Color-Tunable Organic Light Emitting Diodes for Deep Blue Emission by Regulating the Optical Micro-Cavity
Abstract
:1. Introduction
2. Results and Discussion
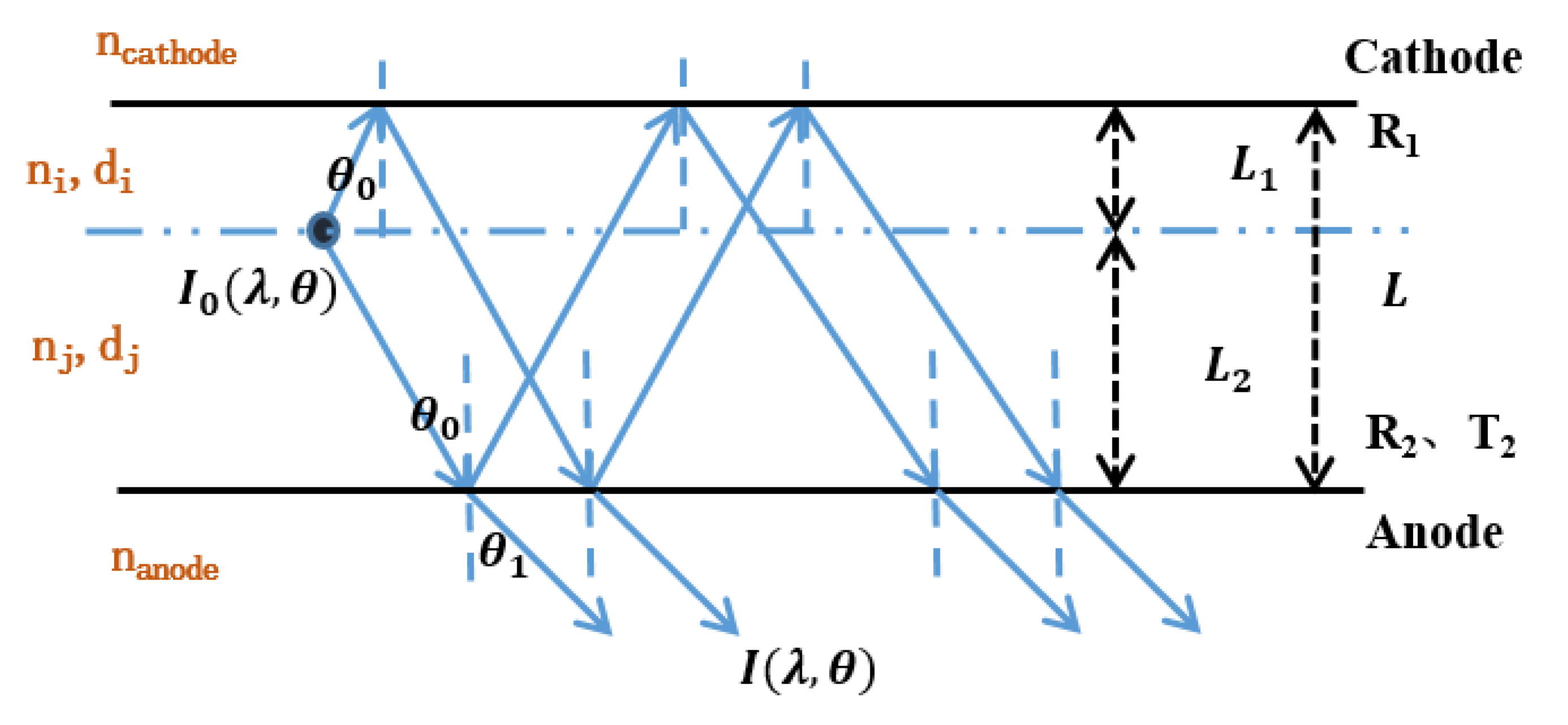
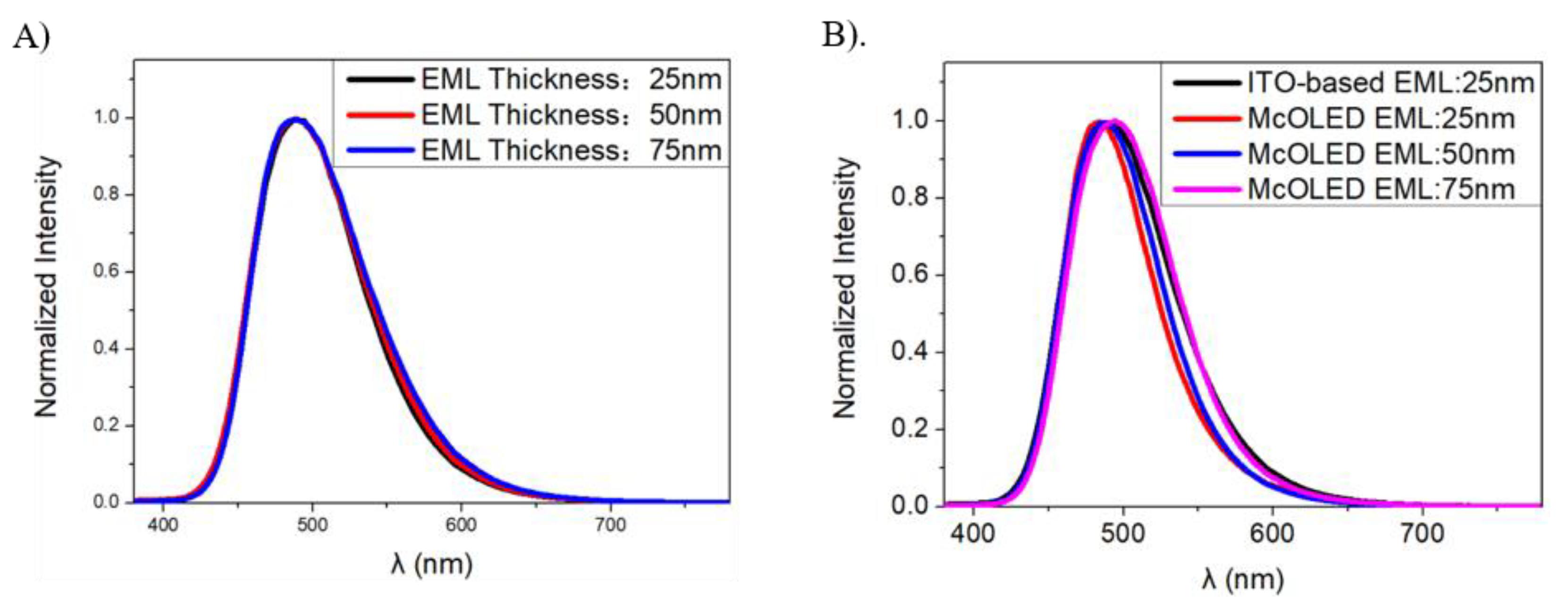
2.1. Optical Properties of the Micro-Cavity in OLEDs
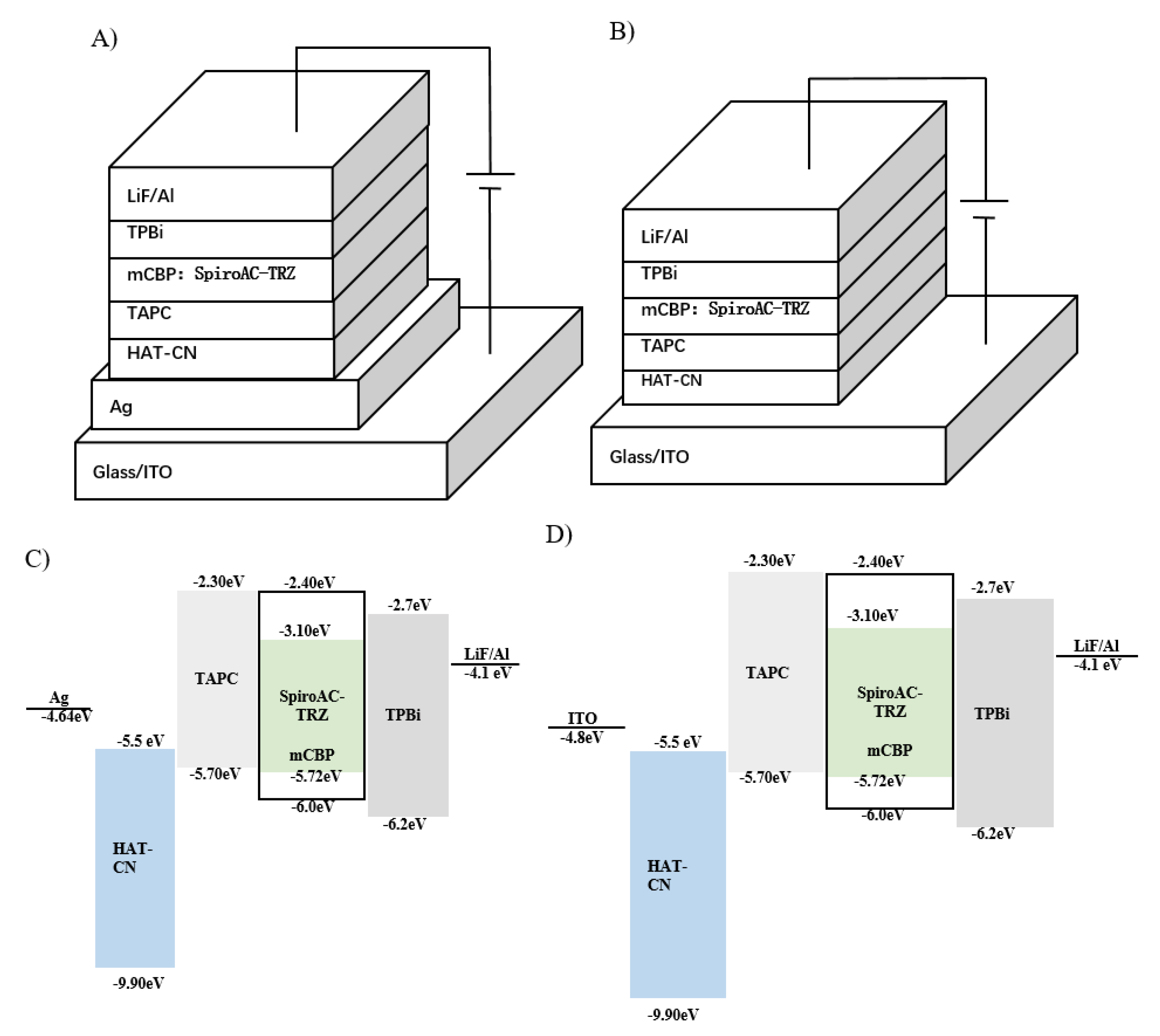
2.2. Design of the Micro-Cavity in Blue OLEDs
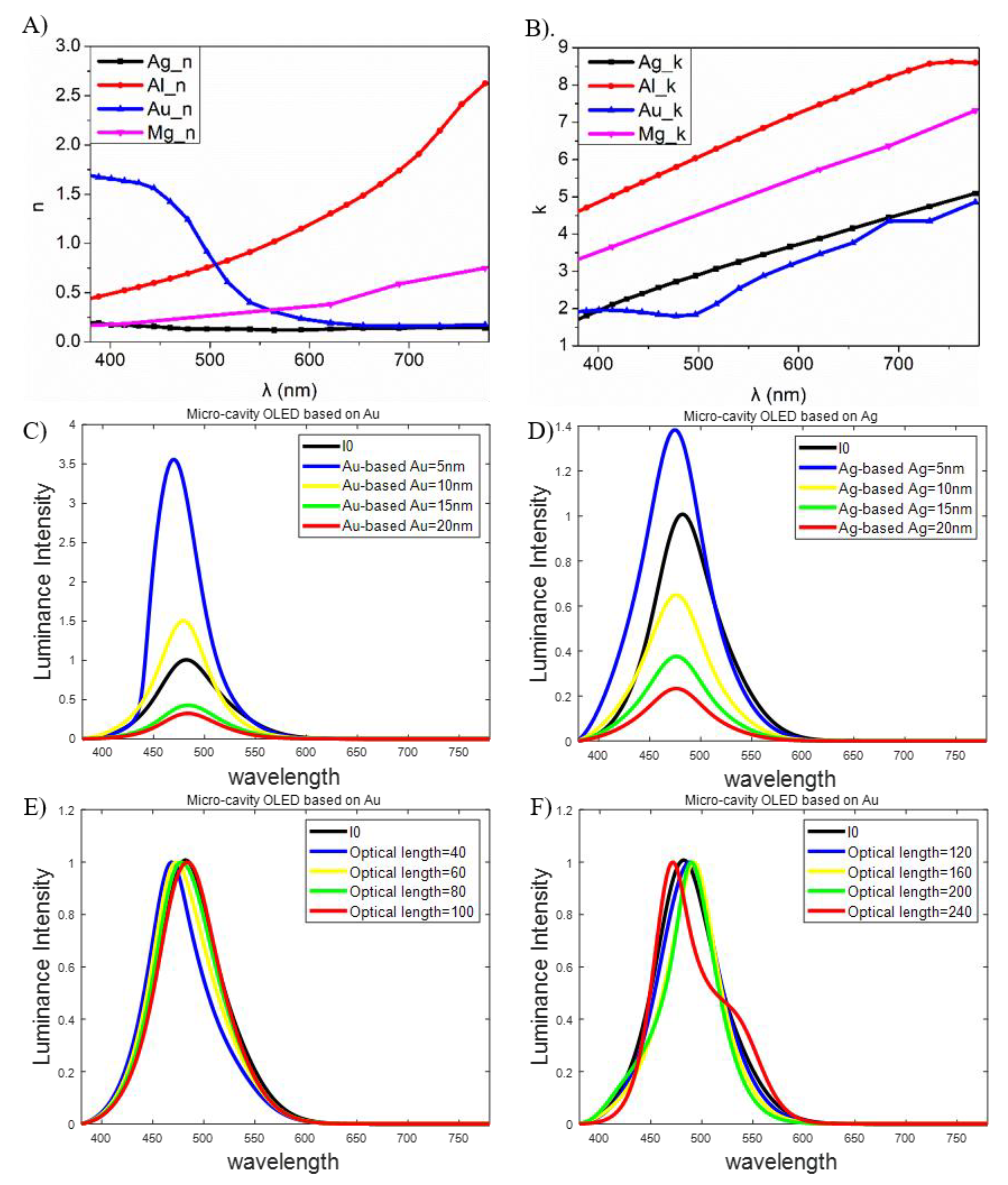
2.3. Theoretical Simulation of the Micro-Cavity in Blue OLEDs
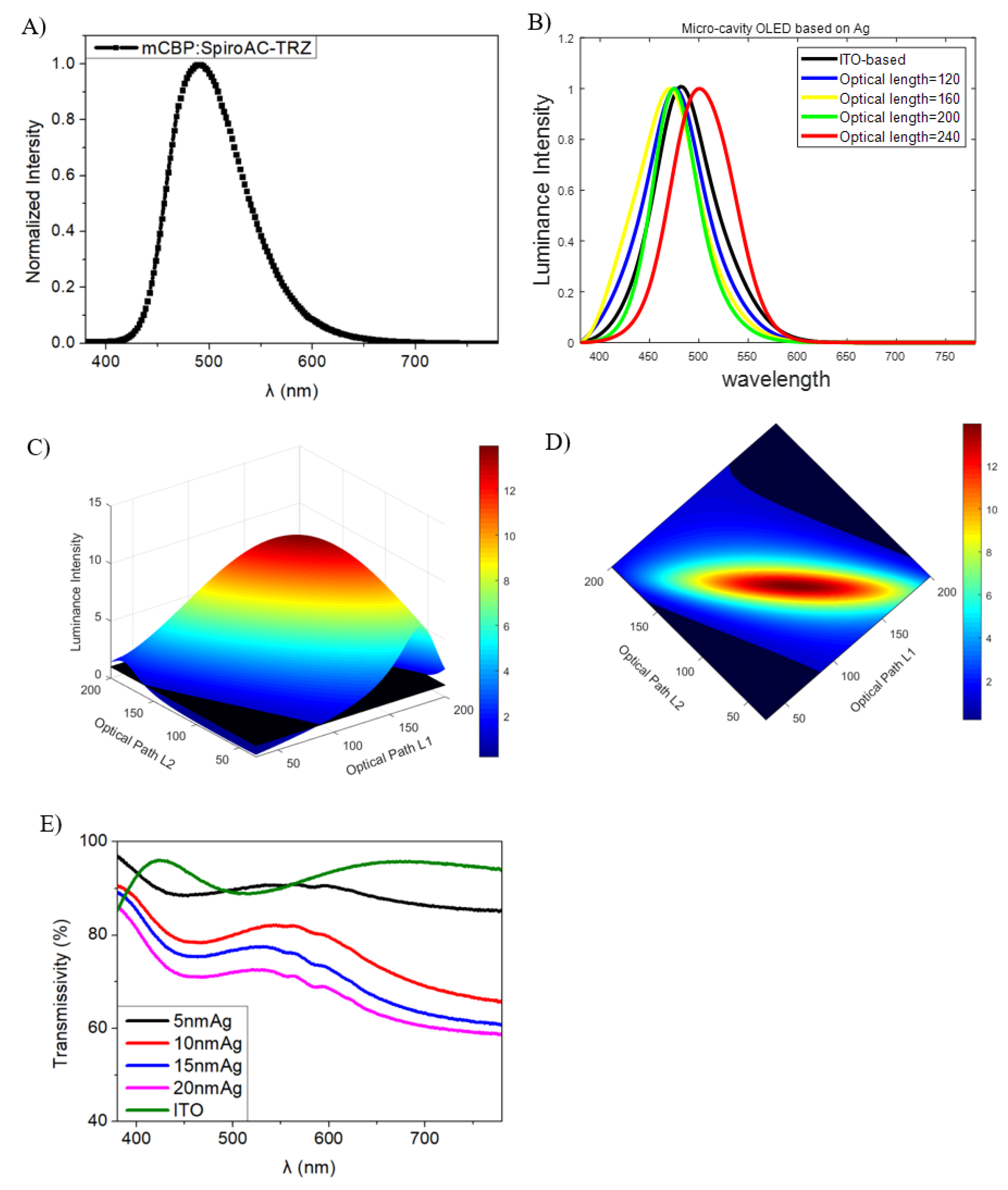
2.4. Deep Blue OLEDs Using Micro-Cavity
3. Materials and Methods
3.1. Materials
3.2. Device Fabrication
3.3. Device Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A.; Chen, C.H. Electroluminescence of doped organic thin films. J. Appl. Phys. 1989, 65, 3610–3616. [Google Scholar] [CrossRef]
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature 2000, 403, 750–753. [Google Scholar] [CrossRef] [PubMed]
- D’Andrade, B.W.; Holmes, R.; Forrest, S.R. Efficient Organic Electrophosphorescent White-Light-Emitting Device with a Triple Doped Emissive Layer. Adv. Mater. 2004, 16, 624–628. [Google Scholar] [CrossRef]
- Gurin, N.T.; Paksyutov, K.V.; Terent’ev, M.A.; Shirokov, A.V. CaO center dot Al2O3 center dot SiO2: Eu Phosphors for Violet/Ultraviolet-to-White Radiation Conversion. Tech. Phys. 2012, 57, 308–310. [Google Scholar] [CrossRef]
- Gao, Z.; Cheng, G.; Shen, F.; Zhang, S.; Zhang, Y.; Lu, P.; Ma, Y. Highly efficient deep blue light emitting devices based on triphenylsilane modified phenanthro[9, 10- d ]imidazole. Laser Photon- Rev. 2013, 8. [Google Scholar] [CrossRef]
- Shin, H.; Ha, Y.H.; Kim, H.; Kim, R.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Controlling Horizontal Dipole Orientation and Emission Spectrum of Ir Complexes by Chemical Design of Ancillary Ligands for Efficient Deep-Blue Organic Light-Emitting Diodes. Adv. Mater. 2019, 31, e1808102. [Google Scholar] [CrossRef]
- Feng, W.; Su, Q.; Ma, Y.; Dzolic, Z.; Huang, F.; Wang, Z.; Chen, S.; Tang, B.Z. Tetraphenylbenzosilole: An AIE Building Block for Deep-Blue Emitters with High Performance in Nondoped Spin-Coating OLEDs. J. Org. Chem. 2019, 85, 158–167. [Google Scholar] [CrossRef]
- Rajamalli, P.; Chen, D.; Li, W.; Samuel, I.D.W.; Cordes, D.B.; Slawin, A.M.Z.; Zysman-Colman, E. Enhanced thermally activated delayed fluorescence through bridge modification in sulfone-based emitters employed in deep blue organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 6664–6671. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Ying, S.; Wang, C.; Hanif, M.; Xu, Y.; Li, Y.; Zhao, R.; Hu, D.; Ma, D.; Ma, Y. Novel 9,9-dimethylfluorene-bridged D–π–A-type fluorophores with a hybridized local and charge-transfer excited state for deep-blue electroluminescence with CIEy ∼ 0.05. J. Mater. Chem. C 2019, 7, 592–600. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Fan, S.; Wu, S.; Zhao, X.; Wang, S.; Li, X. A novel bipolar carbazole/ phenanthroimidazole derivative for high efficiency nondoped deep-blue organic light-emitting diodes. Org. Electron. 2019, 64, 259–265. [Google Scholar] [CrossRef]
- Kondo, Y.; Yoshiura, K.; Kitera, S.; Nishi, H.; Oda, S.; Gotoh, H.; Sasada, Y.; Yanai, M.; Hatakeyama, T. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photonic 2019, 13, 678–682. [Google Scholar] [CrossRef]
- Jou, J.-H.; Li, J.-L.; Sahoo, S.; Dubey, D.K.; Yadav, R.A.K.; Joseph, V.; Thomas, K.R.J.; Wang, C.-W.; Jayakumar, J.; Cheng, C.-H. Enabling a 6.5% External Quantum Efficiency Deep-Blue Organic Light-Emitting Diode with a Solution-Processable Carbazole-Based Emitter. J. Phys. Chem. C 2018, 122, 24295–24303. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Lee, D.R.; Yook, K.S.; Lee, J.Y. Recent Progress of Singlet-Exciton-Harvesting Fluorescent Organic Light-Emitting Diodes by Energy Transfer Processes. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Yuan, Y.; Wu, G.-F.; Wei, H.-X.; Tang, L.; Tong, Q.-X.; Wong, F.-L.; Lee, C. Staggered Face-to-Face Molecular Stacking as a Strategy for Designing Deep-Blue Electroluminescent Materials with High Carrier Mobility. Adv. Opt. Mater. 2014, 2, 626–631. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, C.-H.; Lee, P.-H.; Lin, H.-Y.; Leung, M.-K.; Chiu, T.-L.; Lin, C.-F. Blue organic light-emitting diodes: Current status, challenges, and future outlook. J. Mater. Chem. C 2019, 7, 5874–5888. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, B.Z.; Wang, Y.; Ma, D. Recent advances in high performance blue organic light-emitting diodes based on fluorescence emitters. J. Mater. Chem. C 2020, 8, 2614–2642. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 18020. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, L.; Tianyu, Z.; Xie, W.; Zhang, H. High-contrast and high-efficiency microcavity top-emitting white organic light-emitting devices. Org. Electron. 2010, 11, 202–206. [Google Scholar] [CrossRef]
- Schwab, T.; Schubert, S.; Müller-Meskamp, L.; Leo, K.; Gather, M.C. Eliminating Micro-Cavity Effects in White Top-Emitting OLEDs by Ultra-Thin Metallic Top Electrodes. Adv. Opt. Mater. 2013, 1, 921–925. [Google Scholar] [CrossRef]
- Li, J.-Y.; Chen, S.-P.; Siao, H.-J.; Wu, J.-H.; Chen, G.-Y.; Chen, C.-C.; Ho, S.-Y.; Lin, Y.-P.; Hsu, H.-H.; Lin, J.-S.; et al. Significant color space blue-shift of green OLED emitter with sustaining lifetime and substantial efficiency enhancement. Appl. Phys. Lett. 2017, 111, 093301. [Google Scholar] [CrossRef]
- Genco, A.; Giordano, G.; Carallo, S.; Accorsi, G.; Duan, Y.; Gambino, S.; Mazzeo, M. High quality factor microcavity OLED employing metal-free electrically active Bragg mirrors. Org. Electron. 2018, 62, 174–180. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, D.; Lee, T.-W.; Jeong, E.G.; Lee, H.S.; Choi, K.C. Ultra-High-Resolution Organic Light-Emitting Diodes with Color Conversion Electrode. ACS Photonic 2018, 5, 1891–1897. [Google Scholar] [CrossRef]
- Christogiannis, N.; Somaschi, N.; Michetti, P.; Coles, D.M.; Savvidis, P.G.; Lagoudakis, P.G.; Lidzey, D.G. Characterizing the Electroluminescence Emission from a Strongly Coupled Organic Semiconductor Microcavity LED. Adv. Opt. Mater. 2013, 1, 503–509. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Zhang, H.; Ding, H.; Guo, K.; Wei, B.; Zheng, Y.-Q.; Zhang, Z. Sunlight-like white organic light-emitting diodes with inorganic/organic nanolaminate distributed Bragg reflector (DBR) anode microcavity by using atomic layer deposition. Org. Electron. 2016, 33, 88–94. [Google Scholar] [CrossRef]
- Meister, S.; Bruckner, R.; Sūdžius, M.; Fröb, H.; Leo, K. Optically pumped lasing of an electrically active hybrid OLED-microcavity. Appl. Phys. Lett. 2018, 112, 113301. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lin, H.-W.; Wu, C.-C. Examining microcavity organic light-emitting devices having two metal mirrors. Appl. Phys. Lett. 2005, 87, 21101. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.-F.; Yang, F.-S.; Tsai, Z.-J.; Feng, G.-W.; Wen, T.-C.; Hsieh, S.-N.; Chung, C.-T.; Wu, C.-I. High-brightness top-emissive polymer light-emitting diodes utilizing organic oxide/Al∕Ag composite cathode. Appl. Phys. Lett. 2006, 89, 051103. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Sun, J.; Zhu, X.; Yu, X.; Wong, M.; Kwok, H.-S. High-efficiency microcavity top-emitting organic light-emitting diodes using silver anode. Appl. Phys. Lett. 2006, 88, 73517. [Google Scholar] [CrossRef] [Green Version]
- Poitras, D.; Kuo, C.-C.; Py, C. Design of high-contrast OLEDs with microcavity effect. Opt. Express 2008, 16, 8003–8015. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Device | EML (nm) | Lmax (cd) | CE (cd/A) | Peak Wavelength (nm) | FMWH (nm) |
|---|---|---|---|---|---|
| ITO-based 1 | 25 | 5100 | 3.64 | 487 | 86 |
| ITO-based 2 | 50 | 13,684 | 8.73 | 487 | 89 |
| ITO-based 3 | 75 | 16,835 | 12 | 481 | 87 |
| McOLED 1 | 25 | 1245.4 | 0.683 | 479 | 69.8 |
| McOLED 2 | 50 | 8413.8 | 5.41 | 487 | 75.5 |
| McOLED 3 | 75 | 8450 | 5.62 | 493 | 83.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zheng, W.; Chen, J.; Xu, Z.; Song, D.; Qiao, B.; Zhao, S. Color-Tunable Organic Light Emitting Diodes for Deep Blue Emission by Regulating the Optical Micro-Cavity. Molecules 2020, 25, 2867. https://doi.org/10.3390/molecules25122867
Jiang J, Zheng W, Chen J, Xu Z, Song D, Qiao B, Zhao S. Color-Tunable Organic Light Emitting Diodes for Deep Blue Emission by Regulating the Optical Micro-Cavity. Molecules. 2020; 25(12):2867. https://doi.org/10.3390/molecules25122867
Chicago/Turabian StyleJiang, Jixin, Weiye Zheng, Junfei Chen, Zheng Xu, Dandan Song, Bo Qiao, and Suling Zhao. 2020. "Color-Tunable Organic Light Emitting Diodes for Deep Blue Emission by Regulating the Optical Micro-Cavity" Molecules 25, no. 12: 2867. https://doi.org/10.3390/molecules25122867






