Insights on the Larvicidal Mechanism of Action of Fractions and Compounds from Aerial Parts of Helicteres velutina K. Schum against Aedes aegypti L.
Abstract
:1. Introduction
2. Results
2.1. Larvae (L4) Survival Time after Exposure to Test Substances
2.2. Measurement of Nitric Oxide
2.3. Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Larvae Survival Time Exposed to Test Substances
4.3. Measurement of Nitric Oxide
4.4. Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Souza, T.M.; Farias, D.F.; Soares, B.M.; Viana, M.P.; Lima, G.; Machado, L.; Morais, S.M.; Carvalho, A. Toxicity of Brazilian Plant Seed Extracts to Two Strains of Aedes aegypti (Diptera: Culicidae) and Nontarget Animals. J. Med. Entomol. 2011, 48, 846–851. [Google Scholar] [CrossRef]
- Reegan, D.; Gandhi, M.R.; Paulraj, M.G.; Balakrishna, K.; Ignacimuthu, S. Effect of niloticin, a protolimonoid isolated from Limonia acidissima L. (Rutaceae) on the immature stages of dengue vector Aedes aegypti L. (Diptera: Culicidae). Acta Trop. 2014, 139, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, I.; Dos Santos, E.R.; Mafud, A.C.; Annies, V.; Da Silva, M.A.N.; Malta, V.R.D.S.; Gambardella, M.T.D.P.; Marques, F.D.A.; Carlos, R.M. Synthesis and characterization of Mn(I) complexes and their larvicidal activity against Aedes aegypti, vector of dengue fever. Inorg. Chem. Commun. 2017, 84, 49–55. [Google Scholar] [CrossRef]
- Mendes, L.A.; Martins, G.F.; Valbon, W.; Souza, T.; Menini, L.; Ferreira, A.; Ferreira, M.F.D.S. Larvicidal effect of essential oils from Brazilian cultivars of guava on Aedes aegypti L. Ind. Crop. Prod. 2017, 108, 684–689. [Google Scholar] [CrossRef]
- Faraldo, A.C.; Sá-Nunes, A.; Del Bel, E.; Faccioli, L.; Lello, E. Nitric oxide production in blowfly hemolymph after yeast inoculation. Nitric Oxide 2005, 13, 240–246. [Google Scholar] [CrossRef]
- Galloway, T.S.; Depledge, M.H. Immunotoxicity in invertebrates: Measurement and ecotoxicological relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Bennett, J.W. A common fungal volatile organic compound induces a nitric oxide mediated inflammatory response in Drosophila melanogaster. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nunes, F.C.; Leite, J.A.; Oliveira, L.H.G.; Sousa, P.A.P.S.; Menezes, M.C.; Moraes, J.P.S.; Mascarenhas, S.R.; Braga, V.A. The larvicidal activity of Agave sisalana against L4 larvae of Aedes aegypti is mediated by internal necrosis and inhibition of nitric oxide production. Parasitol. Res. 2014, 114, 543–549. [Google Scholar] [CrossRef]
- Nappi, A.J.; Ottaviani, E. Cytoxicity and cytotoxic molecules in invertebrates. BioEssays 2000, 22, 469–480. [Google Scholar] [CrossRef]
- Dos Santos, E.A.; De Carvalho, C.M.; Costa, A.L.S.; Conceição, A.S.; Moura, F.D.B.P.; Santana, A.E.G. Bioactivity Evaluation of Plant Extracts Used in Indigenous Medicine against the Snail, Biomphalaria glabrata, and the Larvae of Aedes aegypti. Evid.-Based Complement. Altern. Med. 2011, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A.; Souza, M.; Teles, Y.C.F.; Oliveira, L.H.G.; Lima, J.B.; Conceição, A.S.; Nunes, F.C.; Silva, T.M.S. New Sulphated Flavonoids and Larvicidal Activity of Helicteres velutina K. Schum (Sterculiaceae). Molecules 2018, 23, 2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, D.A.; Barros, R.P.C.; Teles, Y.C.F.; Oliveira, L.H.G.; Lima, J.B.; Scotti, M.T.; Nunes, F.C.; Conceição, A.S.; Souza, M.D.F.V. Larvicidal Compounds Extracted from Helicteres velutina K. Schum (Sterculiaceae) Evaluated against Aedes aegypti L. Molecules 2019, 24, 2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, D.; De Assis, E.; Souza, M.; De Souza, P.; Helicteres, L. SPECIES (Malvaceae SENSU LATO) AS SOURCE OF NEW DRUGS: A REVIEW. Química Nova 2020, 1–17. [Google Scholar] [CrossRef]
- Silva, B.G. Quantificação de Apoptose e Necrose Mediante Corantes Fluorescentes e Análise de Imagens no Cultivo de Células de Inseto: O caso da Drosophila Melanogaster S2. Master’s Thesis, Universidade de São Carlos, São Paulo, Brazil, 2007. [Google Scholar]
- Araújo, H.R.C. Caracterização Morfológica dos Hemócitos do Aedes aegypti e do Aedes albopictus e a Resposta Imune dos Hemócitos do Aedes aegypti após a Infecção Pelo Dengue Vírus. Master’s Thesis, Universidade Federal de Belo Horizonte, Minas Gerais, Brazil, 2011. [Google Scholar]
- Chagas, J.M. Avaliação do Potencial Inseticida de Extratos Salinos de Sementes de Seis Espécies de Plantas (Família Fabaceae) contra Aedes (Stegomyia) aegypti (Diptera: Culicidae) L. em Diferentes Estágios do Ciclo Biológico. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil, 2016. [Google Scholar]
- Nunes, F.C. Estudo da Atividade Larvicida da Agave sisalana contra Larvas de Aedes aegypti. Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Paraiba, Brazil, 2013. [Google Scholar]
- Santos, D.B. Atividade larvicida da Copaifera lagsdorffii (Leguminosae), Evidenciada Pelas Alterações Morfohistológicas em Aedes aegypti (Diptera: Culicidae). Master’s Thesis, Universidade Federal de Goiás, Goiânia, Goiás, Brazil, 2015. [Google Scholar]
- Ragavendran, C.; Mariappan, T.; Natarajan, D. Larvicidal, Histopathological Efficacy of Penicillium daleae against Larvae of Culex quinquefasciatus and Aedes aegypti Plus Biotoxicity on Artemia nauplii a Non-target Aquatic Organism. Front. Pharmacol. 2017, 8, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Ragavendran, C.; Manigandan, V.; Kamaraj, C.; Balasubramani, G.; Prakash, J.S.; Perumal, P.; Natarajan, D. Larvicidal, Histopathological, Antibacterial Activity of Indigenous Fungus Penicillium sp. against Aedes aegypti L. and Culex quinquefasciatus (Say) (Diptera: Culicidae) and Its Acetylcholinesterase Inhibition and Toxicity Assessment of Zebrafish (Danio rerio). Front. Microbiol. 2019, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, L.H.G.; De Sousa, P.A.P.S.; Hilario, F.F.; Nascimento, G.J.; Morais, J.P.S.; De Medeiros, E.P.; Nunes, F.C. Agave sisalana extract induces cell death in Aedes aegypti hemocytes increasing nitric oxide production. Asian Pac. J. Trop. Biomed. 2016, 6, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.M.S. Respostas Imunológicas e Mecânicas em População Suscetível e Resistente Plutella xylostella (L.) (Lepidoptera: Plutellidae) Frente a Formulações Comerciais à Base de Bacillus thuringiensis berliner. Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Pernambuco, Brazil, 2010. [Google Scholar]
- Bergstein, T.G.; Weiss, R.R.; Bicudo, S.D. Técnicas de análise de sêmen. Rev. Bras. Reprod. Anim. 2014, 38, 189–194. [Google Scholar]
- Brandão, D.C. Avaliação Biológica In Vitro e In Vivo de Compostos Fluorescentes Derivados do Benzotiadiazol Produzidos para o Imageamento Celular. Master’s Thesis, Universidade de Brasilia, Brasilia, Brazil, 2017. [Google Scholar]
- Araújo, H.; Cavalcanti, M.; Santos, S.; Alves, L.; Brayner, F. Hemocytes ultrastructure of Aedes aegypti (Diptera: Culicidae). Micron 2008, 39, 184–189. [Google Scholar] [CrossRef]
- Green, L.C.; De Luzuriaga, K.R.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Quintero, S.; López-Angulo, G.; Montes-Avila, J.; Díaz-Camacho, S.P.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Salazar-Salas, N.Y.; Delgado-Vargas, F. Chemical composition and biological activities of Helicteres vegae and Heliopsis sinaloensis. Pharm. Biol. 2017, 55, 1473–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of all isolated compounds are available from the authors. |
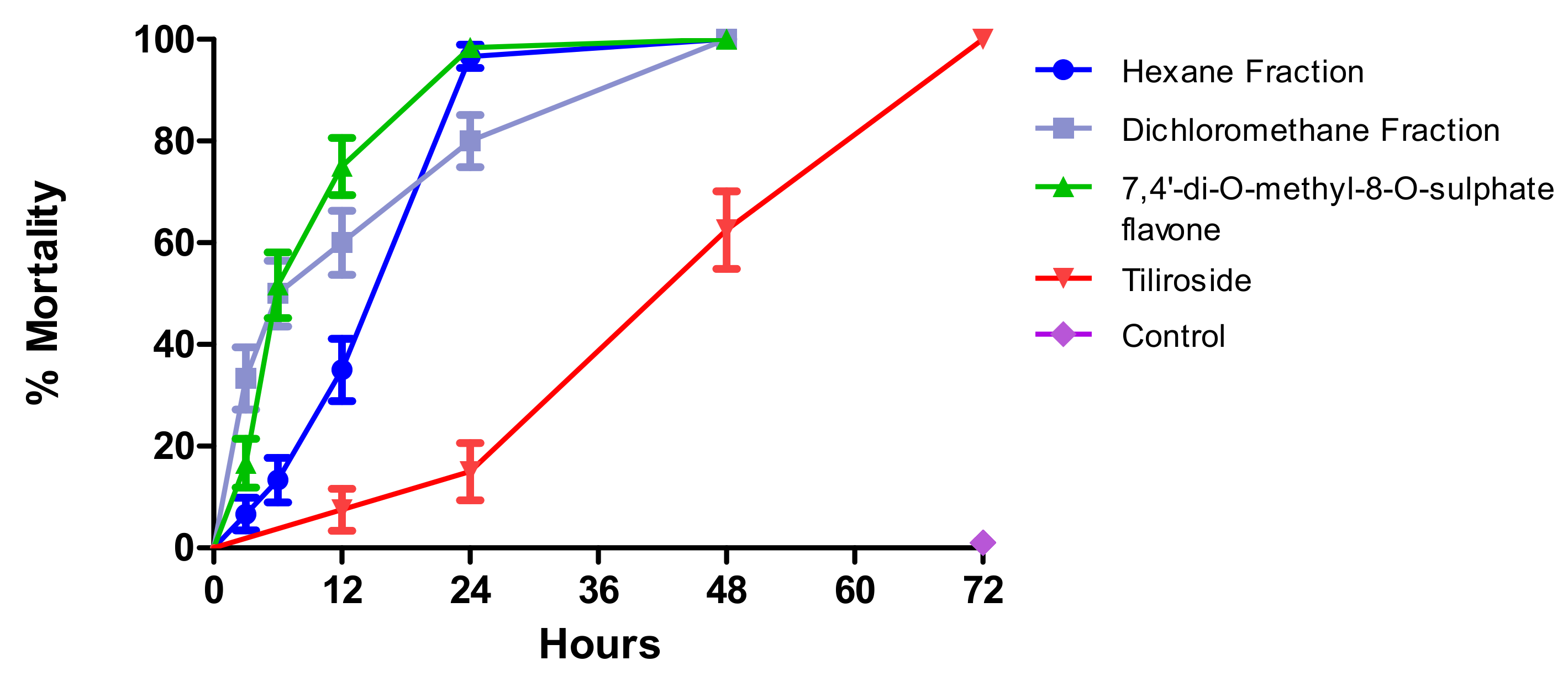

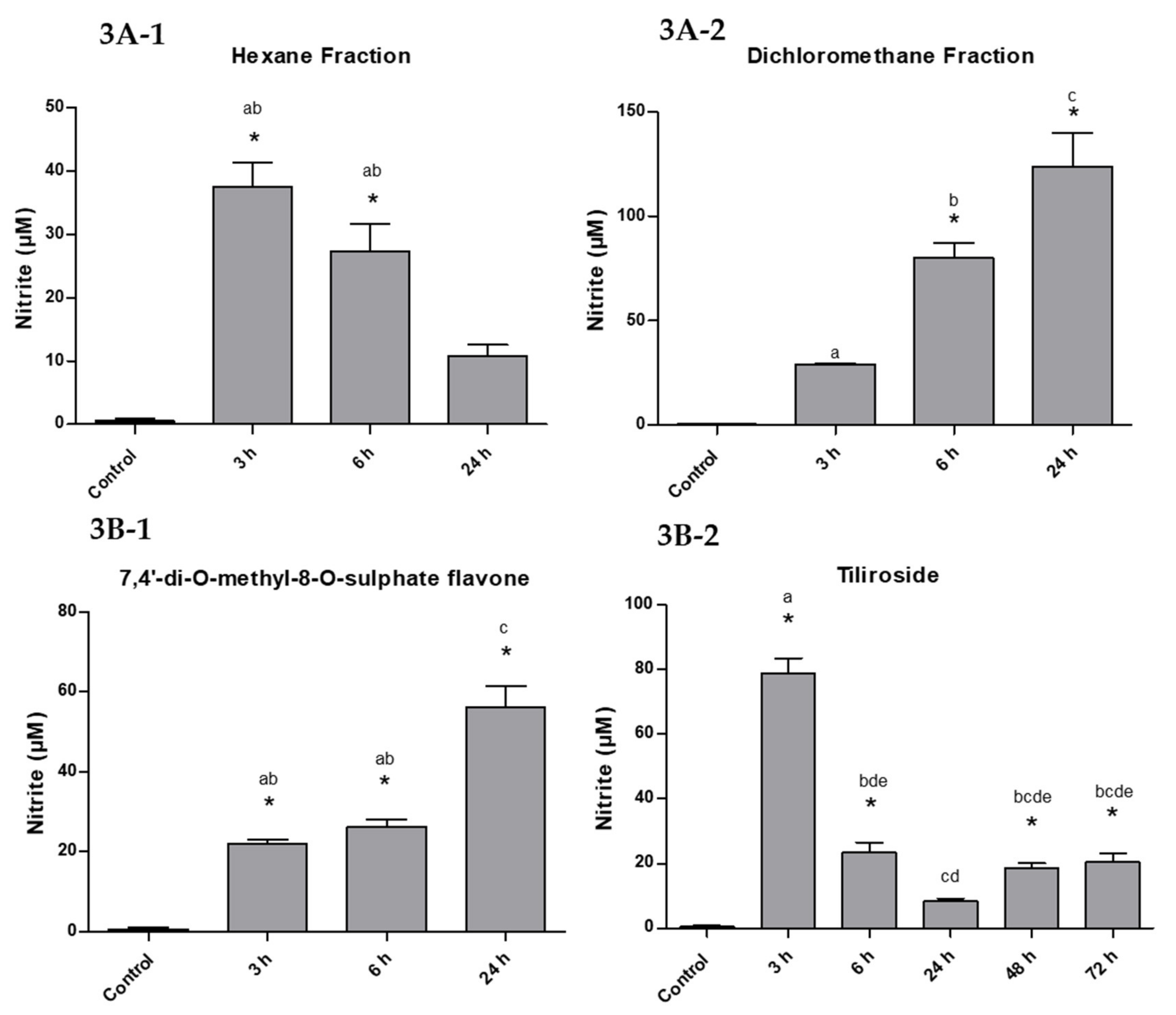
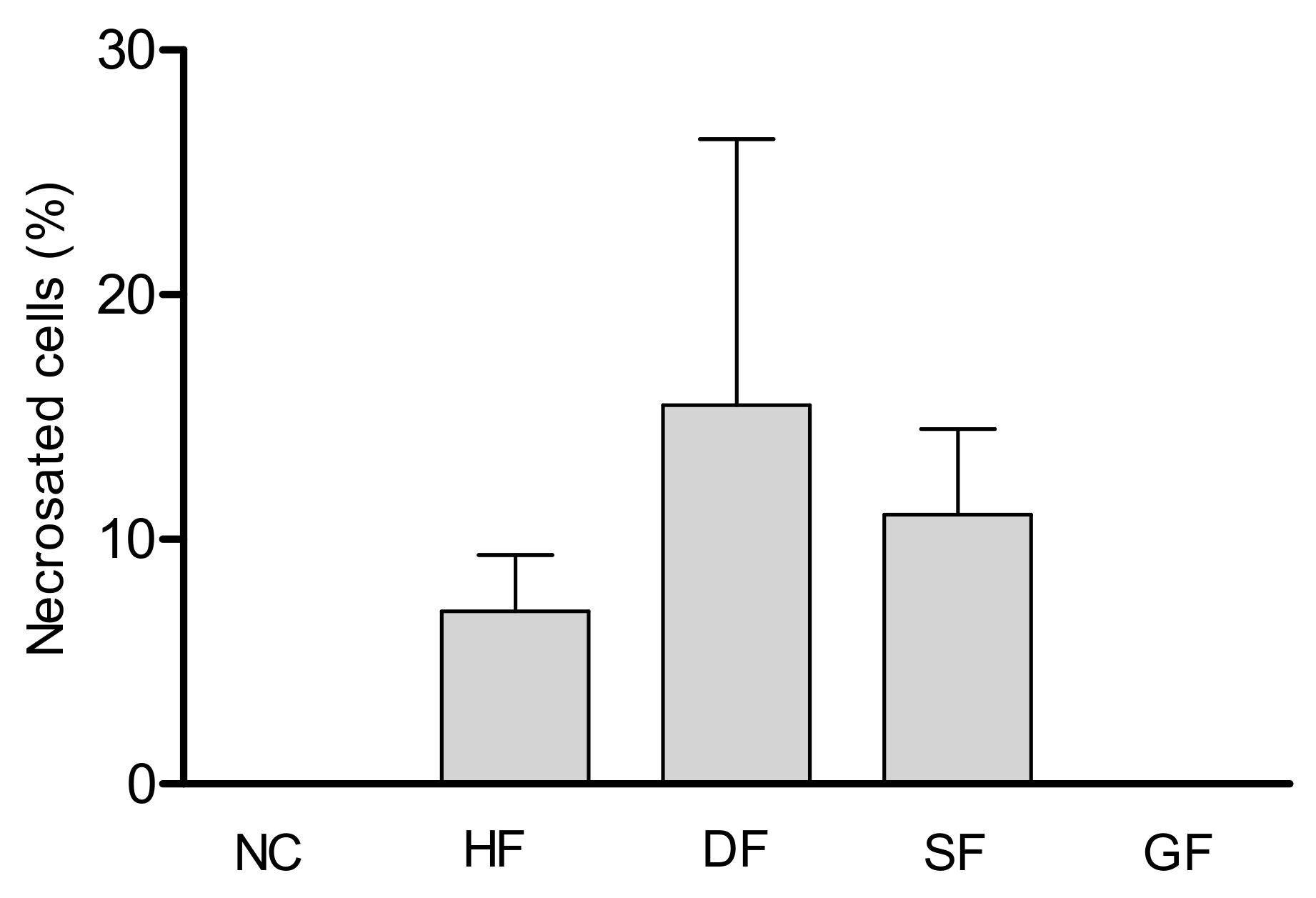


| Comparison of Survival Curves | ||||||
|---|---|---|---|---|---|---|
| HF and DH | HF and SF | HF and GF | DF and SF | DF and GF | SF and GF | |
| Chi square | 1.498 | 21.13 | 57.72 | 2.977 | 49.51 | 73.52 |
| df | 1 | 1 | 1 | 1 | 1 | 1 |
| P value | 0.2210 | <0.0001 | <0.0001 | 0.0845 | <0.0001 | <0.0001 |
| Test Substances | Total of Cells/mL | N° of Viable Cells/mL | % of Viable Cells |
|---|---|---|---|
| Control (−) | 1.2 × 106 | 1.2 × 106 | 100.0% |
| Hexane fraction | 1.32 × 106 | 1.3 × 106 | 98.1% |
| Dichloromethane fraction | 5.5 × 106 | 5.15 × 106 | 93.6% |
| Tiliroside | 9.2 × 105 | 9.2 × 105 | 100.0% |
| 7,4′-di-O-methyl-8-O-sulphate flavone | 2.5 × 106 | 2.46 × 106 | 98.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, D.A.; Oliveira, L.H.G.; Rique, H.L.; Souza, M.d.F.V.d.; Nunes, F.d.C. Insights on the Larvicidal Mechanism of Action of Fractions and Compounds from Aerial Parts of Helicteres velutina K. Schum against Aedes aegypti L. Molecules 2020, 25, 3015. https://doi.org/10.3390/molecules25133015
Fernandes DA, Oliveira LHG, Rique HL, Souza MdFVd, Nunes FdC. Insights on the Larvicidal Mechanism of Action of Fractions and Compounds from Aerial Parts of Helicteres velutina K. Schum against Aedes aegypti L. Molecules. 2020; 25(13):3015. https://doi.org/10.3390/molecules25133015
Chicago/Turabian StyleFernandes, Diégina A., Louise H. G. Oliveira, Hyago L. Rique, Maria de Fátima Vanderlei de Souza, and Fabíola da Cruz Nunes. 2020. "Insights on the Larvicidal Mechanism of Action of Fractions and Compounds from Aerial Parts of Helicteres velutina K. Schum against Aedes aegypti L." Molecules 25, no. 13: 3015. https://doi.org/10.3390/molecules25133015







