Fabrication and Biological Analysis of Highly Porous PEEK Bionanocomposites Incorporated with Carbon and Hydroxyapatite Nanoparticles for Biological Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mechanical Properties
2.2. Porosity of Bionanocomposites and Structural Analysis
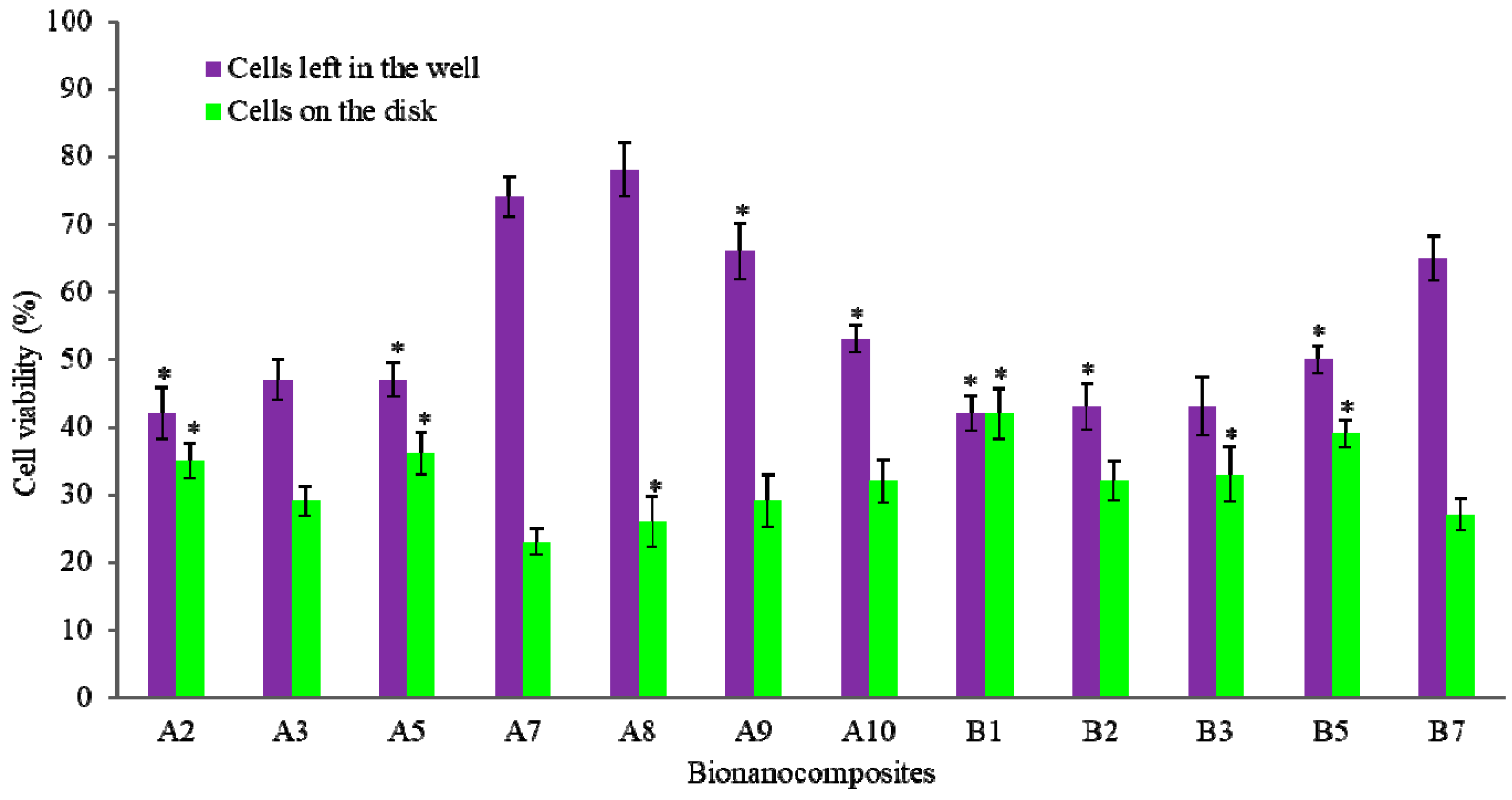
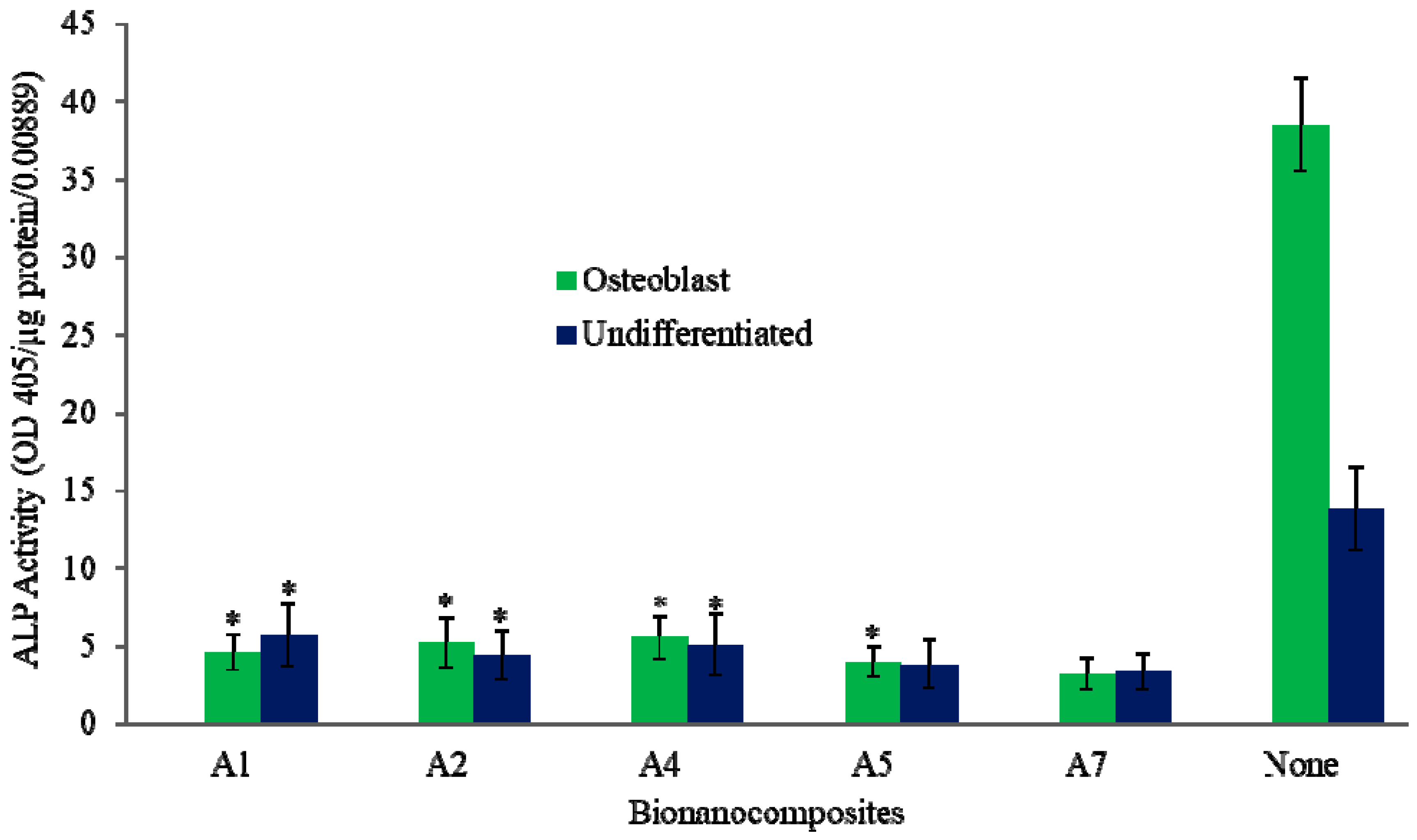
2.3. Biological Properties
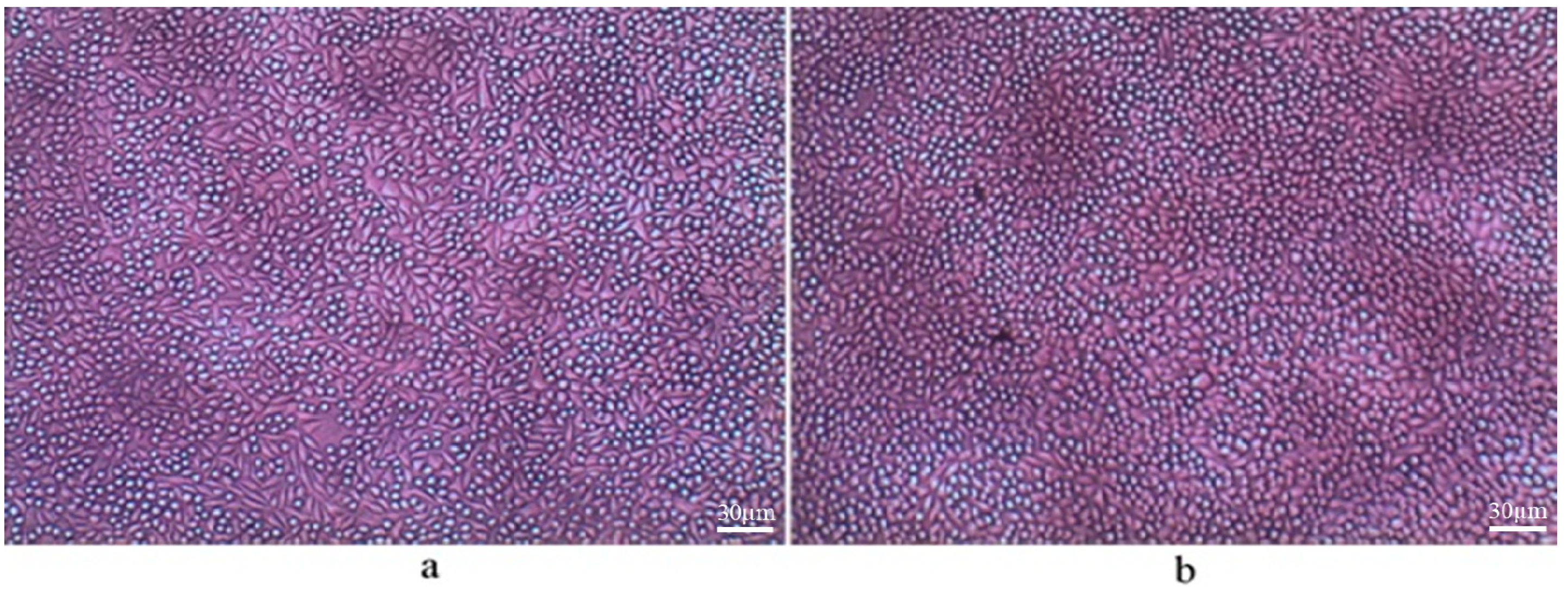
2.4. Bone Marrow and Raw Cell Cultures on Bionanocomposites
3. Materials and Methods
3.1. Materials
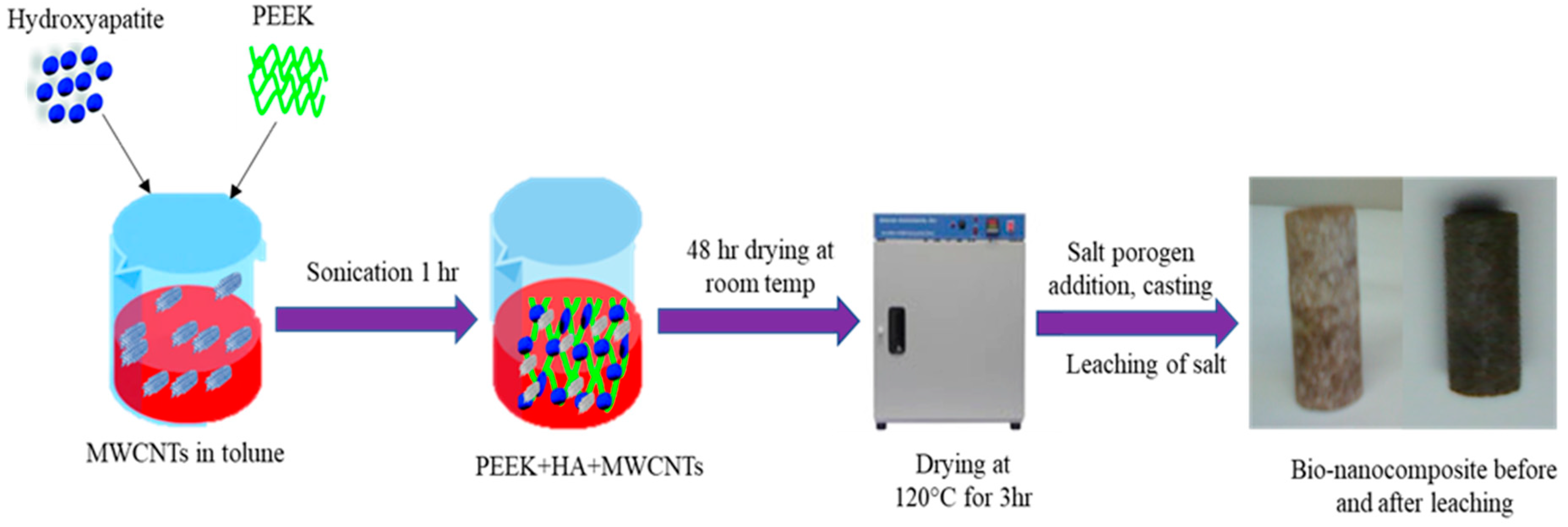
3.2. Fabrication of PEEK Bionanocomposite
3.2.1. Functionalization of CF/CNTs
3.2.2. Fabrication of PEEK Bionanocomposite
3.2.3. Leaching of Salt Porogen
3.3. Morphology, Cytotoxicity, and Cell Viability Tests
3.4. Bone Marrow and Raw Cell Culture on Bionanocomposite Materials
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Naderi, H.; Matin, M.M.; Bahrami, A.R. Review paper: Critical Issues in Tissue Engineering: Biomaterials, Cell Sources, Angiogenesis, and Drug Delivery Systems. J. Biomater. Appl. 2011, 26, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, L.M.; Mueller, T.L.; Bourban, P.-E.; Pioletti, D.; Müller, R.; Månson, J.-A.E. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, C.; Cádiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; Von Marttens, A. Bionanocomposite scaffolds based on chitosan–gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J. Biomater. Appl. 2018, 32, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Dhanasekaran, P.S.; Asmatulu, R. Synthesis, Characterization, and Applications of Polymer Based Biomaterials. In Advances in Nanotechnology; Nova Science Publishers Inc.: New York, NY, USA, 2019; Volume 22. [Google Scholar]
- Feng, P.; Kong, Y.; Yu, L.; Li, Y.; Gao, C.; Peng, S.; Pan, H.; Zhao, Z.; Shuai, C. Molybdenum disulfide nanosheets embedded with nanodiamond particles: Co-dispersion nanostructures as reinforcements for polymer scaffolds. Appl. Mater. Today 2019, 17, 216–226. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Uddin, N.; Le, L.; Nair, R.; Asmatulu, R. Effects of Graphene Oxide Thin Films and Nanocomposite Coatings on Flame Retardancy and Thermal Stability of Aircraft Composites: A Comparative Study. J. Eng. Mater. Technol. 2019, 141, 1–20. [Google Scholar] [CrossRef]
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar] [CrossRef] [Green Version]
- Asmatulu, R.; Veisi, Z.; Uddin, N.; Mahapatro, A. Highly Sensitive and Reliable Electrospun Polyaniline Nanofiber Based Biosensor as a Robust Platform for COX-2 Enzyme Detections. Fibers Polym. 2019, 20, 966–974. [Google Scholar] [CrossRef]
- Seraz, M.S.; Uddin, M.N.; Yolyang, S.Y.; Asmatulu, R. Investigating electrochemical behavior of antibacterial polyelectrte-coated magnesium alloys for biomedical applications. J. Environ. Sci. Eng. Technol. 2017, 5, 23–33. [Google Scholar] [CrossRef]
- Uddin, N.; Gandy, H.T.N.; Rahman, M.M.; Asmatulu, R. Adhesiveless honeycomb sandwich structures of prepreg carbon fiber composites for primary structural applications. Adv. Compos. Hybrid Mater. 2019, 2, 339–350. [Google Scholar] [CrossRef]
- Gan, K.; Liu, H.; Liu, X.; Niu, D. Research progress of polyether ether ketone biocomposites. Ann. Mater. Sci. Eng. 2015, 2, 1020. [Google Scholar]
- Almasi, D.; Iqbal, N.; Sadeghi, M.; Izman, S.; Kadir, M.R.A.; Kamarul, T. Preparation Methods for Improving PEEK’s Bioactivity for Orthopedic and Dental Application: A Review. Int. J. Biomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, E.A.B.; Parkes, A.; Williams, R.L.; Jenkins, M.J.; Grover, L.M. Formulation of a covalently bonded hydroxyapatite and poly(ether ether ketone) composite. J. Tissue Eng. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladapo, B.I.; Zahedi, S.A.; Chong, S.; Omigbodun, F.T.; Malachi, I.O. 3D printing of surface characterisation and finite element analysis improvement of PEEK-HAP-GO in bone implant. Int. J. Adv. Manuf. Technol. 2019, 106, 829–841. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H.; Jing, Y.; Sui, G.; Zhang, Z. The finite element analysis of polyetheretherketone/hydroxyapatite/carbon fiber cage. J. Biomed. Eng. 2013, 30, 873–878. [Google Scholar]
- Liu, X.; Deng, C.; Liu, J.; Li, J.; Sui, G. Research on the extracorporeal cytocompatibility of a composite of HA, carbon fiber and polyetheretherket-one. J. Biomed. Eng. 2011, 28, 1159–1164. [Google Scholar]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Ma, R.; Li, Q.; Wang, L.; Zhang, X.; Fang, L.; Luo, Z.; Xue, B.; Ma, L. Mechanical properties and in vivo study of modified-hydroxyapatite/polyetheretherketone biocomposites. Mater. Sci. Eng. C 2017, 73, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Shuai, C.; Wu, P.; Yuan, F.; Feng, P.; Yang, Y.; Guo, W.; Fan, X.; Su, T.; Peng, S.; et al. Characterization and Bioactivity Evaluation of (Polyetheretherketone/Polyglycolicacid)-Hydroyapatite Scaffolds for Tissue Regeneration. Materials 2016, 9, 934. [Google Scholar] [CrossRef]
- Wong, K.L.; Wong, C.T.; Liu, W.C.; Pan, H.; Fong, M.; Lam, W.; Cheung, W.; Tang, W.; Chiu, K.; Luk, K.; et al. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials 2009, 30, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Dhanasekaran, P.S.; Asmatulu, R. Mechanical properties of highly porous PEEK bionanocomposites incorporated with carbon and hydroxyapatite nanoparticles for scaffold applications. Prog. Biomater. 2019, 8, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Pascual, A.M.; Naffakh, M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. High-performance nanocomposites based on polyetherketones. Prog. Mater. Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]
- Michael, F.M.; Khalid, M.; Walvekar, R.; Ratnam, C.T.; Ramarad, S.; Siddiqui, H.; Hoque, M.E. Effect of nanofillers on the physico-mechanical properties of load bearing bone implants. Mater. Sci. Eng. C 2016, 67, 792–806. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Grabinski, C.; Hussain, S.; Lafdi, K.; Braydich-Stolle, L.; Schlager, J. Effect of particle dimension on biocompatibility of carbon nanomaterials. Carbon 2007, 45, 2828–2835. [Google Scholar] [CrossRef]
- Tjong, S.C.; Yeung, K.W.K.; Wong, H.M.; Liao, C.Z. The development, fabrication, and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers. Int. J. Nanomed. 2014, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.W.; Liao, C.Z.; Wong, H.M.; Yeung, K.W.K.; Tjong, S. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016, 6, 19417–19429. [Google Scholar] [CrossRef]
- Li, X.; Gao, H.; Uo, M.; Sato, Y.; Akasaka, T.; Feng, Q.; Cui, F.; Liu, X.; Watari, F. Effect of carbon nanotubes on cellular functionsin vitro. J. Biomed. Mater. Res. Part A 2009, 91, 132–139. [Google Scholar] [CrossRef]
- Elias, K.L.; Price, R.L.; Webster, T.J. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials 2002, 23, 3279–3287. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Sample Code | Sample Composition | Test Performed |
|---|---|---|
| A1 | PEEK/HA (20 wt%)/CNT (0.5 wt%), porosity 75% | ALP, Mechanical |
| A2 | PEEK/HA (20 wt%)/CNT (1.0 wt%), porosity 75% | ALP, Mechanical, Cell viability |
| A3 | PEEK/HA (20 wt%)/CNTs (2 wt%), porosity 75% | Cell viability, Mechanical |
| A4 | PEEK/HA (20 wt%)/CF (0.5 wt%), porosity 75% | ALP, Mechanical |
| A5 | PEEK/HA (20 wt%)/CF (1.0 wt%), porosity 75% | ALP, Mechanical, Cell viability |
| A6 | PEEK/HA (20 wt%) /CF (2 wt%), porosity 75% | Mechanical |
| A7 | PEEK only, porosity 75% | ALP, Mechanical, cell viability, |
| A8 | PEEK/HA (10 wt%), porosity 75% | Cell viability |
| A9 | PEEK/HA (15 wt%), porosity 75% | Cell viability |
| A10 | PEEK/HA (20 wt%), porosity 75% | Cell viability, Mechanical |
| B1 | PEEK/HA (20 wt%)/CNTs (0.5 wt%), porosity 85% | Cell viability, Mechanical |
| B2 | PEEK/HA (20 wt%)/CNTs (1 wt%), porosity 85% | Cell viability, Mechanical |
| B3 | PEEK/HA (20 wt%)/CNTs (2 wt%), porosity 85% | cell viability, Mechanical |
| B4 | PEEK/HA (20 wt%)/CF (0.5 wt%), porosity 85% | Mechanical |
| B5 | PEEK/HA (20 wt%)/CF (1 wt%), porosity 85% | Cell viability, Mechanical |
| B6 | PEEK/HA (20 wt%)/CF (2 wt%), porosity 85% | Mechanical |
| B7 | PEEK only, porosity 85% | Cell viability |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swaminathan, P.D.; Uddin, M.N.; Wooley, P.; Asmatulu, R. Fabrication and Biological Analysis of Highly Porous PEEK Bionanocomposites Incorporated with Carbon and Hydroxyapatite Nanoparticles for Biological Applications. Molecules 2020, 25, 3572. https://doi.org/10.3390/molecules25163572
Swaminathan PD, Uddin MN, Wooley P, Asmatulu R. Fabrication and Biological Analysis of Highly Porous PEEK Bionanocomposites Incorporated with Carbon and Hydroxyapatite Nanoparticles for Biological Applications. Molecules. 2020; 25(16):3572. https://doi.org/10.3390/molecules25163572
Chicago/Turabian StyleSwaminathan, P. D., Md. Nizam Uddin, P. Wooley, and Ramazan Asmatulu. 2020. "Fabrication and Biological Analysis of Highly Porous PEEK Bionanocomposites Incorporated with Carbon and Hydroxyapatite Nanoparticles for Biological Applications" Molecules 25, no. 16: 3572. https://doi.org/10.3390/molecules25163572






