Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole
Abstract
:1. Introduction
2. Results and Discussion
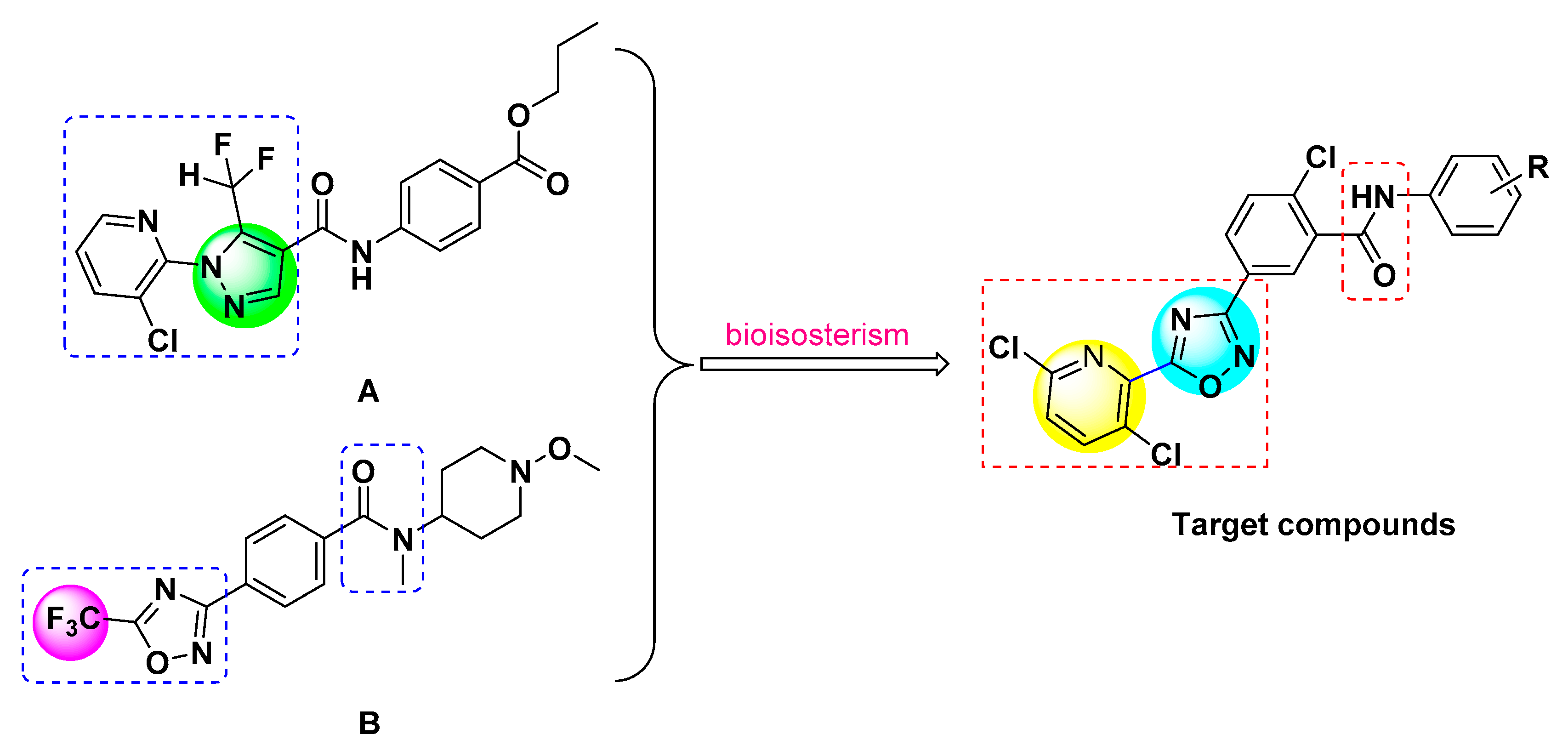
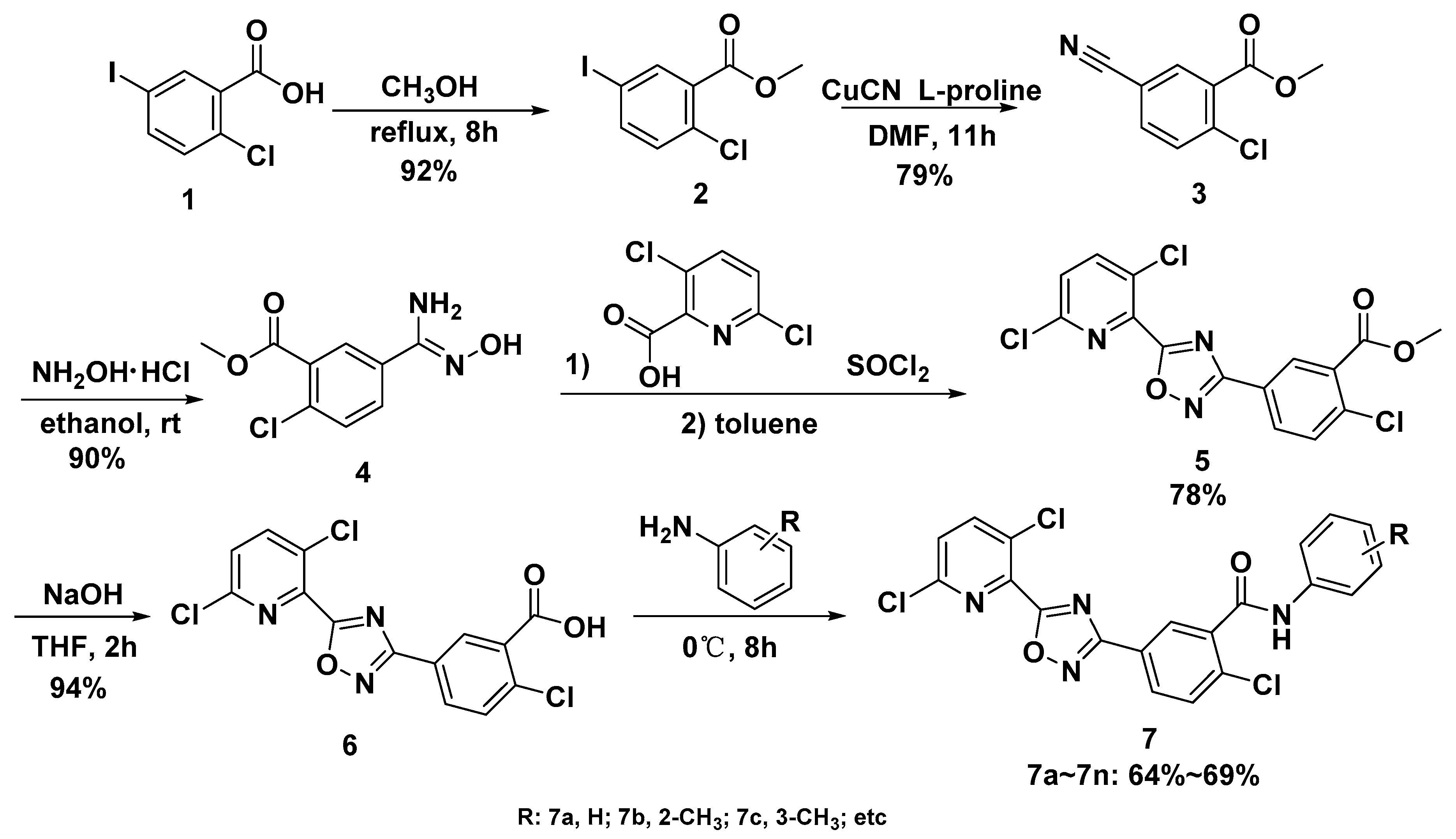
2.1. Synthesis of Target Compounds
2.2. Spectrum Analysis of Target Compounds
2.3. Biological Activities of Target Compounds
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Methyl 2-chloro-5-iodobenzoate 2
3.2.2. Methyl 2-chloro-5-cyanobenzoate 3
3.2.3. Methyl 2-chloro-5-(N′-hydroxycarbamimidoyl)benzoate 4
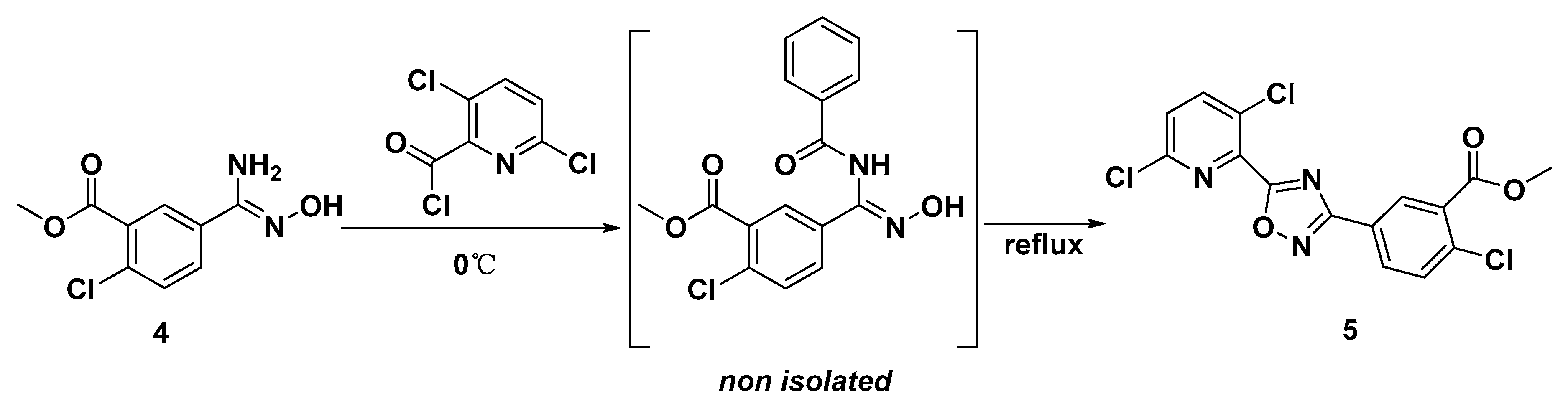
3.2.4. Methyl 2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzoate 5
3.2.5. 2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzoic acid 6
3.2.6. Preparation of Target Compound 7
3.3. Biological Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.H.; Yu, W.; Min, L.J.; Wedge, D.E.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Cantrell, C.L.; Bajsa-Hischel, J.; Hua, X.W.; et al. Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Alnufaie, R.; Alsup, N.; Whitt, J.; Chambers, A.S.; Gilmore, D.; Alam, A.M. Synthesis and antimicrobial studies of coumarin-substituted pyrazole derivatives as potent anti-staphylococcus aureus agents. Molecules 2020, 25, 2758. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.X.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Li, B.J.; Sun, N.B. Design, Synthesis, DFT Study and Antifungal Activity of Pyrazolecarboxamide Derivatives. Molecules 2016, 21, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.H.; Qiao, L.; Zhai, Z.W.; Cai, P.P.; Cantrell, C.L.; Tan, C.X.; Weng, J.Q.; Han, L.; Wu, H.K. Novel 4-Pyrazole Carboxamide Derivatives Containing Flexible Chain Motif: Design, Synthesis and Antifungal Activity. Pest Manag. Sci. 2019, 75, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, F.Y.; Zhu, J.; Dong, K.K.; Wang, Y.L.; Chen, T. Synthesis and antibacterial activities of pleuromutilin derivatives with thiazole-5-carboxamide and thioether moiety. J. Chem. Res. 2011, 35, 313–316. [Google Scholar] [CrossRef]
- Fu, Q.; Cai, P.P.; Cheng, L.; Zhong, L.K.; Tan, C.X.; Shen, Z.H.; Han, L.; Liu, X.H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020, 76, 868–879. [Google Scholar] [CrossRef]
- King, W.F.; Wheeler, R.E. Substituted Oxadiazoles and Their Use as Corn Root Worm Insecticides. U.S. Patent US4237121A, 2 December 1981. [Google Scholar]
- Haugwitz, R.D.; Martinez, A.J.; Venslavsky, J.; Angel, R.G.; Maurer, B.V.; Jacobs, G.A.; Narayanan, V.L.; Cruthers, L.R.; Szanto, J. Synyhesis and anthelmintic acyivities of novel isothiocyanatophenyl-1,2,4-oxadiazoles. J. Med. Chem. 1985, 28, 1234–1241. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, R.; Gao, S.; Ma, S.H.; Tang, H.J.; Diao, Y.M.; Wang, H.L.; Zhu, H.J. Structure-based bioisosterism design, synthesis, insecticidal activity and structure-activity relationship (SAR) of anthranilic diamide analogues containing 1,2,4-oxadiazole rings. Pest Manag. Sci. 2017, 73, 917–924. [Google Scholar] [CrossRef]
- Terteryan-Seiser, V.; Grammenos, W.; Wiebe, C.; Kretschmer, M.; Craig, I.R.; Escribano, C.A.; Marcus, F.; Tobias, M.; Palomar, M.A.Q.; Grote, T.; et al. Substituted Oxadiazoles for Combating Phytopathogenic Fungi. WO Patent WO2017178245A1, 19 October 2017. [Google Scholar]
- Iwata, J.; Nakamura, Y.; Hayashi, T.; Watanabe, S.; Sano, H. Oxadiazole Compound and Fungicide for Agricultural and Horticultural Use. WO Patent WO2019022061A1, 31 January 2019. [Google Scholar]
- Ryu, E.K.; Chung, K.H.; Lee, W.H.; Kim, J.N.; Hong, K.S. Herbicidal Quinolinyloxadiazoles. WO Patent WO9404530A1, 3 March 1994. [Google Scholar]
- Nosalova, G.; Strapkova, A.; Korpas, J. Studies of the antitussive effect of prenoxdiazine of experimentally induced cough. Bratisl. Lek. Listy 1982, 78, 47–54. [Google Scholar]
- Sakai, K.; Mizusawa, H.; Araki, H.; Higuchi, M.; Yoshikawa, Y.; Tomomatsu, E.; Okajima, Y.; Furukawa, T.; Sakanashi, M.; Atobe, Y. Effects of 5[[2-(diethylamino)-ethyl]amino]-3-phenyl-1, 2, 4-oxadiazole dihydrochloride (DEPO) on cardiovascular system. Oyo Yakuri 1979, 18, 667–672. [Google Scholar]
- Zhao, W.; Shen, Z.H.; Xing, J.H.; Yang, G.; Xu, T.M.; Peng, W.L.; Liu, X.H. Synthesis characterization nematocidal activity and docking study of novel pyrazole-4-carboxamide derivatives. Chin. J. Struct. Chem. 2017, 36, 423–428. [Google Scholar]
- Hua, X.W.; Liu, W.R.; Su, Y.Y.; Liu, X.H.; Liu, J.B.; Liu, N.N.; Wang, G.Q.; Jiao, X.Q.; Fan, X.Y.; Xue, C.M.; et al. Studies on the Novel Pyridine Sulfide Containing SDH Based Heterocyclic Amide Fungicide. Pest Manag. Sci. 2020, 76, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shen, Z.H.; Xing, J.H.; Xu, T.M.; Peng, W.L.; Liu, X.H. Synthesis and nematocidal activity of novel pyrazole carboxamide derivatives against meloidogyne incognita. Lett. Drug Des. Discov. 2017, 14, 323–329. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Xu, T.M.; Peng, W.L. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017, 125, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wang, Q.; Sun, Z.H.; Wedge, D.E.; Becnel, J.J.; Estep, A.S.; Tan, C.X.; Weng, J.Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag. Sci. 2017, 73, 953–959. [Google Scholar] [CrossRef]
- Vishnoi, S.; Agrawal, V.; Kasana, V.K. Synthesis and structure-Activity relationships of substituted cinnamic acids and amide analogues: A new class of herbicides. J. Agric. Food Chem. 2009, 57, 3261–3265. [Google Scholar] [CrossRef]
- Ji, W.J.; Xu, T.M.; Zheng, Z.W.; Zhu, B.C.; Li, J.; Hu, W.Q.; Kong, X.L. Synthesis and fungicidal activity of 1-(3-chloropyridin-2-yl)-5-difluorometyl-1H-pyrazole-4-carboxamide derivtives. Chin. J. Pestic. Sci. 2013, 15, 393–397. [Google Scholar]
- Bou-Hamdan, F.; Stierli, D.; Jeanmart, S.A.M.; Godfrey, C.R.A.; Hoffman, T.J.; Beaudegnies, R.; Pouliot, M. Oxadiazole Derivatives for Use as Pesticides and Fungicides and Their Preparation. WO Patent WO2017174158A1, 12 October 2017. [Google Scholar]
- Hoffman, T.J.; Stierli, D.; Beaudegnies, R.; Pouliot, M.; Pitterna, T. Fungicidal Oxadiazole Derivatives. WO Patent WO2018065414A1, 12 April 2018. [Google Scholar]
- Schweizer, E.; Hoffmann-Roeder, A.; Olsen, J.A.; Seiler, P.; Obst-Sander, U.; Wagner, B.; Kansy, M.; Banner, D.W.; Diederich, F. Multipolar interacetions in the D pocket of thrombin:large differences between tricyclic imide and lactam inhibitors. Org. Biomol. Chem. 2006, 4, 2364–2375. [Google Scholar] [CrossRef]
- Hamann, L.G.; Manfredi, M.C.; Sun, C.Q.; Krystek, S.R.; Huang, Y.T.; Bi, Y.Z.; Augeri, D.J.; Wang, T.; Zou, Y.; Betebenner, D.A.; et al. Tandem optimization of target activity and elimination of mutagenic potential in a potent series of N-aryl bicyclic hydantoin-based selective androgen receptor modulators. Bioorg. Med. Chem. Lett. 2007, 17, 1860–1864. [Google Scholar] [CrossRef]
- Esvan, Y.J.; Zeinyeh, W.; Boibessot, T.; Nauton, L.; Thery, V.; Knapp, S.; Chaikuad, A.; Loaec, N.; Meijer, L.; Anizon, F.; et al. Discovery of pyrido [3,4-g]quinazoline derivatives as CMGC family protein kinase inhibitors: Design, synthesis, inhibitory potency and X-ray co-crystal structure. Eur. J. Med. Chem. 2016, 118, 170–177. [Google Scholar] [CrossRef]
- Patrick, D.A.; Bakunov, S.A.; Bakunova, S.M.; Kumar, E.V.K.S.; Lombardy, R.J.; Jones, S.K.; Bridges, A.S.; Zhirnov, O.; Hall, J.E.; Wenzler, T.; et al. Med. Chem. 2007, 50, 2468–2485.
- Zhang, Y.; Zhu, H.W.; Shang, J.F.; Wang, B.L.; Li, Z.M. Synthesis and Biological Activities of Novel 3-(((3-Bromo1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)methylene)amino)-substituted-benzo [d] [1,2,3] triazin-4(3H)-ones. Chin. J. Org. Chem. 2019, 39, 861–866. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Shi, Y.X.; Yang, M.Y.; Zhao, W.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Microwave Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-triazolo [4,3-a] pyridine. Moiety. Lett. Drug Des. Discov. 2016, 13, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.T.; Wang, Q.; Min, L.J.; Wu, H.K.; Weng, J.Q.; Tan, C.X.; Zhang, Y.G.; Hu, B.Z.; Liu, X.H. Synthesis, Crystal Structure, Fungicidal Activity, Molecular Docking, and Density Functional Theory Study of 2-Chloro-N-(p-tolylcarbamoyl) nicotinamide. Indian J. Heterocycl. Chem. 2019, 29, 429–435. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Entry | Catalyst | Condition | Yield/% |
|---|---|---|---|
| 1 | no | 100 °C for 11 h | hardly react |
| 2 | l-proline | 100 °C for 11 h | 66 |
| 3 | l-proline | 70 °C for 2 h, further 100 °C for 9 h | 79 |
| 4 | l-proline | 70 °C for 3 h, further 100 °C for 7 h | 73 |
| 5 | l-proline | 80 °C for 2 h, further 100 °C for 9 h | 75 |
| Compounds | Insecticidal Activities (Death Rates %) | |||
|---|---|---|---|---|
| Concentration | Mythimna sepatara | Helicoverpa armigera | Pyrausta nubilalis | |
| (mg/L) | ||||
| 7a | 500 | 25 | 20 | 10 |
| 7b | 500 | 5 | 0 | 0 |
| 7c | 500 | 20 | 15 | 5 |
| 7d | 500 | 30 | 10 | 15 |
| 7e | 500 | 40 | 25 | 20 |
| 7f | 500 | 25 | 20 | 15 |
| 7g | 500 | 25 | 15 | 10 |
| 7h | 500 | 15 | 10 | 5 |
| 7i | 500 | 10 | 5 | 10 |
| 7j | 500 | 0 | 0 | 0 |
| 7k | 500 | 20 | 10 | 15 |
| 7l | 500 | 0 | 0 | 0 |
| 7m | 500 | 25 | 10 | 20 |
| 7n | 500 | 30 | 5 | 15 |
| Etoxazole | 500 | 100 | 100 | 100 |
| Compounds | Larvicidal Activities (Death Rates %) | |
|---|---|---|
| Concentration | Mosquito Larvae | |
| (mg/L) | ||
| 7a | 10 | 100 |
| 5 | 100 | |
| 2 | 100 | |
| 1 | 40 | |
| 7b | 10 | 10 |
| 7c | 10 | 0 |
| 7d | 10 | 0 |
| 7e | 10 | 0 |
| 7f | 10 | 100 |
| 5 | 55 | |
| 7g | 10 | 5 |
| 7h | 10 | 20 |
| 7i | 10 | 15 |
| 7j | 10 | 25 |
| 7k | 10 | 30 |
| 7l | 10 | 0 |
| 7m | 10 | 45 |
| 7n | 10 | 25 |
| Etoxazole | 10 | 100 |
| 5 | 35 | |
| Compounds | Fungicidal Activities (Inhibition Rate %) | |||||||
|---|---|---|---|---|---|---|---|---|
| AS | FG | CA | PC | SS | BC | TC | FO | |
| 7a | 44.4 | 36.1 | 6.7 | 8.3 | 30.8 | 33.3 | 50.0 | 4.5 |
| 7b | 16.7 | 27.8 | 6.7 | 25.0 | 46.2 | 19.0 | 30.3 | 4.5 |
| 7c | 16.7 | 27.8 | 6.7 | 25.0 | 30.8 | 33.3 | 27.3 | 4.5 |
| 7d | 27.8 | 36.1 | 13.3 | 8.3 | 30.8 | 66.7 | 40.9 | 22.7 |
| 7e | 30.8 | 40.4 | 6.7 | 28.7 | 41.4 | 63.1 | 44.5 | 31.6 |
| 7f | 11.1 | 22.2 | 18.2 | 33.3 | 15.4 | 28.6 | 15.2 | 13.6 |
| 7g | 33.3 | 25.0 | 33.3 | 8.3 | 15.4 | 23.8 | 50.0 | 9.1 |
| 7h | 50.0 | 44.4 | 40.0 | 8.3 | 80.8 | 90.5 | 84.8 | 22.7 |
| 7i | 27.8 | 36.1 | 13.3 | 16.7 | 42.3 | 47.6 | 25.8 | 18.2 |
| 7j | 27.8 | 22.2 | 6.7 | 16.7 | 73.1 | 38.1 | 54.5 | 22.7 |
| 7k | 11.1 | 30.6 | 13.3 | 8.3 | 15.4 | 23.8 | 15.2 | 18.2 |
| 7l | 16.7 | 5.6 | 13.3 | 16.7 | 46.2 | 33.3 | 22.7 | 18.2 |
| 7m | 38.9 | 41.7 | 26.7 | 25.0 | 38.5 | 38.1 | 60.6 | 13.6 |
| 7n | 22.2 | 22.2 | 6.7 | 8.3 | 46.2 | 9.5 | 22.7 | 9.1 |
| Fluxapyroxad | 88.9 | 30.3 | 100 | 38.1 | 96.4 | 63.6 | 88.4 | 44.4 |
| Fungus | y = a + bx | r2 | EC50/(μg·mL−1) |
|---|---|---|---|
| SS | y = 1.5805x + 3.3168 | 0.9836 | 11.61 |
| BC | y = 2.1065x + 2.3871 | 0.9758 | 17.39 |
| TC | y = 1.8992x + 2.6489 | 0.9815 | 17.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Tian, X.-Y.; Ma, T.-Y.; Dai, L.; Ren, C.-L.; Mei, J.-C.; Liu, X.-H.; Tan, C.-X. Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole. Molecules 2020, 25, 3500. https://doi.org/10.3390/molecules25153500
Yang S, Tian X-Y, Ma T-Y, Dai L, Ren C-L, Mei J-C, Liu X-H, Tan C-X. Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole. Molecules. 2020; 25(15):3500. https://doi.org/10.3390/molecules25153500
Chicago/Turabian StyleYang, Sen, Xiao-Yu Tian, Tian-Yang Ma, Li Dai, Chao-Li Ren, Jun-Chang Mei, Xing-Hai Liu, and Cheng-Xia Tan. 2020. "Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole" Molecules 25, no. 15: 3500. https://doi.org/10.3390/molecules25153500




