LC–MS-Based Lipidomic Analysis of Serum Samples from Spontaneously Hypertensive Rats Treated with an Extract of Acanthopanax sessiliflorus Fruits
Abstract
:1. Introduction
2. Results and Discussion
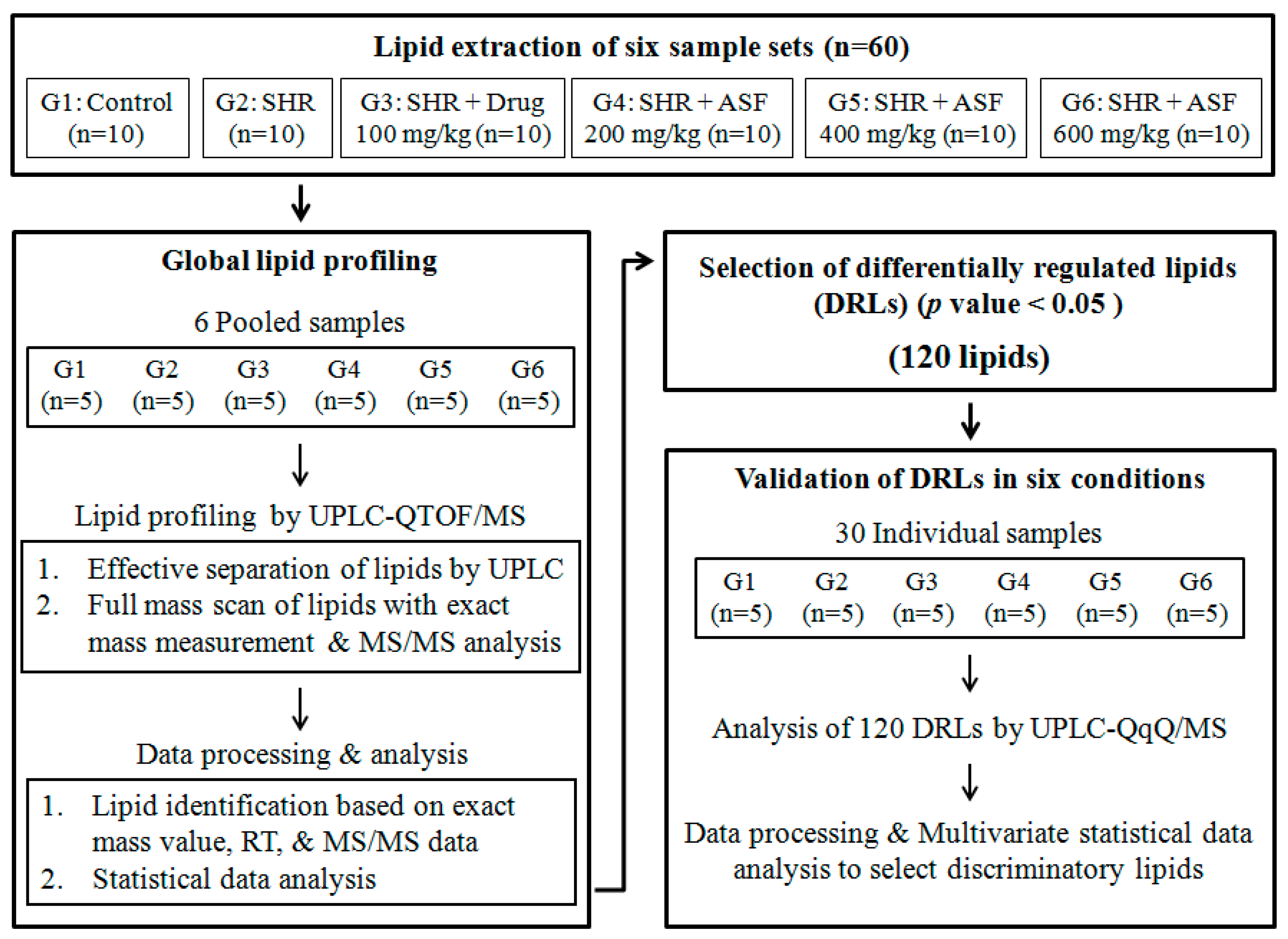
2.1. Overall Workflow of the Study
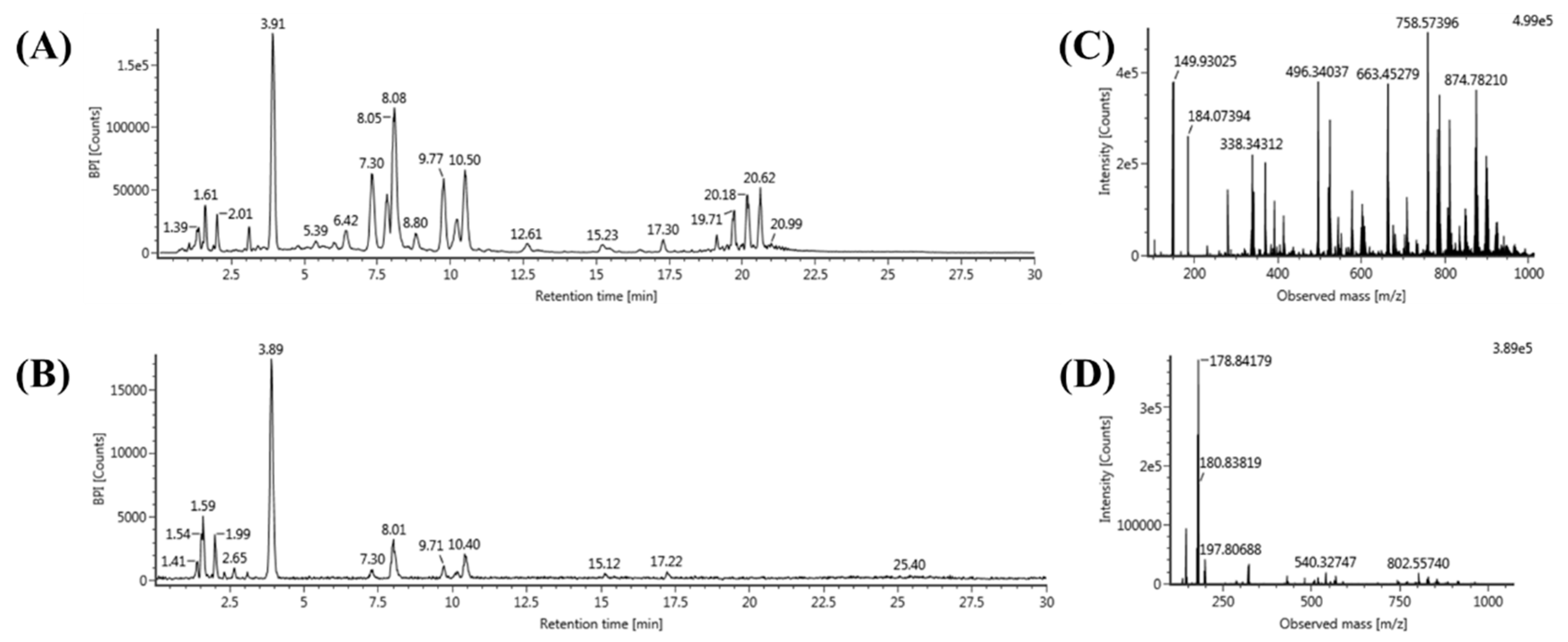
2.2. Global Lipid Profiling of Rat Serum by UPLC–QTOF/MS
2.3. Statistical Data Analysis and Selection of DRLs of the Six Groups
2.4. Validation of Selected DRLs by the Target Profiling of the Six Groups
3. Materials and Methods
3.1. Standard Constituents and Reagents
3.2. Preparation of the Extract of A. sessiliflorus Fruits (ASF)
3.3. Animal Study
3.4. Sample Preparation
3.5. Lipid Extraction
3.6. UPLC–QTOF/MS Analysis
3.7. UPLC–QqQ/MS Analysis
3.8. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance, |
| ASF | Extract of Acanthopanax sessiliflorus fruits, |
| CVD | cardiovascular disease, |
| Cer | Ceramide, |
| DG | Diacylglycerol, |
| DRL | Differentially regulated lipid, |
| LPA | Lysophosphatidic acid, |
| LPC | Lysophosphatidylcholine, |
| LPE | Lysophosphatidylethanolamine, |
| LPG | Lysophosphatidylglycerol, |
| LPI | Lysophosphatidylinositol, |
| LPS | Lysophosphatidylserine, |
| MRM | Multiple reaction monitoring, |
| MS | Mass spectrometry, |
| PA | Phosphatidic acid, |
| PC | Phosphatidylcholine, |
| PCA | Principal component analysis, |
| PE | Phosphatidylethanolamine, |
| PG | Phosphatidylglycerol, |
| PI | Phosphatidylinositol, |
| PS | Phosphatidylserine, |
| RSD | Relative standard deviation, |
| SHR | Spontaneously hypertensive rat, |
| SM | Sphingomyelin, |
| TG | Triacylglycerol, |
| UPLC | Ultra-performance liquid chromatography, |
| sPLS-DA | Sparse projection to latent structure discriminant analysis, |
| vDRLs | validated DRLs. |
References
- Joffres, M.; Falaschetti, E.; Gillespie, C.; Robitaille, C.; Loustalot, F.; Poulter, N.; McAlister, F.A.; Johansen, H.; Baclic, O.; Campbell, N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open 2013, 3, e003423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.L.; Cheung, B.M.; Man, Y.B.; Lau, C.P.; Lam, K.S. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 2007, 49, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.-C.; Wang, W.-H.; Chan, P.; Lin, L.-J.; Wang, C.-H.; Tomlinson, B.; Hsieh, M.-H.; Yang, H.-Y.; Liu, J.-C. The Beneficial Effects of Tai Chi Chuan on Blood Pressure and Lipid Profile and Anxiety Status in a Randomized Controlled Trial. J. Altern. Complement. Med. 2003, 9, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, U.; Trachsel, M.; Von Känel, R.; Abbruzzese, E.; Ehlert, U.; Wirtz, P.H. Attributional styles and stress-related atherogenic plasma lipid reactivity in essential hypertension. J. Psychosom. Res. 2014, 77, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Nabha, L.; Garbern, J.C.; Buller, C.L.; Charpie, J.R. Vascular Oxidative Stress Precedes High Blood Pressure in Spontaneously Hypertensive Rats. Clin. Exp. Hypertens. 2005, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Schiffrin, E.L. Vascular inflammation in hypertension and diabetes: Molecular mechanisms and therapeutic interventions. Clin. Sci. 2007, 112, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA 1996, 275, 1571–1576. [Google Scholar] [CrossRef]
- Lima, S.T.; Souza, B.S.; França, A.K.; Salgado, J.V.; Salgado-Filho, N.; Sichieri, R. Reductions in glycemic and lipid profiles in hypertensive patients undergoing the Brazilian Dietary Approach to Break Hypertension: A randomized clinical trial. Nutr. Res. 2014, 34, 682–687. [Google Scholar] [CrossRef] [Green Version]
- Fenger, M.; Linneberg, A.; Torben, J.; Madsbad, S.; Søbye, K.; Eugen-Olsen, J.; Jeppesen, J. Genetics of the ceramide/sphingosine-1-phosphate rheostat in blood pressure regulation and hypertension. BMC Genet. 2011, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Elsen, L.V.D.; Spijkers, L.J.; Akker, R.F.V.D.; Van Winssen, A.M.; Balvers, M.; Wijesinghe, D.S.; Chalfant, C.E.; Garssen, J.; Willemsen, L.; Alewijnse, A.E.; et al. Dietary fish oil improves endothelial function and lowers blood pressure via suppression of sphingolipid-mediated contractions in spontaneously hypertensive rats. J. Hypertens. 2014, 32, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Nie, L.; Li, Y.; Xie, J. Application of ultra-performance liquid chromatography coupled with mass spectrometry to metabonomic study on spontaneously hypertensive rats and intervention effects of Ping Gan prescription. J. Sep. Sci. 2012, 35, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, J.; Xie, H.; Sun, F.; Yang, K.; Wang, J.; Ke, C.; Lou, G.; Li, K. Differential plasma lipids profiling and lipid signatures as biomarkers in the early diagnosis of ovarian carcinoma using UPLC-MS. Metabolomics 2015, 12, 18. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, Y.-Y.; Goruk, S.; Oilund, K.; Field, C.J.; Jacobs, R.; Curtis, J.M. Validation of an LC–MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J. Chromatogr. B 2012, 911, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Armando, A.M.; Quehenberger, O.; Yan, C.; Dennis, E.A. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J. Chromatogr. A 2014, 1359, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, Q.; Meng, D.; Isaac, G.; Zhao, R.; Fillmore, T.L.; Chu, R.K.; Zhou, J.; Tang, K.; Hu, Z.; et al. A reversed-phase capillary ultra-performance liquid chromatography–mass spectrometry (UPLC-MS) method for comprehensive top-down/bottom-up lipid profiling. Anal. Bioanal. Chem. 2012, 402, 2923–2933. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, J.Y.; Kim, O.Y.; Ham, B.M.; Kim, H.-J.; Kwon, D.Y.; Jang, Y.; Lee, J.H. Metabolic Profiling of Plasma in Overweight/Obese and Lean Men using Ultra Performance Liquid Chromatography and Q-TOF Mass Spectrometry (UPLC−Q-TOF MS). J. Proteome Res. 2010, 9, 4368–4375. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.-T.; Park, J.H.; Yang, H.J.; Kim, M.-S.; Kwon, D.Y.; et al. Metabolomic Analysis of Livers and Serum from High-Fat Diet Induced Obese Mice. J. Proteome Res. 2011, 10, 722–731. [Google Scholar] [CrossRef]
- Kortz, L.; Dorow, J.; Ceglarek, U. Liquid chromatography–tandem mass spectrometry for the analysis of eicosanoids and related lipids in human biological matrices: A review. J. Chromatogr. B 2014, 964, 1–11. [Google Scholar] [CrossRef]
- Lee, J.W.; Nishiumi, S.; Yoshida, M.; Fukusaki, E.; Bamba, T. Simultaneous profiling of polar lipids by supercritical fluid chromatography/tandem mass spectrometry with methylation. J. Chromatogr. A 2013, 1279, 98–107. [Google Scholar] [CrossRef]
- Syed, A.A.; Lahiri, S.; Mohan, D.; Valicherla, G.R.; Gupta, A.P.; Riyazuddin, M.; Kumar, S.; Maurya, R.; Hanif, K.; Gayen, J.R. Evaluation of anti-hypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt- and L-NAME-induced hypertensive rats. J. Ethnopharmacol. 2016, 193, 555–565. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Seo, K.-H.; Jeong, R.-H.; Lee, S.-M.; Kim, G.-S.; Noh, H.-J.; Kim, S.-Y.; Kim, G.-W.; Kim, J.-Y.; Baek, N.-I. Anti-inflammatory Lignans from the Fruits of Acanthopanax sessiliflorus. Molecules 2012, 18, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.; Shin, M. Hyang Yak Dae Sa Jeon, 3rd ed.; Young Lim Sa Publisher: Seoul, Korea, 2003; p. 432. [Google Scholar]
- Song, L.; Wu, Y.; Hu, L.; Zhang, G.; Xu, G.; Xiao, P.; Ling, Y.; Ding, X.; Cao, C.; Li, Y. Zhong Hua Ben Cao, 3rd ed.; Shanghai Scientific Technologic Publisher: Shanghai, China, 1999; p. 5. [Google Scholar]
- Yang, S.; Chun-Juan, Y.; Kai, Y.; Fa-Mei, L. In vivo antithrombotic and antiplatelet activities of a quantified Acanthopanax sessiliflorus fruit extract. Chin. J. Nat. Med. 2011, 9, 141–145. [Google Scholar]
- Tousoulis, D.; Paroutoglou, I.P.; Papageorgiou, N.; Charakida, M.; Stefanadis, C. Recent therapeutic approaches to platelet activation in coronary artery disease. Pharmacol. Ther. 2010, 127, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.H.; Kim, S.E.; Lee, Y.-G.; Kim, D.H.; Kim, H.; Kim, G.-S.; Baek, N.-I.; Lee, D.Y. Antihypertensive Effect of Ethanolic Extract from Acanthopanax sessiliflorus Fruits and Quality Control of Active Compounds. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surinova, S.; Schiess, R.; Huettenhain, R.; Cerciello, F.; Wollscheid, B.; Aebersold, R. On the Development of Plasma Protein Biomarkers. J. Proteome Res. 2011, 10, 5–16. [Google Scholar] [CrossRef] [PubMed]



| No. | Compounds | p Value | No. | Compounds | p Value |
|---|---|---|---|---|---|
| 1 | TG(14:0-14:0-14:0) | <0.001 | 35 | TG(24:0-20:3-16:1) | 0.005 |
| 2 | TG(16:0-16:1-14:0) | <0.001 | 36 | TG(22:6-20:4-18:2) | <0.001 |
| 3 | TG(16:1-16:1-14:0) | 0.006 | 37 | TG(22:2-20:7-18:3) | <0.001 |
| 4 | TG(18:0-16:1-14:0) | <0.001 | 38 | TG(22:4-20:7-18:2) | <0.001 |
| 5 | TG(18:1-16:1-14:0) | <0.001 | 39 | DG(32:0) | 0.019 |
| 6 | TG(18:2-16:1-14:0) | 0.004 | 40 | DG(34:0) | 0.039 |
| 7 | TG(18:1-16:1-16:0) | 0.033 | 41 | DG(36:3) | 0.012 |
| 8 | TG(18:2-16:1-16:0) | 0.006 | 42 | DG(38:4) | 0.003 |
| 9 | TG(18:3-16:1-16:0) | 0.024 | 43 | LPC(14:0) | 0.021 |
| 10 | TG(20:0-18:1-16:0) | 0.011 | 44 | LPC(20:0) | <0.001 |
| 11 | TG(20:1-18:1-16:0) | 0.031 | 45 | LPC(20:1) | 0.001 |
| 12 | TG(20:2-18:1-16:0) | 0.009 | 46 | LPC(20:2) | <0.001 |
| 13 | TG(22:5-16:0-16:0) | 0.001 | 47 | LPC(20:3) | 0.007 |
| 14 | TG(20:3-18:2-16:0) | <0.001 | 48 | LPC(22:0) | <0.001 |
| 15 | TG(20:5-18:0-16:1) | 0.001 | 49 | PC(26:0) | <0.001 |
| 16 | TG(20:4-18:2-16:0) | 0.001 | 50 | PC(30:0) | <0.001 |
| 17 | TG(20:6-18:1-16:0) | 0.029 | 51 | PC(32:0) | <0.001 |
| 18 | TG(20:5-18:2-16:1) | 0.047 | 52 | PC(32:2) | <0.001 |
| 19 | TG(20:0-20:0-16:0) | 0.006 | 53 | PC(34:0) | 0.003 |
| 20 | TG(22:0-18:1-16:0) | 0.003 | 54 | PC(34:1) | <0.001 |
| 21 | TG(20:2-18:1-18:0) | <0.001 | 55 | PC(34:2) | <0.001 |
| 22 | TG(20:2-18:1-18:1) | 0.001 | 56 | PC(34:3) | <0.001 |
| 23 | TG(22:3-20:1-14:1) | 0.001 | 57 | PC(34:4) | <0.001 |
| 24 | TG(20:5-20:1-16:0) | <0.001 | 58 | PC(36:0) | <0.001 |
| 25 | TG(20:3-18:1-18:3) | <0.001 | 59 | PC(36:1) | <0.001 |
| 26 | TG(20:4-18:2-18:2) | <0.001 | 60 | PC(36:2) | <0.001 |
| 27 | TG(20:6-20:1-16:2) | <0.001 | 61 | PC(36:3) | <0.001 |
| 28 | TG(20:0-20:2-18:0) | 0.001 | 62 | PC(36:5) | <0.001 |
| 29 | TG(20:1-20:2-18:0) | 0.009 | 63 | PC(38:2) | <0.001 |
| 30 | TG(20:1-20:6-18:0) | <0.001 | 64 | PC(38:6) | <0.001 |
| 31 | TG(20:3-20:4-18:1) | <0.001 | 65 | PC(40:5) | <0.001 |
| 32 | TG(20:4-20:5-18:1) | <0.001 | 66 | PE(16:0) | <0.001 |
| 33 | TG(20:3-20:5-18:3) | <0.001 | 67 | PE(40:6) | <0.001 |
| 34 | TG(22:0-20:1-18:2) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.Y.; Choi, B.-R.; Yoon, D.; Kim, H.-G.; Lee, M.-H.; Kim, G.-S.; Lee, Y.-S. LC–MS-Based Lipidomic Analysis of Serum Samples from Spontaneously Hypertensive Rats Treated with an Extract of Acanthopanax sessiliflorus Fruits. Molecules 2020, 25, 3269. https://doi.org/10.3390/molecules25143269
Lee DY, Choi B-R, Yoon D, Kim H-G, Lee M-H, Kim G-S, Lee Y-S. LC–MS-Based Lipidomic Analysis of Serum Samples from Spontaneously Hypertensive Rats Treated with an Extract of Acanthopanax sessiliflorus Fruits. Molecules. 2020; 25(14):3269. https://doi.org/10.3390/molecules25143269
Chicago/Turabian StyleLee, Dae Young, Bo-Ram Choi, Dahye Yoon, Hyoung-Geun Kim, Min-Ho Lee, Geum-Soog Kim, and Young-Seob Lee. 2020. "LC–MS-Based Lipidomic Analysis of Serum Samples from Spontaneously Hypertensive Rats Treated with an Extract of Acanthopanax sessiliflorus Fruits" Molecules 25, no. 14: 3269. https://doi.org/10.3390/molecules25143269






