Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization
Abstract
:1. Introduction
2. Models of CoNV
3. Synthetic Small Molecules
3.1. Tyrosine Kinase Inhibitors
3.2. Repurposed Antimicrobials
3.3. Other Synthetics
4. Natural Products
4.1. Polyphenols: Flavonoids
4.2. Non-Flavonoid Phytochemicals
4.3. Immunosuppressants
Macrolides
4.4. Vitamins and Photoactivatable Compounds
4.5. HDAC Inhibitors
5. Discussion/Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar]
- Chang, J.H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Blindness and Vision Impairment Prevention. Available online: https://www.who.int/blindness/causes/priority/en/index8.html (accessed on 29 May 2020).
- Pineda, R. World corneal blindness. In Foundations of Corneal Disease; Springer: New York, NY, USA, 2020; pp. 299–305. [Google Scholar]
- Gupta, D.; Illingworth, C. Treatments for corneal neovascularization: A review. Cornea 2011, 30, 927–938. [Google Scholar] [CrossRef]
- Cursiefen, C.; Wenkel, H.; Martus, P.; Langenbucher, A.; Nguyen, N.X.; Seitz, B.; Kuchle, M.; Naumann, G.O. Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 514–521. [Google Scholar] [CrossRef]
- Al-Torbak, A.; Al-Amri, A.; Wagoner, M.D. Deep corneal neovascularization after implantation with intrastromal corneal ring segments. Am. J. Ophthalmol. 2005, 140, 926–927. [Google Scholar] [CrossRef]
- Krebs, I.; Lie, S.; Stolba, U.; Zeiler, F.; Felke, S.; Binder, S. Efficacy of intravitreal bevacizumab (Avastin) therapy for early and advanced neovascular age-related macular degeneration. Acta Ophthalmol. 2009, 87, 611–617. [Google Scholar] [CrossRef]
- Kim, S.W.; Ha, B.J.; Kim, E.K.; Tchah, H.; Kim, T.I. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology 2008, 115, e33–e38. [Google Scholar] [CrossRef]
- Ozdemir, O.; Altintas, O.; Altintas, L.; Ozkan, B.; Akdag, C.; Yuksel, N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq. Bras. Oftalmol. 2014, 77, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-H.; Garg, N.K.; Lunde, E.; Han, K.-Y.; Jain, S.; Azar, D.T. Corneal neovascularization: An anti-VEGF therapy review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329. [Google Scholar] [CrossRef] [Green Version]
- Madri, J.A.; Pratt, B.M.; Tucker, A.M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 1988, 106, 1375–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, M.S.; Birsner, A.E.; D’Amato, R.J. The mouse cornea micropocket angiogenesis assay. Nat. Protoc. 2007, 2, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Montezuma, S.R.; Vavvas, D.; Miller, J.W. Review of the ocular angiogenesis animal models. Semin. Ophthalmol. 2009, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Beecken, W.D.; Moromizato, Y.; Schwartz, A.; Kirchhof, B.; Poulaki, V. Inhibition of inflammatory corneal angiogenesis by TNP-470. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2510–2516. [Google Scholar]
- Mahoney, J.M.; Waterbury, L.D. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr. Eye Res. 1985, 4, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Tadvalkar, G.; Jurjus, R.A.; Zieske, J.D.; Stepp, M.A. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 2008, 87, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Gotink, K.J.; Verheul, H.M. Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action? Angiogenesis 2010, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Detry, B.; Blacher, S.; Erpicum, C.; Paupert, J.; Maertens, L.; Maillard, C.; Munaut, C.; Sounni, N.E.; Lambert, V.; Foidart, J.M.; et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3082–3093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Santonja, J.J.; Campos-Mollo, E.; Lledo-Riquelme, M.; Javaloy, J.; Alio, J.L. Inhibition of corneal neovascularization by topical bevacizumab (anti-VEGF) and sunitinib (anti-VEGF and anti-PDGF) in an animal model. Am. J. Ophthalmol. 2010, 150, 519–528.e1. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.Y.; Kim, Y.S.; Baek, S.G.; Lee, G.W.; Kim, J.M.; Jean, W.S.; Lee, N.S.; Kang, J. Inhibition of corneal neovascularization by subconjunctival and topical bevacizumab and sunitinib in a rabbit model. Cornea 2013, 32, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; Kalen, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar]
- Dell, S.; Peters, S.; Muther, P.; Kociok, N.; Joussen, A.M. The role of PDGF receptor inhibitors and PI3-kinase signaling in the pathogenesis of corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1928–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hos, D.; Bock, F.; Dietrich, T.; Onderka, J.; Kruse, F.E.; Thierauch, K.H.; Cursiefen, C. Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1836–1842. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [Green Version]
- Kane, R.C.; Farrell, A.T.; Madabushi, R.; Booth, B.; Chattopadhyay, S.; Sridhara, R.; Justice, R.; Pazdur, R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009, 14, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Chung, S.H.; Choi, J.S.; Joo, C.K. Inhibition of corneal neovascularization in rats by systemic administration of sorafenib. Cornea 2012, 31, 907–912. [Google Scholar] [CrossRef]
- Fong, T.A.; Shawver, L.K.; Sun, L.; Tang, C.; App, H.; Powell, T.J.; Kim, Y.H.; Schreck, R.; Wang, X.; Risau, W.; et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999, 59, 99–106. [Google Scholar]
- Keskin, U.; Totan, Y.; Karadag, R.; Erdurmus, M.; Aydin, B. Inhibitory effects of SU5416, a selective vascular endothelial growth factor receptor tyrosine kinase inhibitor, on experimental corneal neovascularization. Ophthalmic Res. 2012, 47, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kuenen, B.C.; Rosen, L.; Smit, E.F.; Parson, M.R.; Levi, M.; Ruijter, R.; Huisman, H.; Kedde, M.A.; Noordhuis, P.; Van Der Vijgh, W.J. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J. Clin. Oncol. 2002, 20, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Hata, Y.; Shiose, S.; Sassa, Y.; Honda, M.; Fujisawa, K.; Sakamoto, T.; Ishibashi, T. Suppression of experimental choroidal neovascularization utilizing KDR selective receptor tyrosine kinase inhibitor. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Woo, J.M.; Ji, Y.S.; Yoon, K.C. Comparison of the therapeutic efficacies of topical rivoceranib and topical bevacizumab in a murine model of corneal neovascularization. Medicina (Kaunas) 2019, 55, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, T.; Joensuu, H.; Nathan, P.D.; Harper, P.G.; Wojtukiewicz, M.Z.; Nicholson, S.; Bahl, A.; Tomczak, P.; Pyrhonen, S.; Fife, K.; et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: A single-group phase 2 trial. Lancet Oncol. 2012, 13, 1055–1062. [Google Scholar] [CrossRef]
- Onder, H.I.; Erdurmus, M.; Bucak, Y.Y.; Simavli, H.; Oktay, M.; Kukner, A.S. Inhibitory effects of regorafenib, a multiple tyrosine kinase inhibitor, on corneal neovascularization. Int. J. Ophthalmol. 2014, 7, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Konecny, G.E.; Pegram, M.D.; Venkatesan, N.; Finn, R.; Yang, G.; Rahmeh, M.; Untch, M.; Rusnak, D.W.; Spehar, G.; Mullin, R.J.; et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006, 66, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Rusnak, D.W.; Lackey, K.; Affleck, K.; Wood, E.R.; Alligood, K.J.; Rhodes, N.; Keith, B.R.; Murray, D.M.; Knight, W.B.; Mullin, R.J.; et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer 2001, 1, 85–94. [Google Scholar]
- Kaya, M.K.; Demir, T.; Bulut, H.; Akpolat, N.; Turgut, B. Effects of lapatinib and trastuzumab on vascular endothelial growth factor in experimental corneal neovascularization. Clin. Exp. Ophthalmol. 2015, 43, 449–457. [Google Scholar] [CrossRef]
- Pfizer. Inlyta (Axitinib) Tablets for Oral Administration. Available online: http://labeling.pfizer.com/ShowLabeling.aspx?id=759 (accessed on 29 May 2020).
- Lledo Riquelme, M.; Campos-Mollo, E.; Fernandez-Sanchez, L. Topical axitinib is a potent inhibitor of corneal neovascularization. Clin. Exp. Ophthalmol. 2018, 46, 1063–1074. [Google Scholar] [CrossRef]
- Lee, S.H.; Lopes de Menezes, D.; Vora, J.; Harris, A.; Ye, H.; Nordahl, L.; Garrett, E.; Samara, E.; Aukerman, S.L.; Gelb, A.B.; et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin. Cancer Res. 2005, 11, 3633–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahan, B.; Ciftci, F.; Eyuboglu, S.; Yaba, A.; Yilmaz, B.; Yalvac, B.I. Comparison of the effects of dovitinib and bevacizumab on reducing neovascularization in an experimental rat corneal neovascularization model. Cornea 2019, 38, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Ekim, Y.; Kara, S.; Gencer, B.; Karaca, T. Efficacy of sunitinib, sunitinib-hesperetin, and sunitinib-doxycycline combinations on experimentally-induced corneal neovascularization. Curr. Eye Res. 2019, 44, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.J.; Pryszlak, M.; Smith, L.; Yanchus, C.; Kurji, N.; Shahani, V.M.; Molinski, S.V. Giving drugs a second chance: Overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front. Oncol. 2017, 7, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Derm. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Dan, L.; Shi-long, Y.; Miao-li, L.; Yong-ping, L.; Hong-jie, M.; Ying, Z.; Xiang-gui, W. Inhibitory effect of oral doxycycline on neovascularization in a rat corneal alkali burn model of angiogenesis. Curr. Eye Res. 2008, 33, 653–660. [Google Scholar] [CrossRef]
- Aydin, E.; Kivilcim, M.; Peyman, G.A.; Esfahani, M.R.; Kazi, A.A.; Sanders, D.R. Inhibition of experimental angiogenesis of cornea by various doses of doxycycline and combination of triamcinolone acetonide with low-molecular-weight heparin and doxycycline. Cornea 2008, 27, 446–453. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Li, F.; Chen, X.; Wan, Q.; Liang, D. Doxycycline-mediated inhibition of corneal angiogenesis: An MMP-independent mechanism. Investig. Ophthalmol. Vis. Sci. 2013, 54, 783–788. [Google Scholar] [CrossRef]
- Yao, J.S.; Shen, F.; Young, W.L.; Yang, G.Y. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem. Int. 2007, 50, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Xiao, O.; Xie, Z.L.; Lin, B.W.; Yin, X.F.; Pi, R.B.; Zhou, S.Y. Minocycline inhibits alkali burn-induced corneal neovascularization in mice. PLoS ONE 2012, 7, e41858. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.R. A review of tigecycline—The first glycylcycline. Int. J. Antimicrob. Agents 2008, 32 (Suppl. S4), S215–S222. [Google Scholar] [CrossRef]
- Goktas, S.; Erdogan, E.; Sakarya, R.; Sakarya, Y.; Yilmaz, M.; Ozcimen, M.; Unlukal, N.; Alpfidan, I.; Tas, F.; Erdogan, E.; et al. Inhibition of corneal neovascularization by topical and subconjunctival tigecycline. J. Ophthalmol. 2014, 2014, 452685. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Xu, J.; Lu, J.; Bhat, S.; Sullivan, D.J., Jr.; Liu, J.O. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem. Biol. 2007, 2, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Goktas, S.; Sakarya, R.; Erdogan, E.; Sakarya, Y.; Ozcimen, M.; Dursunoglu, D.; Kocacan, M.; Alpfidan, I.; Erdogan, E.; Bukus, A.; et al. Antiangiogenic effect of itraconazole on corneal neovascularization: A pilot experimental investigation. Ophthalmic Res. 2014, 52, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.H.; Zhou, H.J.; Fang, X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharm Res. 2003, 48, 231–236. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Zhang, H.F.; Zhong, J.X.; Bai, L.; Lu, X.H. Topical dihydroartemisinin inhibits suture-induced neovascularization in rat corneas through ERK1/2 and p38 pathways. Int. J. Ophthalmol. 2011, 4, 150–155. [Google Scholar] [CrossRef]
- Francis, S.E.; Goh, K.L.; Hodivala-Dilke, K.; Bader, B.L.; Stark, M.; Davidson, D.; Hynes, R.O. Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arter. Thromb. Vasc. Biol. 2002, 22, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Muether, P.S.; Dell, S.; Kociok, N.; Zahn, G.; Stragies, R.; Vossmeyer, D.; Joussen, A.M. The role of integrin α5β1 in the regulation of corneal neovascularization. Exp. Eye Res. 2007, 85, 356–365. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.; Choi, J.S.; Park, D.Y.; Song, H.Y.; Park, T.K.; Cho, C.H.; Ye, S.K.; Joo, C.K.; Koh, G.Y.; et al. Imidazole-based alkaloid derivative LCB54–0009 suppresses ocular angiogenesis and lymphangiogenesis in models of experimental retinopathy and corneal neovascularization. Br. J. Pharm. 2015, 172, 3875–3889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, A.S., Jr. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, M.; Shimmura, S.; Kubota, S.; Miyashita, H.; Kato, N.; Noda, K.; Ozawa, Y.; Usui, T.; Ishida, S.; Umezawa, K.; et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennikov, A.; Hiraoka, M.; Abe, A.; Ohno, S.; Fujikawa, T.; Itai, A.; Ohguro, H. IκB kinase-β inhibitor IMD-0354 beneficially suppresses retinal vascular permeability in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6365–6373. [Google Scholar] [CrossRef] [Green Version]
- Lennikov, A.; Mirabelli, P.; Mukwaya, A.; Schaupper, M.; Thangavelu, M.; Lachota, M.; Ali, Z.; Jensen, L.; Lagali, N. Selective IKK2 inhibitor IMD0354 disrupts NF-κB signaling to suppress corneal inflammation and angiogenesis. Angiogenesis 2018, 21, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Recio, S.; Gascon, P. Biological and pharmacological aspects of the NK1-receptor. Biomed. Res. Int. 2015, 2015, 495704. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Hong, H.; Kim, J. Identification of substance-P as an early inductive cytokine of corneal wound and its possible role in the mobilization of mesenchymal stem cell and corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1423. [Google Scholar]
- Bignami, F.; Giacomini, C.; Lorusso, A.; Aramini, A.; Rama, P.; Ferrari, G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6783–6794. [Google Scholar] [CrossRef] [Green Version]
- Crane, I.J.; Wallace, C.A.; McKillop-Smith, S.; Forrester, J.V. CXCR4 receptor expression on human retinal pigment epithelial cells from the blood-retina barrier leads to chemokine secretion and migration in response to stromal cell-derived factor 1 alpha. J. Immunol. 2000, 165, 4372–4378. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Baffi, J.Z.; Kleinman, M.E.; Cho, W.G.; Nozaki, M.; Yamada, K.; Kaneko, H.; Albuquerque, R.J.; Dridi, S.; Saito, K.; et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 2009, 460, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.J.; Liu, G.Q.; Li, L.B.; Zhang, X.G.; Lu, P.R. Inhibitory effect of CCR3 signal on alkali-induced corneal neovascularization. Int. J. Ophthalmol. 2012, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Jin, D.; Rafii, S. The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007, 28, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.H.; Shen, W.; Yong, W.; Lu, L.; Liu, L. Effects of AMD3100 subconjunctival injection on alkali burn induced corneal neovascularization in mice. Int. J. Ophthalmol. 2011, 4, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.I.; Jefferies, K.C.; Panjwani, N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 2011, 286, 29913–29921. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.S.; Cao, Z.; Leffler, H.; Nilsson, U.J.; Panjwani, N. Galectin-3 inhibition by a small-molecule inhibitor reduces both pathological corneal neovascularization and fibrosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 9–20. [Google Scholar] [CrossRef]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef]
- Sin, N.; Meng, L.; Wang, M.Q.; Wen, J.J.; Bornmann, W.G.; Crews, C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 1997, 94, 6099–6103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Griffith, E.C.; Sage, J.; Jacks, T.; Liu, J.O. Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc. Natl. Acad. Sci. USA 2000, 97, 6427–6432. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima-Yuasa, A.; Hua, J.J.; Kennedy, D.O.; Matsui-Yuasa, I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003, 73, 1299–1313. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Moriwaki, H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG. Int. J. Mol. Sci. 2008, 9, 1034–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valcic, S.; Muders, A.; Jacobsen, N.E.; Liebler, D.C.; Timmermann, B.N. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (-)-epigallocatechin gallate with peroxyl radicals. Chem. Res. Toxicol. 1999, 12, 382–386. [Google Scholar] [CrossRef]
- Koh, C.H.; Lee, H.S.; Chung, S.K. Effect of topical epigallocatechin gallate on corneal neovascularization in rabbits. Cornea 2014, 33, 527–532. [Google Scholar] [CrossRef]
- Castro, M.R.; Lutz, D.; Edelman, J.L. Effect of COX inhibitors on VEGF-induced retinal vascular leakage and experimental corneal and choroidal neovascularization. Exp. Eye Res. 2004, 79, 275–285. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.-H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Chuang, Y.-L.; Fang, H.-W.; Ajitsaria, A.; Chen, K.-H.; Su, C.-Y.; Liu, G.-S.; Tseng, C.-L. Development of kaempferol-loaded gelatin nanoparticles for the treatment of corneal neovascularization in mice. Pharmaceutics 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Liu, H.; Law, K.; Lee, V.Y.; Huang, S.F.; Pang, C.P.; Yam, G.H. Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models. Br. J. Ophthalmol. 2011, 95, 1309–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joussen, A.M.; Rohrschneider, K.; Reichling, J.; Kirchhof, B.; Kruse, F.E. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp. Eye Res. 2000, 71, 483–487. [Google Scholar] [CrossRef]
- Shao, Z.M.; Wu, J.; Shen, Z.Z.; Barsky, S.H. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998, 58, 4851–4857. [Google Scholar]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Mizokami, S.S.; Borghi, S.M.; Bordignon, J.; Silva, R.L.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J. Nutr. Biochem. 2016, 33, 8–14. [Google Scholar] [CrossRef]
- Xu, X.R.; Yu, H.T.; Hang, L.; Shao, Y.; Ding, S.H.; Yang, X.W. Preparation of naringenin/β-cyclodextrin complex and its more potent alleviative effect on choroidal neovascularization in rats. Biomed. Res. Int. 2014, 2014, 623509. [Google Scholar] [CrossRef]
- Oguido, A.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Crespigio, J.; Domiciano, T.P.; Verri, W.A., Jr.; Casella, A.M.B. Naringenin eye drops inhibit corneal neovascularization by anti-inflammatory and antioxidant mechanisms. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5764–5776. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef]
- Haynes, W.L.; Proia, A.D.; Klintworth, G.K. Effect of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1588–1593. [Google Scholar]
- Hope, W.C.; Welton, A.F.; Fiedler-Nagy, C.; Batula-Bernardo, C.; Coffey, J.W. In vitro inhibition of the biosynthesis of slow reacting substance of anaphylaxis (SRS-A) and lipoxygenase activity by quercetin. Biochem. Pharm. 1983, 32, 367–371. [Google Scholar] [CrossRef]
- Neichi, T.; Koshihara, Y.; Murota, S. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim. Biophys. Acta 1983, 753, 130–132. [Google Scholar] [PubMed]
- Araujo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995, 95, 221–225. [Google Scholar] [CrossRef]
- Phan, T.T.; See, P.; Lee, S.T.; Chan, S.Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J. Trauma 2001, 51, 927–931. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, J.S.; Chung, S.K. The effect of curcumin on corneal neovascularization in rabbit eyes. Curr. Eye Res. 2010, 35, 274–280. [Google Scholar] [CrossRef]
- Bian, F.; Zhang, M.C.; Zhu, Y. Inhibitory effect of curcumin on corneal neovascularization in vitro and in vivo. Ophthalmologica 2008, 222, 178–186. [Google Scholar] [CrossRef]
- Pradhan, N.; Guha, R.; Chowdhury, S.; Nandi, S.; Konar, A.; Hazra, S. Curcumin nanoparticles inhibit corneal neovascularization. J. Mol. Med. (Berl.) 2015, 93, 1095–1106. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Bhat, K.P.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakenhielm, E.; Cao, R.; Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001, 15, 1798–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doganay, S.; Firat, P.G.; Cankaya, C.; Kirimlioglu, H. Evaluation of the effects of resveratrol and bevacizumab on experimental corneal alkali burn. Burns 2013, 39, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Hammers, H.J.; Bargagna-Mohan, P.; Zhan, X.H.; Herbstritt, C.J.; Ruiz, A.; Zhang, L.; Hanson, A.D.; Conner, B.P.; Rougas, J.; et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Ho, Y.K.A.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Chandel, S.; Bagai, U.; Vashishat, N. Antiplasmodial activity of Xanthium strumarium against Plasmodium berghei-infected BALB/c mice. Parasitol. Res. 2012, 110, 1179–1183. [Google Scholar] [CrossRef]
- Li, W.D.; Wu, Y.; Zhang, L.; Yan, L.G.; Yin, F.Z.; Ruan, J.S.; Chen, Z.P.; Yang, G.M.; Yan, C.P.; Zhao, D.; et al. Characterization of xanthatin: Anticancer properties and mechanisms of inhibited murine melanoma in vitro and in vivo. Phytomedicine 2013, 20, 865–873. [Google Scholar] [CrossRef]
- Pinel, B.; Landreau, A.; Seraphin, D.; Larcher, G.; Bouchara, J.P.; Richomme, P. Synthesis of reduced xanthatin derivatives and in vitro evaluation of their antifungal activity. J. Enzym. Inhib. Med. Chem. 2005, 20, 575–579. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Pei, C.G.; Li, Y.Y.; Tu, P.; Gao, G.P.; Shao, Y. Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 10355–10364. [Google Scholar]
- Shen, M.; Zhou, X.Z.; Ye, L.; Yuan, Q.; Shi, C.; Zhu, P.W.; Jiang, N.; Ma, M.Y.; Yang, Q.C.; Shao, Y. Xanthatin inhibits corneal neovascularization by inhibiting the VEGFR2 mediated STAT3/PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018, 42, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Kiviharju, T.M.; Lecane, P.S.; Sellers, R.G.; Peehl, D.M. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer Res. 2002, 8, 2666–2674. [Google Scholar] [PubMed]
- Qiu, D.; Kao, P.N. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D 2003, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, N.; Lv, X.; Ahmed, N.; Li, X.; Liu, H.; Ma, J.; Zhang, Y. Triptolide suppresses alkali burn-induced corneal angiogenesis along with a downregulation of VEGFA and VEGFC expression. Anat. Rec. 2017, 300, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef]
- Al-Ghamdi, M. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Roessner, A.; Schneider-Stock, R. Thymoquinone: A promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006, 38, 1249–1253. [Google Scholar] [CrossRef]
- Erdurmus, M.; Yagci, R.; Yilmaz, B.; Hepsen, I.F.; Turkmen, C.; Aydin, B.; Karadag, R. Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea 2007, 26, 715–719. [Google Scholar] [CrossRef]
- Calò, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22phox in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evid.-Based Complementary Altern. Med. 2018, 2018, 8438101. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Chen, Z.; Pan, X.; Xia, L.; Chen, P.; Yang, Y.; Hu, H.; Zhang, J.; Li, K.; Ge, J. Inhibition of angiogenesis, fibrosis and thrombosis by tetramethylpyrazine: Mechanisms contributing to the SDF-1/CXCR4 axis. PLoS ONE 2014, 9, e88176. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-g.; Jiang, C. Ligustrazine as a salvage agent for patients with relapsed or refractory non-Hodgkin’s lymphoma. Chin. Med. J. 2010, 123, 3206–3211. [Google Scholar] [PubMed]
- Yu, K.; Zhuang, J.; Kaminski, J.M.; Ambati, B.; Gao, Q.; Ma, P.; Liao, D.; Li, F.; Liu, X.; Ge, J. CXCR4 down-regulation by small interfering RNA inhibits invasion and tubule formation of human retinal microvascular endothelial cells. Biochem. Biophys. Res. Comm. 2007, 358, 990–996. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Yang, Y.; Qiu, J.; Yang, M.; Wu, C.; Lai, Z.; Tang, M.; Ge, J.; Yu, K. Tetramethylpyrazine (TMP) ameliorates corneal neovascularization via regulating cell infiltration into cornea after alkali burn. Biomed. Pharm. 2019, 109, 1041–1051. [Google Scholar] [CrossRef]
- Tian, H.; Cronstein, B.N. Understanding the mechanisms of action of methotrexate. Bull. NYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar]
- Mizusawa, S.; Kondoh, Y.; Murakami, M.; Nakamichi, H.; Sasaki, H.; Komatsu, K.; Takahashi, A.; Kudoh, Y.; Watanabe, K.; Ono, Y.; et al. Effect of methotrexate on local cerebral blood flow in conscious rats. Jpn. J. Pharm. 1988, 48, 499–501. [Google Scholar] [CrossRef] [Green Version]
- Byun, Y.S.; Chung, S.K. The effect of methotrexate on corneal neovascularization in rabbits. Cornea 2011, 30, 442–446. [Google Scholar] [CrossRef]
- Joussen, A.M.; Kruse, F.E.; Volcker, H.E.; Kirchhof, B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 920–927. [Google Scholar] [CrossRef]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Kohler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl). Arzneimittelforschung 1979, 29, 1640–1642. [Google Scholar]
- Joussen, A.M.; Germann, T.; Kirchhof, B. Effect of thalidomide and structurally related compounds on corneal angiogenesis is comparable to their teratological potency. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 952–961. [Google Scholar] [CrossRef]
- Kruse, F.E.; Joussen, A.M.; Rohrschneider, K.; Becker, M.D.; Volcker, H.E. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the thalidomide chirality in biological processes by the self-disproportionation of enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef]
- Marriott, J.B.; Westby, M.; Cookson, S.; Guckian, M.; Goodbourn, S.; Muller, G.; Shire, M.G.; Stirling, D.; Dalgleish, A.G. CC-3052: A water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-α production. J. Immunol. 1998, 161, 4236–4243. [Google Scholar]
- Lee, Y.K.; Chung, S.K. The inhibitory effect of thalidomide analogue on corneal neovascularization in rabbits. Cornea 2013, 32, 1142–1148. [Google Scholar] [CrossRef]
- Li, P.-K.; Pandit, B.; Sackett, D.L.; Hu, Z.; Zink, J.; Zhi, J.; Freeman, D.; Robey, R.W.; Werbovetz, K.; Lewis, A. A thalidomide analogue with in vitro antiproliferative, antimitotic, and microtubule-stabilizing activities. Mol. Cancer 2006, 5, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, P.-K.; Ma, J.-X.; Chen, D. Therapeutic effects of a novel phenylphthalimide analog for corneal neovascularization and retinal vascular leakage. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3630–3642. [Google Scholar] [CrossRef]
- Lima, L.d.M.; Castro, P.; Machado, A.L.; Fraga, C.A.M.; Lugnier, C.; de Moraes, V.L.G.; Barreiro, E.J. Synthesis and anti-inflammatory activity of phthalimide derivatives, designed as new thalidomide analogues. Bioorg. Med. Chem. 2002, 10, 3067–3073. [Google Scholar] [CrossRef]
- Rocco, P.; Momesso, D.; Figueira, R.; Ferreira, H.; Cadete, R.; Légora-Machado, A.; Koatz, V.; Lima, L.; Barreiro, E.; Zin, W. Therapeutic potential of a new phosphodiesterase inhibitor in acute lung injury. Eur. Respir. J. 2003, 22, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.C.M.; Fechine, F.V.; Ribeiro, M.Z.M.; Barreiro, E.J.; Lima, L.M.; Ricardo, N.M.; De Moraes, M.E.A.; De Moraes, M.O. Potential inhibitory effect of LASSBio-596, a new thalidomide hybrid, on inflammatory corneal angiogenesis in rabbits. Ophthalmic Res. 2012, 48, 177–185. [Google Scholar] [CrossRef]
- Benelli, U.; Ross, J.R.; Nardi, M.; Klintworth, G.K. Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A. Investig. Ophthalmol. Vis. Sci. 1997, 38, 274–282. [Google Scholar]
- Bucak, Y.Y.; Erdurmus, M.; Terzi, E.H.; Kükner, A.; Çelebi, S. Inhibitory effects of topical cyclosporine A 0.05% on immune-mediated corneal neovascularization in rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2555–2561. [Google Scholar] [CrossRef]
- Durrani, K.; Zakka, F.R.; Ahmed, M.; Memon, M.; Siddique, S.S.; Foster, C.S. Systemic therapy with conventional and novel immunomodulatory agents for ocular inflammatory disease. Surv. Ophthalmol. 2011, 56, 474–510. [Google Scholar] [CrossRef]
- Cejkova, J.; Cejka, C.; Trosan, P.; Zajicova, A.; Sykova, E.; Holan, V. Treatment of alkali-injured cornea by cyclosporine A-loaded electrospun nanofibers—An alternative mode of therapy. Exp. Eye Res. 2016, 147, 128–137. [Google Scholar] [CrossRef]
- Groth, C.G.; Bäckman, L.; Morales, J.-M.; Calne, R.; Kreis, H.; Lang, P.; Touraine, J.-L.; Claesson, K.; Campistol, J.M.; Durand, D. Sirolimus (rapamycin)-based therapy in human renal transplantation: Similar efficacy and different toxicity compared with cyclosporine 1, 2. Transplantation 1999, 67, 1036–1042. [Google Scholar] [CrossRef]
- Sonpavde, G.; Choueiri, T.K. Biomarkers: The next therapeutic hurdle in metastatic renal cell carcinoma. Br. J. Cancer 2012, 107, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Hong, H.S.; Kim, J.C.; Shin, J.S.; Son, Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2005, 46, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.J.; Hyon, J.Y.; Choi, W.S.; Yi, K.; Chung, E.S.; Chung, T.Y.; Wee, W.R. Chemical injury-induced corneal opacity and neovascularization reduced by rapamycin via TGF-β1/ERK pathways regulation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4452–4458. [Google Scholar] [CrossRef] [Green Version]
- Çakmak, H.; Ergin, K.; Bozkurt, G.; Kocatürk, T.; Evliçoğlu, G.E. The effects of topical everolimus and sunitinib on corneal neovascularization. Cutan. Ocul. Toxicol. 2016, 35, 97–103. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. (Tokyo) 1987, 40, 1249–1255. [Google Scholar] [CrossRef] [Green Version]
- Turgut, B.; Guler, M.; Akpolat, N.; Demir, T.; Celiker, U. The impact of tacrolimus on vascular endothelial growth factor in experimental corneal neovascularization. Curr. Eye Res. 2011, 36, 34–40. [Google Scholar] [CrossRef]
- Park, J.H.; Joo, C.K.; Chung, S.K. Comparative study of tacrolimus and bevacizumab on corneal neovascularization in rabbits. Cornea 2015, 34, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. In vitro and in vivo antitumorigenic activity of a mixture of lysine, proline, ascorbic acid, and green tea extract on human breast cancer lines MDA-MB-231 and MCF-7. Med. Oncol. 2005, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Peyman, G.A.; Kivilcim, M.; Morales, A.M.; DellaCroce, J.T.; Conway, M.D. Inhibition of corneal angiogenesis by ascorbic acid in the rat model. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Chung, S.K. Treatment of corneal neovascularization by topical application of ascorbic acid in the rabbit model. Cornea 2012, 31, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Kohlhaas, M.; Spoerl, E.; Speck, A.; Schilde, T.; Sandner, D.; Pillunat, L. A new treatment of keratectasia after LASIK by using collagen with riboflavin/UVA light cross-linking. Klin. Mon. Augenheilkd. 2005, 222, 430. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Spoerl, E.; Mrochen, M.; Sliney, D.; Trokel, S.; Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007, 26, 385–389. [Google Scholar] [CrossRef]
- Hou, Y.; Le, V.N.H.; Toth, G.; Siebelmann, S.; Horstmann, J.; Gabriel, T.; Bock, F.; Cursiefen, C. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am. J. Transpl. 2018, 18, 2873–2884. [Google Scholar] [CrossRef]
- Blinder, K.J.; Blumenkranz, M.S.; Bressler, N.M.; Bressler, S.B.; Donato, G.; Lewis, H.; Lim, J.I.; Menchini, U.; Miller, J.W.; Mones, J.M. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial—VIP report no. 3. Ophthalmology 2003, 110, 667–673. [Google Scholar]
- Weiss, A.; van den Bergh, H.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiogenesis inhibition for the improvement of photodynamic therapy: The revival of a promising idea. Biochim. Biophys. Acta-Rev. Cancer 2012, 1826, 53–70. [Google Scholar] [CrossRef]
- Hou, Y.; Le, V.N.H.; Clahsen, T.; Schneider, A.C.; Bock, F.; Cursiefen, C. Photodynamic therapy leads to time-dependent regression of pathologic corneal (lymph) angiogenesis and promotes high-risk corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5862–5869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokravi, M.T.; Marcus, D.M.; Alroy, J.; Egan, K.; Saornil, M.A.; Albert, D.M. Vitamin D inhibits angiogenesis in transgenic murine retinoblastoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 83–87. [Google Scholar]
- Merrigan, S.L.; Park, B.; Ali, Z.; Jensen, L.D.; Corson, T.W.; Kennedy, B.N. Calcitriol and non-calcemic vitamin D analogue, 22-oxacalcitriol, attenuate developmental and pathological choroidal vasculature angiogenesis ex vivo and in vivo. Oncotarget 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Sano, Y.; Kinoshita, S. Effects of 1α, 25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 154–158. [Google Scholar]
- Ha, C.H.; Jhun, B.S.; Kao, H.Y.; Jin, Z.G. VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis. Arter. Thromb. Vasc. Biol. 2008, 28, 1782–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Luesch, H. Largazole: From discovery to broad-spectrum therapy. Nat. Prod. Rep. 2012, 29, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Jiang, S.; Chen, J.; Ren, X.; Jin, J.; Su, S.B. Largazole, an inhibitor of class I histone deacetylases, attenuates inflammatory corneal neovascularization. Eur. J. Pharm. 2014, 740, 619–626. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, S.; Chen, J.; Su, S.B. Suberoylanilide hydroxamic acid suppresses inflammation-induced neovascularization. Can. J. Physiol. Pharm. 2014, 92, 879–885. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Hanus, J.; Anderson, C.; Zhang, H.; Dellinger, M.; Brekken, R.; Wang, S. Inhibition of multiple pathogenic pathways by histone deacetylase inhibitor SAHA in a corneal alkali-burn injury model. Mol. Pharm. 2013, 10, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.X.; Garneau-Tsodikova, S. What are the drugs of the future? MedChemComm 2018, 9, 757–758. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Engen, J.R.; Mazzeo, J.R.; Jones, G.B. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 2012, 11, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Sugiki, T.; Furuita, K.; Fujiwara, T.; Kojima, C. Current NMR Techniques for structure-based drug discovery. Molecules 2018, 23, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jitendra; Sharma, P.K.; Bansal, S.; Banik, A. Noninvasive routes of proteins and peptides drug delivery. Indian J. Pharm. Sci. 2011, 73, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Patel, S.P.; Vadlapudi, A.D.; Mitra, A.K. Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharm. 2013, 29, 106–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar] [CrossRef] [Green Version]
- EL-Meghawry, E.; Rahman, H.; Abdelkarim, G.; Najda, A. Natural products against cancer angiogenesis. Tumor Biol. 2016, 37, 14513–14536. [Google Scholar]
- Sulaiman, R.S.; Basavarajappa, H.D.; Corson, T.W. Natural product inhibitors of ocular angiogenesis. Exp. Eye Res. 2014, 129, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Basavarajappa, H.D.; Lee, B.; Lee, H.; Sulaiman, R.S.; An, H.; Magaña, C.; Shadmand, M.; Vayl, A.; Rajashekhar, G.; Kim, E.-Y. Synthesis and biological evaluation of novel homoisoflavonoids for retinal neovascularization. J. Med. Chem. 2015, 58, 5015–5027. [Google Scholar]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Jun, H.-O.; Kwon, H.J.; Park, K.H.; Kim, K.-W. Inhibition of choroidal neovascularization by homoisoflavanone, a new angiogenesis inhibitor. Mol. Vis. 2008, 14, 556. [Google Scholar]
- Kim, J.H.; Kim, K.H.; Kim, J.H.; Yu, Y.S.; Kim, Y.-M.; Kim, K.-W.; Kwon, H.J. Homoisoflavanone inhibits retinal neovascularization through cell cycle arrest with decrease of cdc2 expression. Biochem. Biophys. Res. Comm. 2007, 362, 848–852. [Google Scholar] [CrossRef]
- Lee, B.; Basavarajappa, H.D.; Sulaiman, R.S.; Fei, X.; Seo, S.-Y.; Corson, T.W. The first synthesis of the antiangiogenic homoisoflavanone, cremastranone. Org. Biomol. Chem. 2014, 12, 7673–7677. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, R.S.; Merrigan, S.; Quigley, J.; Qi, X.; Lee, B.; Boulton, M.E.; Kennedy, B.; Seo, S.-Y.; Corson, T.W. A novel small molecule ameliorates ocular neovascularisation and synergises with anti-VEGF therapy. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Basavarajappa, H.D.; Sulaiman, R.S.; Qi, X.; Shetty, T.; Sheik Pran Babu, S.; Sishtla, K.L.; Lee, B.; Quigley, J.; Alkhairy, S.; Briggs, C.M.; et al. Ferrochelatase is a therapeutic target for ocular neovascularization. EMBO Mol. Med. 2017, 9, 786–801. [Google Scholar] [CrossRef] [Green Version]
- Pran Babu, S.P.S.; White, D.; Corson, T.W. Ferrochelatase regulates retinal neovascularization. FASEB J. 2020. [Google Scholar] [CrossRef]
- Shetty, T.; Corson, T.W. Mitochondrial heme synthesis enzymes as therapeutic targets in vascular diseases. Front. Pharm. 2020. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Park, B.; Sheik Pran Babu, S.P.; Si, Y.; Kharwadkar, R.; Mitter, S.K.; Lee, B.; Sun, W.; Qi, X.; Boulton, M.E. Chemical proteomics reveals soluble epoxide hydrolase as a therapeutic target for ocular neovascularization. ACS Chem. Biol. 2018, 13, 45–52. [Google Scholar] [CrossRef]
- Park, B.; Corson, T.W. Soluble epoxide hydrolase inhibition for ocular diseases: Vision for the future. Front. Pharm. 2019, 10, 95. [Google Scholar] [CrossRef]
- Chaoran, Z.; Zhirong, L.; Gezhi, X. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves the antiangiogenic efficacy for advanced stage mouse corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1493–1501. [Google Scholar] [CrossRef]

| Tyrosine Kinase Inhibitor | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Sunitinib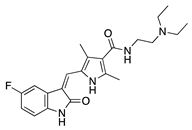 | Synthetic | Inhibition of the VEGFR and PDGFR pathways | Oral | 40 mg/kg | Murine thermal cauterization | [23] |
| Topical | 0.5 mg/mL | Rabbit suture model | [24] | |||
| Subconjunctival Topical | 0.25 mg/0.1 mL 0.5 mg/mL | Rabbit suture model | [25] | |||
AG 1296 | Synthetic | PI3K-RTK inhibition | Systemic via osmotic pump implantation | 10 ng/mL | Murine alkali burn model | [27] |
Vatalanib succinate | Synthetic | VEGFR inhibition | Oral | 75 mg/kg; 2x/day | Murine suture model | [28] |
ZK261991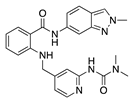 | Synthetic | VEGFR inhibition | Oral | 50 mg/kg; 2x/day | Murine suture model | [28] |
Sorafenib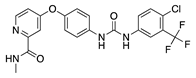 | Synthetic | Inhibition of ERK and VEGFR2 phosphorylation | Oral | 30 mg/kg; 60 mg/kg | Rat silver-nitrate burn model | [31] |
Semaxanib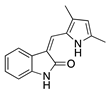 | Synthetic | Selective VEGFR2 inhibition | Intraperitoneal | 25 mg/kg | Rat silver-nitrate burn model | [33] |
Rivoceranib | Synthetic | Selective VEGFR2 inhibition | Topical | 0.1%; 0.5% | Murine alkali burn model | [36] |
Regorafenib | Synthetic | Decreases epithelial and endothelial VEGF levels | Topical | 1 mg/mL | Rat alkali burn model | [38] |
Lapatinib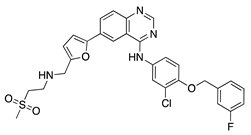 | Synthetic | Decreases corneal epithelial and stromal VEGF expression | Oral | 50 mg/kg | Rat silver-nitrate burn model | [41] |
Axitinib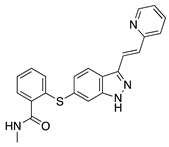 | Synthetic | Inhibition of VEGFR2 and PDGFR | Topical | 0.02, 0.35, 0.5 mg/mL | Rabbit suture model | [43] |
Dovitinib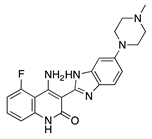 | Synthetic | Inhibition of VEGFRs, PDGFR, FGFR-1 and -3, | Topical | 5 mg/mL; 2x/day | Rat silver-nitrate burn model | [45] |
| Antimicrobial | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Doxycycline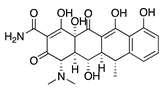 | Semisynthetic | MMP inhibition, and modulation of the MMP-independent PI3K/Akt-eNOS pathway | Oral | 40 mg/kg | Murine alkali burn model | [49] |
| Topical | 0.5 mg/mL | Murine silver-nitrate model | [50] | |||
Minocycline | Semisynthetic | Inhibition of MMP and downregulation of the ERK1/2 and Akt pathways | Intraperitoneal | 30 mg/kg; 60 mg/kg; 2x/day | Murine alkali burn model | [53] |
Tigecycline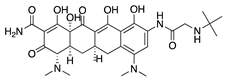 | Synthetic derived from minocycline | Unknown | Topical Subconjunctival | 1 mg/mL 1 mg/mL | Rat silver-nitrate model | [55] |
Itraconazole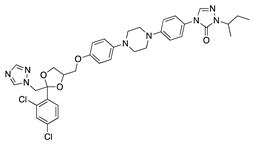 | Synthetic | Inhibition of cholesterol biosynthesis | Topical Subconjunctival Intraperitoneal | 10 mg/mL 10 mg/mL 19 mg/mL | Rat silver-nitrate model | [57] |
Dihydroartemisinin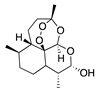 | Semisynthetic derivative of artemisinin | Modulation of the ERK1/2 and p38 pathways | Topical | 5 mg/L, 10 mg/L, 20 mg/L | Rat suture model | [60] |
| Molecule | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
| JSM5562 (Exact structure not reported) | Synthetic | Impairing EC migration, adhesion, and tube formation. Exact mechanism unknown | Systemic via osmotic pump implantation | 0.1 mg/mL, 0.5 mg/mL, 2.5 mg/mL | Murine alkali burn model | [62] |
LCB54–0009 | Imidazole-based alkaloid derivative | Regulation of HIF-1α protein stability and HIF-1α/NF-κB redox sensitivity. Inhibits Ang expression and VEGF signaling cascade | Subconjunctival | 50 µg/ 20 µL | Rat silver-nitrate burn model | [64] |
N-acetyl-l-cysteine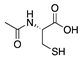 | Synthetic | Antioxidant; downregulates VEGF | Intraperitoneal | 200 mg/kg | Murine alkali burn model | [66] |
IMD0354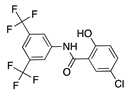 | Synthetic | Inhibition of NF-κB through selective blockage of IKK complex, IKK2 | Systemic | 30 mg/kg | Rat suture model | [68] |
Lanepitant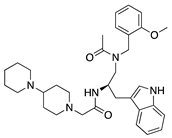 | Synthetic | NK1R antagonist | Topical Subconjunctival | 0.4, 1.6, 6.4 mg/mL 12.8 mg/mL | Murine alkali burn and suture models | [71] |
SB-328437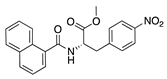 | Synthetic | CCR3 antagonist; reduces MCP-1 and -3. Exact mechanism unknown | Topical | 125 µg/mL, 250 µg/mL, 500 µg/mL | Murine alkali burn model | [74] |
AMD3100 | Synthetic | CXCR4 antagonist; Downregulates VEGF expression and inflammation | Subconjunctival Intraperitoneal | 5 µL 2.5 mg/kg | Murine alkali burn model | [77] |
33-DFTG | Synthetic | Downregulates VEGF through unknown mechanism | Subconjunctival | 50 mM | Murine alkali burn and murine silver-nitrate models | [79] |
TNP-470 | Synthetic analogue of fumagillin | Targets MetAP2 | Topical Subconjunctival injection | 5 ng/nL; 3x/day 30 mg/kg | Murine alkali burn model | [17] |
| Flavonoid | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Epigallocatechin gallate (EGCG)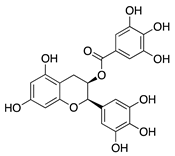 | Green tea (Camellia sinensis) | Unknown; downregulation of VEGF and COX-2 | Topical | 0.01 µg/mL 0.1 µg/mL | Rabbit suture model | [89] |
| Nanoparticle-mediated delivery via eye drops | 30 mg/mL | Murine alkali burn model | [91] | |||
Kaempferol | Fruits and vegetables | Unknown; downregulation of MMP and VEGF | Nanoparticle- mediated delivery via eye drops | 7.5 µg/mL | Murine silver-nitrate/ potassium model | [94] |
Isoliquiritigenin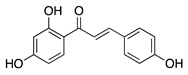 | Licorice root (Glycyrrhiza uralensis) | Unknown; downregulates VEGF and upregulates PEDF | Topical | 0.5, 1, 5, 10, 50 µM | Murine silver-nitrate model | [96] |
Fisetin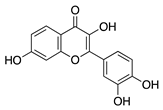 | Fruits and vegetables | Unknown | Topical | 1.0 mg/mL; 4x/day | Rabbit corneal micropocket b-FGF model | [97] |
Luteolin | Fruits and vegetables | Unknown | Topical | 0.5 mg/mL; 4x/day | Rabbit corneal micropocket b-FGF model | [97] |
Genistein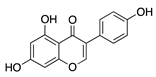 | Soybeans | Unknown; downregulates VEGF and TGF-β | Topical | 0.5 mg/mL; 4x/day | Rabbit corneal micropocket b-FGF model | [97] |
Naringenin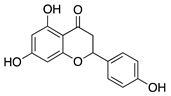 | Citrus fruits and vegetables | Unknown; Downregulates NF-κB activity, proangiogenic factors, and reduces production of cytokines IL-1β and IL-6 | Topical | 0.08, 0.8, 8, 80 µg | Rat alkali burn model | [102] |
| Non-Flavonoid Phytochemical | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Curcumin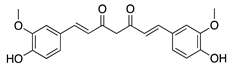 | Turmeric (Curcuma longa) | Unknown; inhibition of several signal transduction pathways, including NF-κB activation | Topical | 40 µM; 2x/day | Rat alkali burn model | [111] |
| Topical | 40, 80, and 160 µM | Rabbit suture model | [110] | |||
| Nanoparticle-mediated delivery via eye drops | 80 mg | Rat silver-nitrate model | [112] | |||
Resveratrol | Grapes and other fruits | Unknown; Downregulates FGF-2 and VEGF | Oral | 48 mg/kg | Murine FGF-2 and VEGF-micropocket model | [115] |
| Subconjunctival | 10 mg/mL | Rabbit alkali burn model | [116] | |||
Withaferin A | Steroidal lactone (Withania somnifera) | Targets and downregulates vimentin | Intraperitoneal | 2 mg/kg | Murine de-epithelializ-ation model using wild type and vimentin-null mice | [118] |
Xanthatin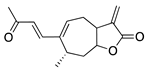 | Sesquiterpene lactone (Xanthium sibiricum) | Inhibition of the VEGFR2-mediated STAT3/PI3K/Akt signaling pathways | Topical | 10 µM; 4x/day | Rat alkali burn model | [123] |
Triptolide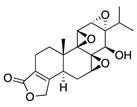 | Tripterygium wilfordii Hook F | Unknown; downregulates VEGF | Topical | 100 nM; 3x/day | Murine alkali burn model | [126] |
Thymoquinone | Volatile oil of black seed (Nigella sativa) | Unknown; Likely related to antioxidant and anti-inflammatory properties | Topical | 0.1%, 0.4% | Rat silver-nitrate model | [131] |
Glycyrrhizin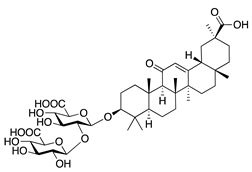 | Saponin from licorice root (Glycyrrhiza glabra) | Unknown | Topical | 1% | Rabbit alkali burn model | [133] |
| Immunosuppressant | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Tetramethylpyrazine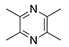 | Bioactive component of chuanxiong (Ligusticum striatum) | Unknown; Downregulates CXCR-4 | Topical | 1.5 mg/mL 4x/day | Murine alkali burn model | [137] |
Methotrexate | Synthetic | Unknown; Downregulates VEGF and IL-6 | Topical Subconjunctival | 2 mg/mL, 4 mg/mL 2 mg/mL | Rabbit suture model | [140] |
CC-3052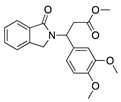 | Thalidomide analogue | Unknown; Downregulates VEGF and TNF-α | Topical Subconjunctival | 0.25%, 0.5%, and 1% 0.5% | Rabbit suture model | [147] |
DAID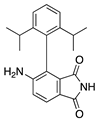 | Thalidomide analogue | Unknown; Downregulates VEGF | Topical | 0.25% | Murine alkali burn model | [149] |
LASSBio-596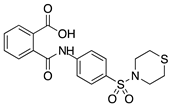 | Thalidomide and arylsulfonamide derivative | Unknown | Topical | 1%; 3x/day | Rabbit alkali burn model | [152] |
Cyclosporine A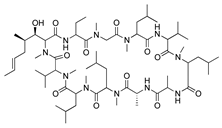 | Secondary metabolite of fungal genus Tolypocladium | Calcineurin inhibition; downregulates MMP-9, VEGF, and iNOS | Topical Subconjunctival | 4% 5 mg/kg | Rat silver-nitrate model | [153] |
| Topical | 0.05% | Rabbit immune-mediated CoNV model | [154] | |||
| Nanofibers | 0.25 mg/mm2 | Rabbit alkali burn model | [156] | |||
Rapamycin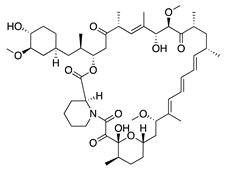 | Product of Streptomyces hygroscopicus | mTOR inhibition; downregulates VEGF, TNF-α, TGF-β, IL-6, and Substance P | Topical Intraperitoneal | 1 mg/mL 0.2 mg/kg | Murine alkali burn model | [160] |
| Intraperitoneal | 2 mg/kg; 1x/day | Murine alkali burn model | [159] | |||
Tacrolimus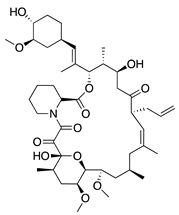 | Product of Streptomyces tsukubaensis | Calcineurin inhibition; downregulates VEGF, TNF-α, IL-1β, and MCP-1 | Topical Subconjunctival | 5 mg/5 mL 0.25 mg/ 0.05 mL | Rabbit suture model | [164] |
| Vitamin/Photoactivatable Compound | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Ascorbic acid | Diet | Unknown; Downregulation of VEGF and MMP-9 | Topical | 0.5, 1, 10 mg/mL | Rabbit suture model | [167] |
Riboflavin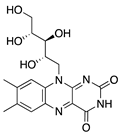 | Diet | Induction of apoptosis in vascular ECs; downregulation of macrophages and CD45+ cells | Topical riboflavin followed by UVA exposure | 0.1% | Murine suture model | [171] |
Verteporfin | Synthetic | Suppressed blood vessels and lymphatic vessels | Intravenous followed by light exposure | 6 mg/m2 | Murine suture model | [174] |
1α,25-dihydroxyvitamin D3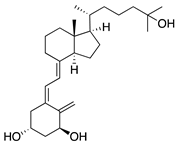 | Diet | Inhibited migration of Langerhans cells into cornea | Topical | 10−7 M, 10−8 M, and 10−9 M | Murine suture model | [177] |
| HDAC Inhibitor | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Largazole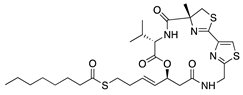 | Macrocyclic depsipeptide from marine cyanobacterium Symploca species | Class I HDAC inhibition; downregulates VEGF, b-FGF, TGF-β1, and EGF; Upregulates Tsp-1, Tsp-2, and ADAMTS-1 | Topical | 5 µL; 2x/day | Murine alkali burn model | [180] |
Vorinostat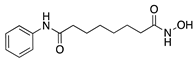 | Synthetic | HDAC inhibition; targets unknown | Topical | 10 µM; 3x/day | Murine alkali burn model | [182] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barry, Z.; Park, B.; Corson, T.W. Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization. Molecules 2020, 25, 3468. https://doi.org/10.3390/molecules25153468
Barry Z, Park B, Corson TW. Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization. Molecules. 2020; 25(15):3468. https://doi.org/10.3390/molecules25153468
Chicago/Turabian StyleBarry, Zachary, Bomina Park, and Timothy W. Corson. 2020. "Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization" Molecules 25, no. 15: 3468. https://doi.org/10.3390/molecules25153468








