Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stability of the Nanoemulsions
2.2. Bioactive Compounds and Antioxidant Activity
2.3. Quantification of Betalains
2.4. Color in Films
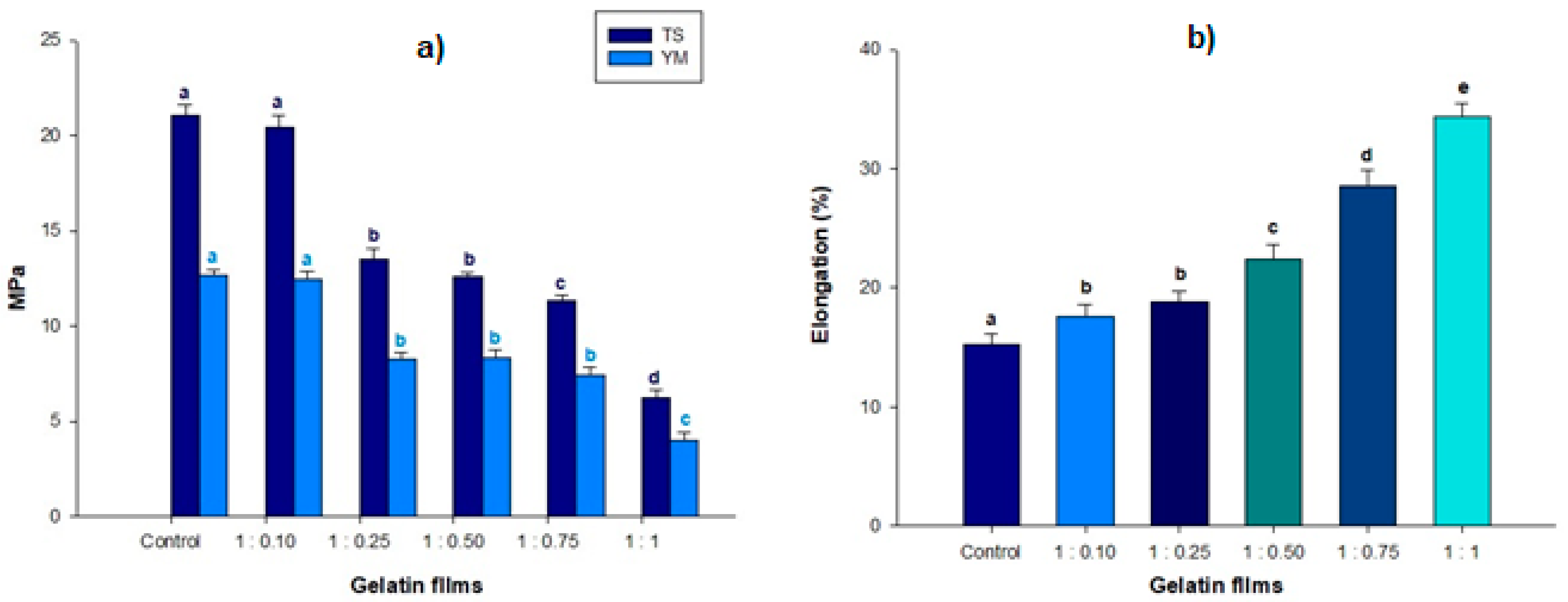
2.5. Mechanical Properties
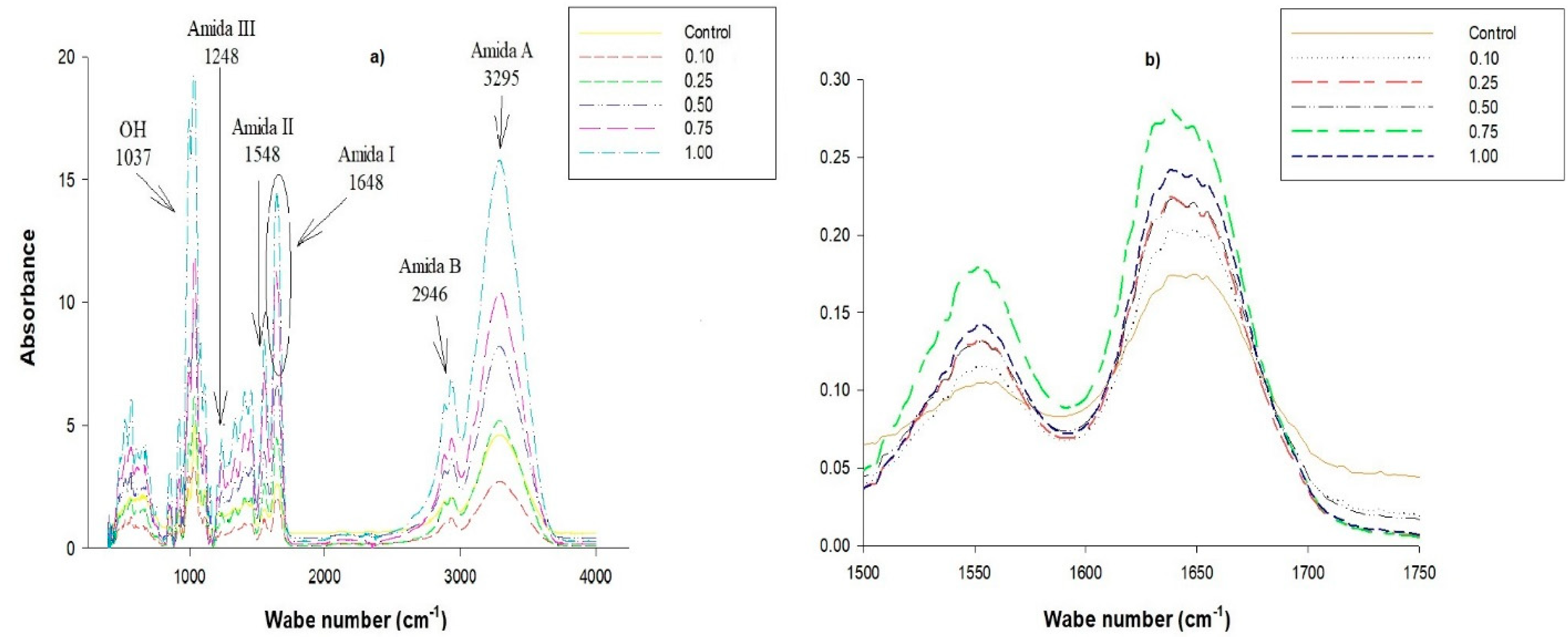
2.6. Thermal Properties and Fourier Transform Infrared (FTIR)
3. Materials and Methods
3.1. Fruits
3.2. Xoconostle Bioactive Compounds Extract
3.3. Preparation and Characterization of the Nanoemulsion
3.4. Film Preparation
3.5. Extraction of Active Compounds from the Films
3.6. Total Phenols
3.7. Total Flavonoids
3.8. Total Betalains Determination
3.9. ABTS Assay
3.10. DPPH Assay
3.11. Color Values of the Films
3.12. Mechanical Properties of Gelatine/Nanoemulsions Films
3.13. Scanning Calorimetry (DSC) and FTIR Measurements
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arora, A.; Padua, G.W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Aguirre-Álvarez, G.; Pimentel, D.; Campos-Montiel, R.G.; Foster, T.; Hill, S.E. The effect of drying temperature on mechanical properties of pig skin gelatin films El efecto de la temperatura de secado sobre las propiedades mecánicas de películas de gelatina de cerdo. CyTA J. Food 2011, 9, 243–249. [Google Scholar] [CrossRef]
- Baziwane, D.; He, Q. Gelatin: The Paramount Food Additive. Food Rev. Int. 2003, 19, 423–435. [Google Scholar] [CrossRef]
- Zhou, P.; Regenstein, J.M. Comparison of Water Gel Desserts from Fish Skin and Pork Gelatins Using Instrumental Measurements. J. Food Sci. 2007, 72, C196–C201. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Pereira, M.; Rogério, P.; Souza, A.; de Miranda, L.; Mendes, E. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-Anan, M. Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control. 2017, 73, 1310–1317. [Google Scholar] [CrossRef]
- Poverenov, E.; Zaitsev, Y.; Arnon, H.; Granit, R.; Alkalai-Tuvia, S.; Perzelan, Y.; Weinberg, T.; Fallik, E. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Boil. Technol. 2014, 96, 106–109. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; A Casari, A.C.; Mariano, M.; Yamashita, F.; Mei, L.H.I.; Soldi, V.; Martelli, S.M. Effect of a gelatin-based edible coating containing cellulose nanocrystals (CNC) on the quality and nutrient retention of fresh strawberries during storage. IOP Conf. Series: Mater. Sci. Eng. 2014, 64, 64. [Google Scholar] [CrossRef]
- Kamer, D.D.A.; Palabiyik, I.; Işık, N.O.; Akyuz, F.; Demirci, A.S.; Gumus, T. Effect of confectionery solutes on the rheological properties of fish (Oncorhynchus mykiss) gelatin. LWT 2019, 101, 499–505. [Google Scholar] [CrossRef]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery gels: Effects of low calorie sweeteners on the rheological properties and microstructure of fish gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Oberholster, A.; Carstens, L.; Du Toit, W. Investigation of the effect of gelatine, egg albumin and cross-flow microfiltration on the phenolic composition of Pinotage wine. Food Chem. 2013, 138, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Decker, E.A.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hydrocoll. 2006, 20, 596–606. [Google Scholar] [CrossRef]
- Alakali, J.; Okonkwo, T.; Iordye, E. Effect of stabilizers on the physico-chemical and sensory attributes of thermized yoghurt. Afr. J. Biotechnol. 2008, 7. [Google Scholar]
- Baygar, T. Bioactivity Potentials of Biodegradable Chitosan/Gelatin Film Forming Solutions Combined with Monoterpenoid Compounds. J. Polym. Environ. 2019, 27, 1686–1692. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Cha, D.S.; Chinnan, M.S. Biopolymer-Based Antimicrobial Packaging: A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Díaz-Monroy, G.; Medina-Pérez, G.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Vieyra-Alberto, R.; Campos-Montiel, R.G. Multiple Emulsions with Extracts of Cactus Pear Added in A Yogurt: Antioxidant Activity, In Vitro Simulated Digestion and Shelf Life. Foods 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Fuentes, A.D.; Trapala-Islas, A.; Gallegos-Vásquez, C.; Campos-Montiel, R.G.; Pinedo-Espinoza, J.M.; Guzmán-Maldonado, S.H. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits 2015, 70, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Medina-Pérez, G.; Hernández-Uribe, J.P.; Fernández-León, D.; Prince, L.; Fernández-Luqueño, F.; Campos-Montiel, R.G. Application of nanoemulsions (W/O) with active compounds of cactus pear fruit in starch films to improve antioxidant activity and incorporate antibacterial property. J. Food Process. Eng. 2019, 42. [Google Scholar] [CrossRef]
- Pimentel, D.; Aguilar-García, M.; Aguirre-Álvarez, G.; Salcedo-Hernández, R.; Guevara-Arauza, J.; Campos-Montiel, R.G. The Process and Maturation Stability of Chihuahua Cheese with Antioxidants in Multiple Emulsions. J. Food Process. Preserv. 2014, 39, 1027–1035. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Campos-Montiel, R.G.; Jiménez-Alvarado, R.; Almaraz-Buendía, I.; Medina-Pérez, G.; Fernández-Luqueño, F. Development and incorporation of nanoemulsions in food. Int. J. Food Stud. 2019, 8, 105–124. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Boil. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Vargas-Arispuro, L.; Sanz, B.I.; Martínez-Tellez, M.A.; Primo-Yúfera, E. Actividad antioxidante de compuestos aislados del residuo no-volátil del aceite esencial de naranja. Grasas y Aceites 1998, 49, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Waters, R.D.; Kesterson, J.W.; Braddock, R.J. Method for determining the α-tocopherol content of citrus essential oils. J. Food Sci. 1976, 41, 370–371. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Iturriaga, L.; Olabarrieta, I.; De Marañón, I.M. Antimicrobial assays of natural extracts and their inhibitory effect against Listeria innocua and fish spoilage bacteria, after incorporation into biopolymer edible films. Int. J. Food Microbiol. 2012, 158, 58–64. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Ocampo-López, J.; Reyes-Munguía, A.; Carrillo-Inungaray, M.L.; Cawood, M.E.; Medina-Pérez, G.; Fernández-Luqueño, F.; Montiel, R.G.C. Influence of Bioactive Compounds Incorporated in a Nanoemulsion as Coating on Avocado Fruits (Persea americana) during Postharvest Storage: Antioxidant Activity, Physicochemical Changes and Structural Evaluation. Antioxidants 2019, 8, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sari, T.; Mann, B.; Kumar, R.; Singh, R.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- McClements, D. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–672. [Google Scholar]
- Schramm, L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; John Wiley & Sons: Toronto, ON, Canada, 2006; pp. 1–353. [Google Scholar]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical Characterization of Lemongrass Essential Oil–Alginate Nanoemulsions: Effect of Ultrasound Processing Parameters. Food Bioprocess Technol. 2012, 6, 2439–2446. [Google Scholar] [CrossRef]
- Dias, D.D.O.; Colombo, M.; Kelmann, R.G.; Kaiser, S.; Lucca, L.G.; Teixeira, H.F.; Limberger, R.P.; Veiga, V.F.; Koester, L. Optimization of Copaiba oil-based nanoemulsions obtained by different preparation methods. Ind. Crop. Prod. 2014, 59, 154–162. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Kwon, Y.J. Characterization of β-carotene nanoemulsions prepared by microfluidization technique. Food Sci. Biotechnol. 2013, 23, 107–113. [Google Scholar] [CrossRef]
- Córdoba, L.J.P.; Norton, I.T.; Batchelor, H.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Mahmoudian, A. Antioxidant and antimicrobial effects of zein edible film impregnated with Zataria multiflora Boiss. essential oil and monolaurin. LWT 2016, 72, 37–43. [Google Scholar] [CrossRef]
- Cervi-Bitencourt, C.; Favaro-Trindade, C.S.; Sobral, P.; De Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Benjakul, S.; Rawdkuen, S. Retardation of lipid oxidation using gelatin film incorporated with longan seed extract compared with BHT. J. Food Sci. Technol. 2014, 52, 5842–5849. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, L.J.P.; Sobral, P.J. Physical and antioxidant properties of films based on gelatin, gelatin-chitosan or gelatin-sodium caseinate blends loaded with nanoemulsified active compounds. J. Food Eng. 2017, 213, 47–53. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Šuput, D.; Lazić, V.; Pezo, L.; Markov, S.; Vaštag, Ž.; Popović, L.; Radulović, A.; Ostojic, S.; Zlatanović, S.; Popović, S. Characterization of Starch Edible Films with Different Essential Oils Addition. Pol. J. Food Nutr. Sci. 2016, 66, 277–285. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Morales, P. Xoconostle fruit (Opuntia matudae Scheinvar cv. Rosa) by-products as potential functional ingredients. Food Chem. 2015, 185, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Muñoz, V.; Roldán, C.A.; Hernández-Fuentes, A.D.; Quintero-Lira, A.; Almaraz-Buendía, I.; Campos-Montiel, R.G. Ultrasonic-Assisted Extraction of Phenols, Flavonoids, and Biocompounds with Inhibitory Effect Against Salmonella Typhimurium and Staphylococcus Aureus from Cactus Pear. J. Food Process. Eng. 2016, 40, e12358. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rahmatollahi, A.; Dadfar, S.M.M.; Purfard, A.M. Effects of essential oil on the water binding capacity, physico-mechanical properties, antioxidant and antibacterial activity of gelatin films. LWT 2014, 57, 556–561. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Dammak, I.; De Carvalho, R.A.; Favaro-Trindade, C.S.; Lourenço, R.V.; Sobral, P.J.D.A. Properties of active gelatin films incorporated with rutin-loaded nanoemulsions. Int. J. Boil. Macromol. 2017, 98, 39–49. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Granada-Restrepo, D.; Medina-Pineda, Y.; Culebras-Rubio, M.; Gomez-Clari, C. Desarrollo y caracterización de una película activa biodegradable con antioxidantes (alfa-tocoferol) a partir de las proteínas del lactosuero. Vitae 2014, 21, 11–19. [Google Scholar]
- Rivero, S.; García, M.; Pinotti, A. Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- De Campo, C.; Costa, T.M.H.; Rios, A.D.O.; Flôres, S.H. Effect of incorporation of nutraceutical capsule waste of safflower oil in the mechanical characteristics of corn starch films. Food Sci. Technol. 2016, 36, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Wang, Q.; Liu, F.; Wei, Z.-H. Characterization of gelatin-based edible films incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Ovissipour, M.; Prodpran, T. Indigenous proteases in the skin of unicorn leatherjacket (Alutherus monoceros) and their influence on characteristic and functional properties of gelatin. Food Chem. 2011, 127, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Chai, Z.; Leng, X. Study of the Physical Properties of Whey Protein Isolate and Gelatin Composite Films. J. Agric. Food Chem. 2010, 58, 5100–5108. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Olszewski, K.; Bajek, A.; Rynkiewicz, A.; Sionkowska, A. Dialysis as a method of obtaining neutral collagen gels. Mater. Sci. Eng. C 2014, 40, 65–70. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey Protein Concentrate Edible Film Activated with Cinnamon Essential Oil. J. Food Process. Preserv. 2013, 38, 1251–1258. [Google Scholar] [CrossRef]
- Nor, M.; Nazmi, N.; Sarbon, N. Effects of plasticizer concentrations on functional properties of chicken skin gelatin films. Int. Food Res. J. 2017, 24, 1910–1918. [Google Scholar]
- Yang, H.; Guo, X.; Chen, X.; Shu, Z. Preparation and characterization of collagen food packaging film. J. Chem. Pharm. Res. 2014, 6, 740–745. [Google Scholar]
- Guler, E.; Barlas, F.B.; Yavuz, M.; Demir, B.; Gumus, Z.P.; Başpınar, Y.; Coşkunol, H.; Timur, S.; Başpınar, Y. Bio-active nanoemulsions enriched with gold nanoparticle, marigold extracts and lipoic acid: In vitro investigations. Colloids Surf. B Biointerfaces 2014, 121, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alonso, C.; Campos-Montiel, R.; Morales-Luna, E.; Reyes-Munguía, A.; Aguirre-Álvarez, G.; Pimentel-González, D. Estabilización de compuestos fenólicos de Opuntia oligacantha Först por microencapsulación con agave SAP (aguamiel). Rev. Mex. Ing. Quím. 2015, 14, 579–588. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.; Supabphol, R.; Gritsanapan, W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia 2009, 80, 442–447. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Designation D 882-95a. Standard test method for tensile properties of thin plastic sheeting. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 1995. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Compounds | Films | |||||
|---|---|---|---|---|---|---|
| Control | 1:0.10 | 1:0.25 | 1:0.50 | 1:0.75 | 1:1 | |
| Phenols (mg GAE/100 g) | 0.89 ± 0.05 a | 10.38 ± 0.92 b | 20.86 ± 4.01 c | 27.14 ± 3.70 d | 34.14 ± 7.08 e | 41.32 ± 3.71 f |
| Flavonoids (mg QE/100g) | 1.76 ± 0.80 a | 8.15 ± 2.20 b | 16.57 ± 0.32 c | 20.49 ± 2.19 d | 23.48 ± 1.11 e | 28.03 ± 3.25 f |
| % inhibition of ABTS radical | 18.44 ± 9.68 a | 62.35 ± 0.24 b | 67.23 ± 0.25 c | 68.79 ± 0.15 c | 71.83 ± 0.12 d | 72.13 ± 0.23 d |
| % inhibition of DPPH radical | 12.08 ± 0.12 a | 63.24 ± 1.90 b | 66.92 ± 4.10 b | 71.50 ± 1.93 b,c | 72.25 ± 5.12 b,c | 82.23 ± 2.71 d |
| Treatment | L* | a* | b* |
|---|---|---|---|
| Control | 49.66 ± 2.19 b | −0.88 ± 0.05 d | 0.71 ± 0.13 e |
| 1:0.1 | 46.40 ± 2.41 a,b | −1.47 ± 0.09 c | 8.16 ± 0.40 d |
| 1:0.25 | 45.26 ± 0.29 a | −1.49 ± 0.08 b | 9.14 ± 0.16 d |
| 1:0.50 | 45.69 ± 0.51 a | −1.93 ± 0.13 a,b | 13.72 ± 0.43 c |
| 1:0.75 | 45.06 ± 0.63 a | −1.61 ± 0.49 b | 15.21 ± 0.31 b |
| 1:1 | 44.67 ± 1.14 a | −1.23 ± 0.54 a | 16.14 ± 0.49 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espino-Manzano, S.O.; León-López, A.; Aguirre-Álvarez, G.; González-Lemus, U.; Prince, L.; Campos-Montiel, R.G. Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films. Molecules 2020, 25, 3487. https://doi.org/10.3390/molecules25153487
Espino-Manzano SO, León-López A, Aguirre-Álvarez G, González-Lemus U, Prince L, Campos-Montiel RG. Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films. Molecules. 2020; 25(15):3487. https://doi.org/10.3390/molecules25153487
Chicago/Turabian StyleEspino-Manzano, Salvador Omar, Arely León-López, Gabriel Aguirre-Álvarez, Uriel González-Lemus, Laurette Prince, and Rafael Germán Campos-Montiel. 2020. "Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films" Molecules 25, no. 15: 3487. https://doi.org/10.3390/molecules25153487







