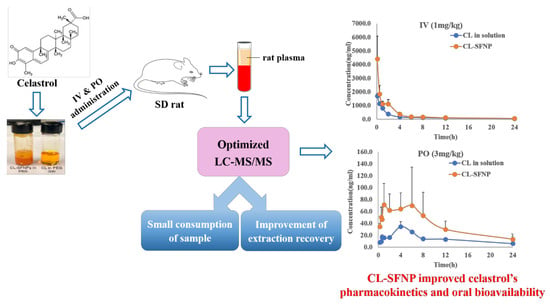
Oral Bioavailability Evaluation of Celastrol-Encapsulated Silk Fibroin Nanoparticles Using an Optimized LC-MS/MS Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
2.2. Chromatographic and Mass Spectrometry Conditions
2.3. Preparation of Calibration Standards and Quality Control Samples
2.4. Sample Preparation
2.5. Method Validation
2.5.1. Specificity
2.5.2. Linearity and Low Limit of Quantification
2.5.3. Precision and Accuracy
2.5.4. Extraction Recovery and Matrix Effect
2.5.5. Stability
2.6. Pharmacokinetic Studies
2.7. Data Analysis
3. Results and Discussion
3.1. LC-MS/MS Method
3.2. Optimization of Sample Preparation
3.3. Method Validation
3.3.1. Specificity
3.3.2. Linearity and LLOQ
3.3.3. Precision and Accuracy
3.3.4. Extraction Recovery and Matrix Effect
3.3.5. Stability
3.4. Pharmacokinetic and Bioavailability Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Mukherjee, T.; Bishayee, A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018, 128, 70–81. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol suppresses experimental autoimmune encephalomyelitis via MAPK/SGK1-regulated mediators of autoimmune pathology. Inflamm. Res. 2019, 68, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cascao, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, K.; Davis, K.C.; Morgan, D.A.; Toth, B.A.; Jiang, J.; Singh, U.; Berglund, E.D.; Grobe, J.L.; Rahmouni, K.; Cui, H. Celastrol Reduces Obesity in MC4R Deficiency and Stimulates Sympathetic Nerve Activity Affecting Metabolic and Cardiovascular Functions. Diabetes 2019, 68, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, A.; Kaur, J.; Tomljanovic, I.; Nistri, A.; Mladinic, M. Pharmacological induction of Heat Shock Protein 70 by celastrol protects motoneurons from excitotoxicity in rat spinal cord in vitro. Eur. J. Neurosci. 2019, 49, 215–231. [Google Scholar] [CrossRef]
- Sanna, V.; Chamcheu, J.C.; Pala, N.; Mukhtar, H.; Sechi, M.; Siddiqui, I.A. Nanoencapsulation of natural triterpenoid celastrol for prostate cancer treatment. Int. J. Nanomed. 2015, 10, 6835–6846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, X.; Jiang, H.; Lu, X.; Zhu, Y.; Chen, B. New strategy of photodynamic treatment of TiO2 nanofibers combined with celastrol for HepG2 proliferation in vitro. Nanoscale 2011, 3, 3115–3122. [Google Scholar] [CrossRef]
- Soe, Z.C.; Thapa, R.K.; Ou, W.; Gautam, M.; Nguyen, H.T.; Jin, S.G.; Ku, S.K.; Oh, K.T.; Choi, H.G.; Yong, C.S.; et al. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surf. B Biointerfaces 2018, 170, 718–728. [Google Scholar] [CrossRef]
- Qu, D.; Wang, L.; Qin, Y.; Guo, M.; Guo, J.; Huang, M.; Liu, Y.; Liu, C.; Li, H.; Chen, Y. Non-triggered sequential-release liposomes enhance anti-breast cancer efficacy of STS and celastrol-based microemulsion. Biomater. Sci. 2018, 6, 3284–3299. [Google Scholar] [CrossRef]
- Li, P.; Zhou, X.; Qu, D.; Guo, M.; Fan, C.; Zhou, T.; Ling, Y. Preliminary study on fabrication, characterization and synergistic anti-lung cancer effects of self-assembled micelles of covalently conjugated celastrol-polyethylene glycol-ginsenoside Rh2. Drug Deliv. 2017, 24, 834–845. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tan, Y.; Meng, T.; Liu, X.; Zhu, Y.; Hong, Y.; Yang, X.; Yuan, H.; Huang, X.; Hu, F. Simultaneous targeting therapy for lung metastasis and breast tumor by blocking the NF-kappaB signaling pathway using Celastrol-loaded micelles. Drug Deliv. 2018, 25, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yao, L.; Li, J.; Zhang, W.; Wu, X.; Liu, Y.; Lin, M.; Su, W.; Li, Y.; Liang, D. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int. J. Nanomed. 2012, 7, 1163–1173. [Google Scholar]
- Niemela, E.; Desai, D.; Nkizinkiko, Y.; Eriksson, J.E.; Rosenholm, J.M. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur. J. Pharm. Biopharm. 2015, 96, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.R.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Luo, S.; Du, Z.; Zhou, M.; Li, P.; Fu, Y.; Sun, X.; Huang, Y.; Zhang, Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Shan, W.G.; Wang, H.G.; Wu, R.; Zhan, Z.J.; Ma, L.F. Synthesis and anti-tumor activity study of water-soluble PEG-celastrol coupling derivatives as self-assembled nanoparticles. Bioorg. Med. Chem. Lett. 2019, 29, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wahid, M.A.; Wang, Z.; Xie, C.; Thakkar, A.; Prabhu, S.; Wang, J. Triptolide and celastrol loaded silk fibroin nanoparticles show synergistic effect against human pancreatic cancer cells. Nanoscale 2017, 9, 11739–11753. [Google Scholar] [CrossRef] [PubMed]
- Onyeabor, F.; Paik, A.; Kovvasu, S.; Ding, B.; Lin, J.; Wahid, M.A.; Prabhu, S.; Betageri, G.; Wang, J. Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat. Molecules 2019, 24, 3271. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Wang, J.; Song, H.; Cui, D.; Li, L.; Li, J.; Lin, L.; Zhou, J.; Liu, Y. Optimized preparation of celastrol-loaded polymeric nanomicelles using rotatable central composite design and response surface methodology. J. Biomed. Nanotechnol. 2012, 8, 491–499. [Google Scholar] [CrossRef]
- Qi, X.; Qin, J.; Ma, N.; Chou, X.; Wu, Z. Solid self-microemulsifying dispersible tablets of celastrol: Formulation development, charaterization and bioavailability evaluation. Int. J. Pharm. 2014, 472, 40–47. [Google Scholar] [CrossRef]
- Freag, M.S.; Saleh, W.M.; Abdallah, O.Y. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int. J. Pharm. 2018, 535, 18–26. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.Y.; Xu, M.J.; Wu, T.; Chu, J.H.; Liu, S.J.; Ju, W.Z. Oral bioavailability and gender-related pharmacokinetics of celastrol following administration of pure celastrol and its related tablets in rats. J. Ethnopharmacol. 2012, 144, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gangu Naidu, C.; Nageswara Rao, R.; Prasada Rao, A.V.; Nagesh Kumar, K.; Padiya, R.; Madhusudhan Rao, V. Supported liquid extraction and LC-MS-MS determination of iloperidone and olanzapine in rat plasma: Application to a pharmacokinetic study. J. Chromatogr. Sci. 2018, 56, 879–887. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, T.; Li, P.; Liu, R.; Li, Q.; Bi, K. Development of two step liquid-liquid extraction tandem UHPLC-MS/MS method for the simultaneous determination of Ginkgo flavonoids, terpene lactones and nimodipine in rat plasma: Application to the pharmacokinetic study of the combination of Ginkgo biloba dispersible tablets and Nimodipine tablets. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1028, 33–41. [Google Scholar] [PubMed]
- Chandu, B.R.; Kanala, K.; Hwisa, N.T.; Katakam, P.; Khagga, M. Bioequivalance and pharmacokinetic study of febuxostat in human plasma by using LC-MS/MS with liquid liquid extraction method. Springerplus 2013, 2, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, G.; Mohammadi, B. An isocratic high performance liquid chromatographic method for quantification of mycophenolic acid and its glucuronide metabolite in human serum using liquid-liquid extraction: Application to human pharmacokinetic studies. Clin. Chim. Acta 2006, 370, 185–190. [Google Scholar] [CrossRef]
- Wang, W.; Liu, K.; Dong, H.; Liu, W. High-performance liquid chromatography spectrometric analysis of tripterin in rat plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 863, 163–166. [Google Scholar] [CrossRef]
- Ouyang, X.K.; Cai, M.Q.; Chen, X.H.; Jin, M.C. Development and validation of a liquid chromatography coupled with atmospheric-pressure chemical ionization ion trap mass spectrometric method for the simultaneous determination of triptolide, tripdiolide, and tripterine in human serum. J. Anal. Toxicol. 2008, 32, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, D.; Wang, Z. Effects of diclofenac on the pharmacokinetics of celastrol in rats and its transport. Pharm. Biol. 2018, 56, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Li, Y.; Xie, M.B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [Green Version]
- Fuster, M.G.; Carissimi, G.; Montalban, M.G.; Villora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perteghella, S.; Sottani, C.; Cocce, V.; Negri, S.; Cavicchini, L.; Alessandri, G.; Cottica, D.; Torre, M.L.; Grignani, E.; Pessina, A. Paclitaxel-Loaded Silk Fibroin Nanoparticles: Method Validation by UHPLC-MS/MS to Assess an Exogenous Approach to Load Cytotoxic Drugs. Pharmaceutics 2019, 11, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalban, M.G.; Coburn, J.M.; Lozano-Perez, A.A.; Cenis, J.L.; Villora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compound celastrol pure drug are available from the authors. |




| Concentration (ng/mL) | One-Step Extraction with 5 min | One-Step Extraction with 10 min | Two-Step Extraction with Each 5 min |
|---|---|---|---|
| 1 | 0.0035 ± 0.0003 | 0.0036 ± 0.0002 | 0.0037 ± 0.0003 |
| 25 | 0.0881 ± 0.0037 | 0.0858 ± 0.0041 | 0.0899 ± 0.0039 |
| 200 | 1.0483 ± 0.1025 | 0.9752 ± 0.0604 | 1.0361 ± 0.0541 |
| Concentration Spiked (ng/mL) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|
| Concentration Measured (ng/mL) | Accuracy (%) | Precision (%) | Concentration Measured (ng/mL) | Accuracy (%) | Precision (%) | |
| 1 | 0.91 ± 0.08 | 91.1 | 9.1 | 1.03 ± 0.07 | 102.6 | 7.2 |
| 25 | 26.77 ± 2.40 | 107.1 | 9.0 | 24.38 ± 2.85 | 97.5 | 11.7 |
| 200 | 220.03 ± 13.15 | 110.0 | 6.0 | 196.76 ± 19.74 | 98.4 | 10.0 |
| Concentration (ng/mL) | Extraction Recovery (%) | Matrix Effect (%) |
|---|---|---|
| 1 | 67.0 ± 8.9 | 101.2 ± 8.8 |
| 25 | 63.5 ± 2.5 | 87.3 ± 5.9 |
| 200 | 74.7 ± 2.3 | 98.0 ± 11.0 |
| Concentration Spiked (ng/mL) | In Autosampler after Preparation for 18 h | After Three Freeze-Thaw Cycles | At −20 °C for 30 Days | ||||
|---|---|---|---|---|---|---|---|
| Concentration Measured (ng/mL) | Deviation (%) | Concentration Measured (ng/mL) | Deviation (%) | Concentration Measured (ng/mL) | Deviation (%) | ||
| 1 | 0.88 ± 0.06 | −12.4 | 0.95 ± 0.09 | −5.1 | 0.97 ± 0.08 | −3.3 | |
| 25 | 25.44 ± 3.14 | 1.8 | 24.28 ± 1.82 | −2.9 | 23.32 ± 2.02 | −6.7 | |
| 200 | 181.99 ± 8.21 | 9.0 | 180.01 ± 10.37 | −10.0 | 221.64 ± 17.32 | 10.8 | |
| Parameters | CL in PEG 300 | CL-SFNP |
|---|---|---|
| ke1 (h−1) | 0.0684 ± 0.0092 | 0.0640 ± 0.0151 |
| T1/2β (h) | 10.27 ± 1.47 | 11.27 ± 2.83 |
| Cmax (ng/mL) | 1701.3 ± 170.7 | 4414.8 ± 1666.1 * |
| AUC0-t (h*ng/mL) | 4124.3 ± 663.8 | 7600.4 ± 1658.8 * |
| AUC0-∞ (h*ng/mL) | 4697.7 ± 723.0 | 8646.1 ± 1998.9 * |
| Vd (mL) | 990.3 ± 272.1 | 544.5 ± 88.7 |
| Cl (mL/h) | 66.3 ± 12.5 | 34.6 ± 9.3 * |
| MRT0-∞ (h) | 9.47 ± 0.77 | 8.83 ± 2.51 |
| Parameters | CL in PEG 300 | CL-SFNP |
|---|---|---|
| T1/2 (h) | 12.02 ± 8.32 | 8.97 ± 2.57 |
| Tmax (h) | 4.67 ± 1.15 | 3.00 ± 2.65 |
| Cmax (ng/mL) | 35.1 ± 7.9 | 90.5 ± 49.2 |
| AUC0-t (h*ng/mL) | 308.9 ± 45.1 | 842.9 ± 567.9 |
| AUC0-∞ (h*ng/mL) | 441.9 ± 82.6 | 1065.5 ± 494.6 * |
| Vd (mL) | 367.1 ± 279.0 | 261.0 ± 73.2 |
| Cl (mL/h) | 20.5 ± 1.4 | 20.2 ± 0.2 |
| MRT0-∞ (h) | 17.01 ± 8.95 | 13.31 ± 2.67 |
| F (%) | 3.14 ± 0.59 | 7.56 ± 3.51 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, S.; Paik, A.; Onyeabor, F.; Ding, B.; Prabhu, S.; Wang, J. Oral Bioavailability Evaluation of Celastrol-Encapsulated Silk Fibroin Nanoparticles Using an Optimized LC-MS/MS Method. Molecules 2020, 25, 3422. https://doi.org/10.3390/molecules25153422
Zhan S, Paik A, Onyeabor F, Ding B, Prabhu S, Wang J. Oral Bioavailability Evaluation of Celastrol-Encapsulated Silk Fibroin Nanoparticles Using an Optimized LC-MS/MS Method. Molecules. 2020; 25(15):3422. https://doi.org/10.3390/molecules25153422
Chicago/Turabian StyleZhan, Shuyu, Amy Paik, Felicia Onyeabor, Baoyue Ding, Sunil Prabhu, and Jeffrey Wang. 2020. "Oral Bioavailability Evaluation of Celastrol-Encapsulated Silk Fibroin Nanoparticles Using an Optimized LC-MS/MS Method" Molecules 25, no. 15: 3422. https://doi.org/10.3390/molecules25153422