A.; Lan, Y.; Wernsdorfer, W.; Busse, M.; Roesky, P.W.; Unterreiner, A.-N.; Powell, A.K.; Junk, P.C.; et al. Novel Lanthanide-Based Polymeric Chains and Corresponding Ultrafast Dynamics in Solution. Inorg. Chem. 2011, 50, 11990–12000. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, A.; Blanco-Rodríguez, A.M.; El Nahhas, A.; Šebera, J.; Záliš, S.; Vlček, A.; Chergui, M. Femtosecond Fluorescence and Intersystem Crossing in Rhenium(I) Carbonyl−Bipyridine Complexes. J. Am. Chem. Soc. 2008, 130, 8967–8974. [Google Scholar] [CrossRef]
- Schnepf, A. [Ge9{Si(SiMe3)3}3]−: Ein löslicher polyedrischer Ge9-Cluster, stabilisiert durch nur drei Silylliganden. Angew. Chem. 2003, 115, 2728–2729. [Google Scholar] [CrossRef]
- Schenk, C.; Henke, F.; Santiso-Quiñones, G.; Krossing, I.; Schnepf, A. [Si(SiMe3)3]6Ge18M (M = Cu, Ag, Au): Metalloid cluster compounds as unusual building blocks for a supramolecular chemistry. Dalton Trans. 2008, 4436–4441. [Google Scholar] [CrossRef]
- Henke, F.; Schenk, C.; Schnepf, A. [Si(SiMe3)3]6Ge18M (M = Zn, Cd, Hg): Neutral metalloid cluster compounds of germanium as highly soluble building blocks for supramolecular chemistry. Dalton Trans. 2009, 9141–9145. [Google Scholar] [CrossRef]
- Li, F.; Sevov, S.C. Rational Synthesis of [Ge−9{Si(SiMe3)3}3]− from Its Parent Zintl Ion Ge−94−. Inorg. Chem. 2012, 51, 2706–2708. [Google Scholar] [CrossRef]
- Kysliak, O.; Schnepf, A. Metalloid Germanium Clusters as Starting Point for New Chemistry. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Schenk, C.; Schnepf, A. [AuGe18{Si(SiMe3)3}6]−: A Soluble Au–Ge Cluster on the Way to a Molecular Cable? Angew. Chem. Int. Ed. 2007, 46, 5314–5316. [Google Scholar] [CrossRef]
- Geitner, F.S.; Fässler, T.F. Introducing Tetrel Zintl Ions to N-Heterocyclic Carbenes – Synthesis of Coinage Metal NHC Complexes of [Ge9{Si(SiMe3)3}3]–. Eur. J. Inorg. Chem. 2016, 2016, 2688–2691. [Google Scholar] [CrossRef]
- Geitner, F.S.; Giebel, M.A.; Pöthig, A.; Fässler, T.F. N-Heterocyclic Carbene Coinage Metal Complexes of the Germanium-Rich Metalloid Clusters [Ge9R3]− and [Ge9RI2]2− with R = Si(iPr)3 and RI = Si(TMS)3. Molecules 2017, 22, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kysliak, O.; Nguyen, D.D.; Clayborne, A.Z.; Schnepf, A. [PtZn2Ge18(Hyp)8] (Hyp = Si(SiMe3)3): A Neutral Polynuclear Chain Compound with Ge9(Hyp)3 Units. Inorg. Chem. 2018, 57, 12603–12609. [Google Scholar] [CrossRef]
- Dorfman, L.M.; Jou, F.Y.; Wageman, R. Solvent Dependence of Optical Absorption Spectrum of Solvated Electron. Berich. Bunsen. Phys. Chem. 1971, 75, 681–685. [Google Scholar] [CrossRef]
- Giling, L.J.; Kloosterboer, J.G.; Rettschnick, R.P.H.; van Voorst, J.D.W. Flash photolysis of negative aromatic ions in liquid solutions. Chem. Phys. Lett. 1971, 8, 457–461. [Google Scholar] [CrossRef]
- Kloosterboer, J.G.; Giling, L.J.; Rettschnick, R.P.H.; van Voorst, J.D.W. Flash photolysis of solutions of sodium in ethers. Chem. Phys. Lett. 1971, 8, 462–466. [Google Scholar] [CrossRef]
- Jou, F.Y.; Dorfman, L.M. Pulse radiolysis studies. XXI. Optical absorption spectrum of the solvated electron in ethers and in binary solutions of these ethers. J. Chem. Phys. 1973, 58, 4715–4723. [Google Scholar] [CrossRef]
- Bockrath, B.; Dorfman, L.M. Pulse radiolysis studies. XXII. Spectrum and kinetics of the sodium cation-electron pair in tetrahydrofuran solutions. J. Phys. Chem. 1973, 77, 1002–1006. [Google Scholar] [CrossRef]
- Bockrath, B.; Dorfman, L.M. Ionic Aggregation of Solvated Electron with Lithium Cation in Tetrahydrofuran Solution. J. Phys. Chem. 1975, 79, 1509–1512. [Google Scholar] [CrossRef] [Green Version]
- Salmon, G.A.; Seddon, W.A.; Fletcher, J.W. Pulse Radiolytic Formation of Solvated Electrons, Ion–pairs, and Alkali Metal Anions in Tetrahydrofuran. Can. J. Chem. 1974, 52, 3259–3268. [Google Scholar] [CrossRef]
- Schweigert, C.; Babii, O.; Afonin, S.; Schober, T.; Leier, J.; Michenfelder, N.C.; Komarov, I.V.; Ulrich, A.S.; Unterreiner, A.N. Real-Time Observation of Diarylethene-Based Photoswitches in a Cyclic Peptide Environment. ChemPhotoChem 2019, 3, 403–410. [Google Scholar] [CrossRef]
- Stähler, J.; Deinert, J.-C.; Wegkamp, D.; Hagen, S.; Wolf, M. Real-Time Measurement of the Vertical Binding Energy during the Birth of a Solvated Electron. J. Am. Chem. Soc. 2015, 137, 3520–3524. [Google Scholar] [CrossRef] [PubMed]
- Martini, I.B.; Barthel, E.R.; Schwartz, B.J. Mechanisms of the ultrafast production and recombination of solvated electrons in weakly polar fluids: Comparison of multiphoton ionization and detachment via the charge-transfer-to-solvent transition of Na− in THF. J. Chem. Phys. 2000, 113, 11245–11257. [Google Scholar] [CrossRef] [Green Version]
- Piotrowiak, P.; Miller, J.R. Spectra of the solvated electron in the presence of sodium cation in tetrahydrofuran and in its.alpha.,.alpha.’-methylated derivatives. J. Am. Chem Soc. 1991, 113, 5086–5087. [Google Scholar] [CrossRef]
- Andre Clayborne, P.; Hakkinen, H. The electronic structure of Ge9[Si(SiMe3)3]3−: A superantiatom complex. Phys. Chem Chem Phys. 2012, 14, 9311–9316. [Google Scholar] [CrossRef]
- Kysliak, O.; Schnepf, A. {Ge9[Si(SiMe3)3]2}2−: A starting point for mixed substituted metalloid germanium clusters. Dalton Trans. 2016, 45, 2404–2408. [Google Scholar] [CrossRef]
- Kiwi, J.; Denisov, N.; Gak, Y.; Ovanesyan, N.; Buffat, P.A.; Suvorova, E.; Gostev, F.; Titov, A.; Sarkisov, O.; Albers, P.; et al. Catalytic Fe3+ clusters and complexes in Nafion active in photo-Fenton processes. High-resolution electron microscopy and femtosecond studies. Langmuir 2002, 18, 9054–9066. [Google Scholar] [CrossRef]
- Baskoutas, S.; Terzis, A.F. Size-dependent band gap of colloidal quantum dots. J. Appl. Phys. 2006, 99, 013708. [Google Scholar] [CrossRef]
- Joly, A.G.; Williams, J.R.; Chambers, S.A.; Xiong, G.; Hess, W.P.; Laman, D.M. Carrier dynamics in α-Fe2O3 (0001) thin films and single crystals probed by femtosecond transient absorption and reflectivity. J. Appl. Phys. 2006, 99, 053521. [Google Scholar] [CrossRef]
- Vohringer, P. Ultrafast dynamics of electrons in ammonia. Annu. Rev. Phys. Chem. 2015, 66, 97–118. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| K(crypt)Ge9 (267 nm) | KGe9 (267 nm) | LiGe9 (267 nm) | Ge9ZnGe9 (267 nm) | Ge9ZnGe9 (400 nm) | |
|---|---|---|---|---|---|
| τ1/ps | 0.1 | 0.5 | 0.5−0.7 | 0.3 | 0.4 |
| τ2/ps | 2.5−3.5 | 3.3 | 2.4 | 2.5−5.8 | 1.8 |
| τ3/ps | 120−140 | 30−50 | 40−60 | 30 | 30 |
| τ4/ps | >1000 | >1000 | >1000 | 700−1200 | 400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michenfelder, N.C.; Gienger, C.; Dilanas, M.; Schnepf, A.; Unterreiner, A.-N. Photoexcitation of Ge9− Clusters in THF: New Insights into the Ultrafast Relaxation Dynamics and the Influence of the Cation. Molecules 2020, 25, 2639. https://doi.org/10.3390/molecules25112639
Michenfelder NC, Gienger C, Dilanas M, Schnepf A, Unterreiner A-N. Photoexcitation of Ge9− Clusters in THF: New Insights into the Ultrafast Relaxation Dynamics and the Influence of the Cation. Molecules. 2020; 25(11):2639. https://doi.org/10.3390/molecules25112639
Chicago/Turabian StyleMichenfelder, Nadine C., Christian Gienger, Melina Dilanas, Andreas Schnepf, and Andreas-Neil Unterreiner. 2020. "Photoexcitation of Ge9− Clusters in THF: New Insights into the Ultrafast Relaxation Dynamics and the Influence of the Cation" Molecules 25, no. 11: 2639. https://doi.org/10.3390/molecules25112639