Developing 3D-Printable Cathode Electrode for Monolithically Printed Microbial Fuel Cells (MFCs)
Abstract
:1. Introduction
2. Results
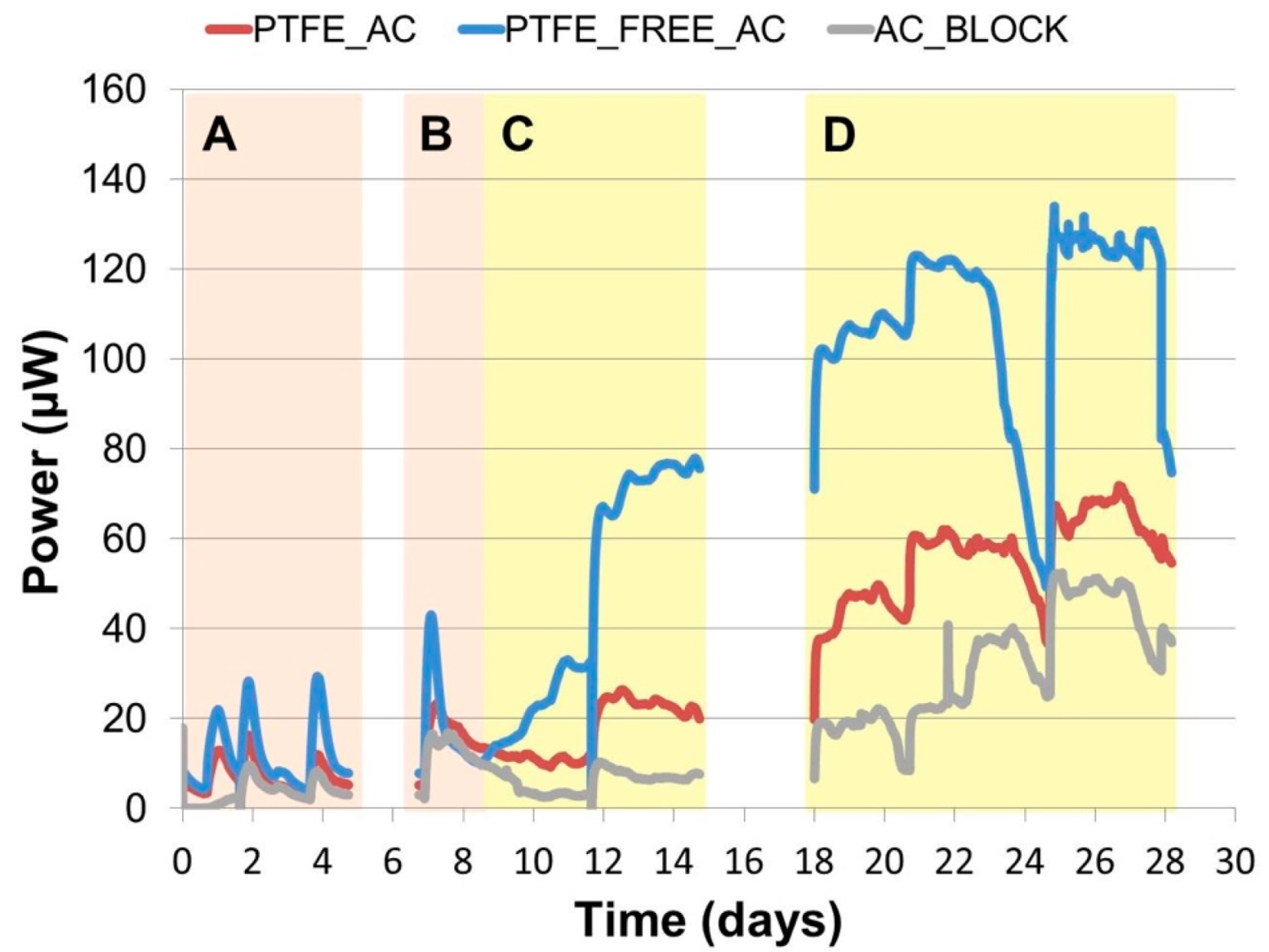
2.1. Continuous Power Output
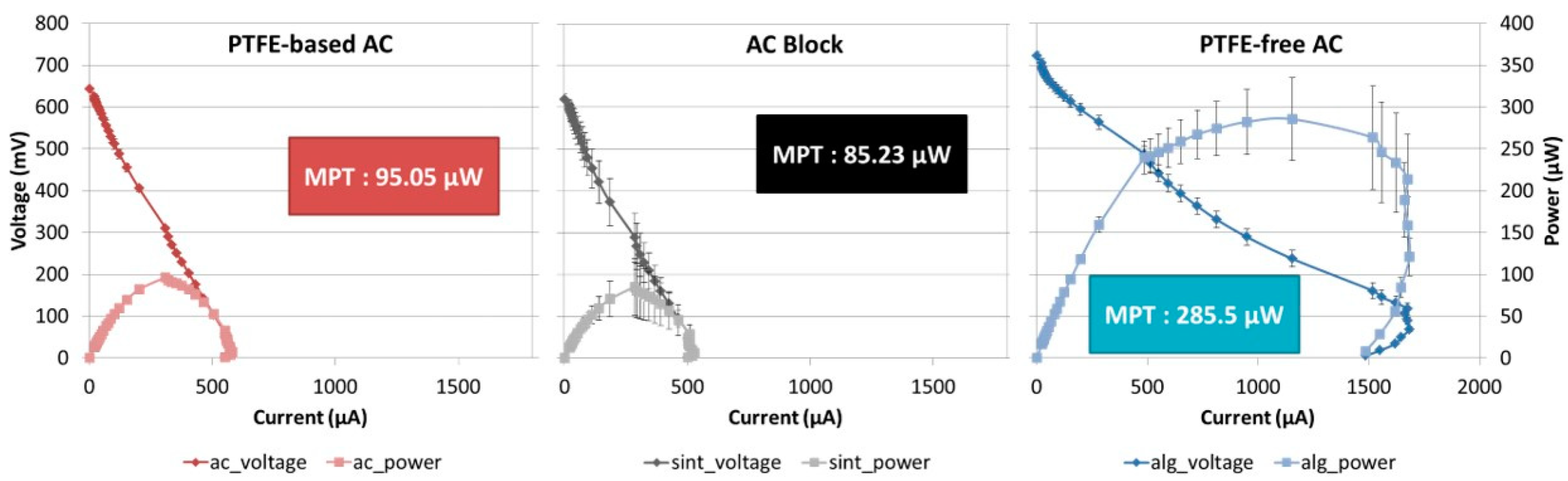
2.2. Polarisation Results
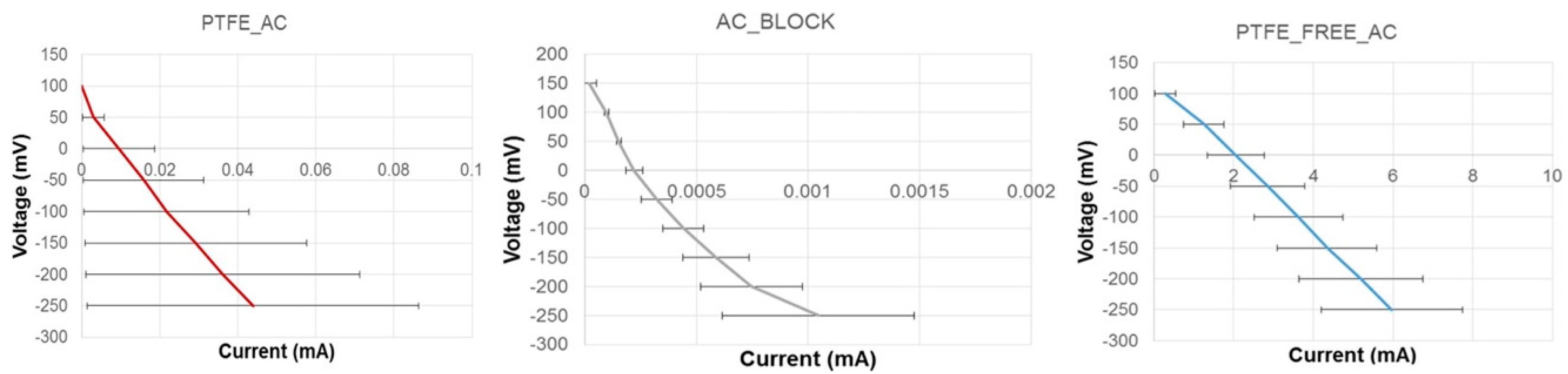
2.3. Cathode Electrode Lineal Sweep Voltammetry (LSV)
3. Discussion
3.1. Material Selection Rationale
3.1.1. Activated Carbon
3.1.2. Sodium Alginate
3.1.3. Carbon Block
3.2. Cost Analysis
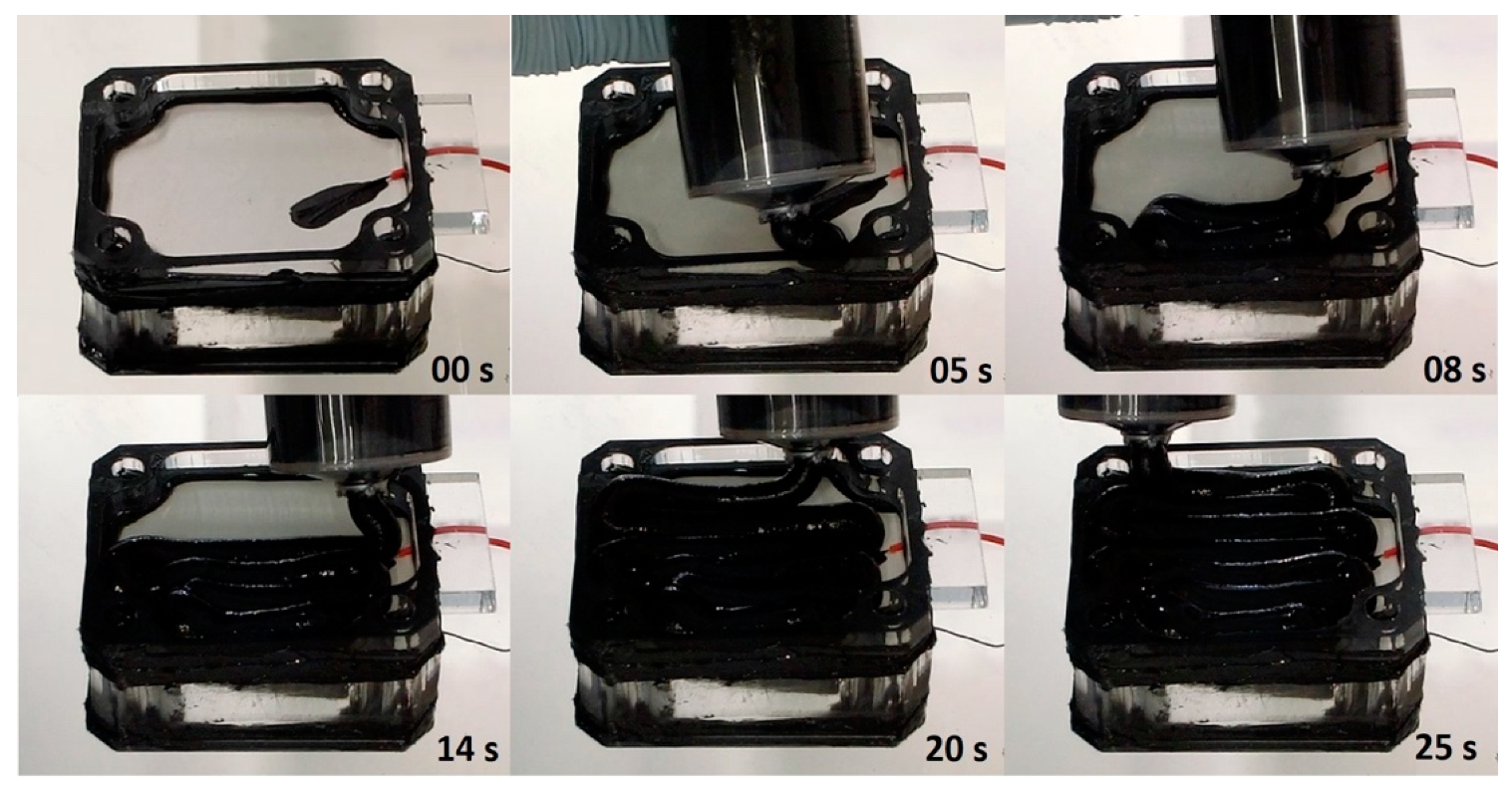
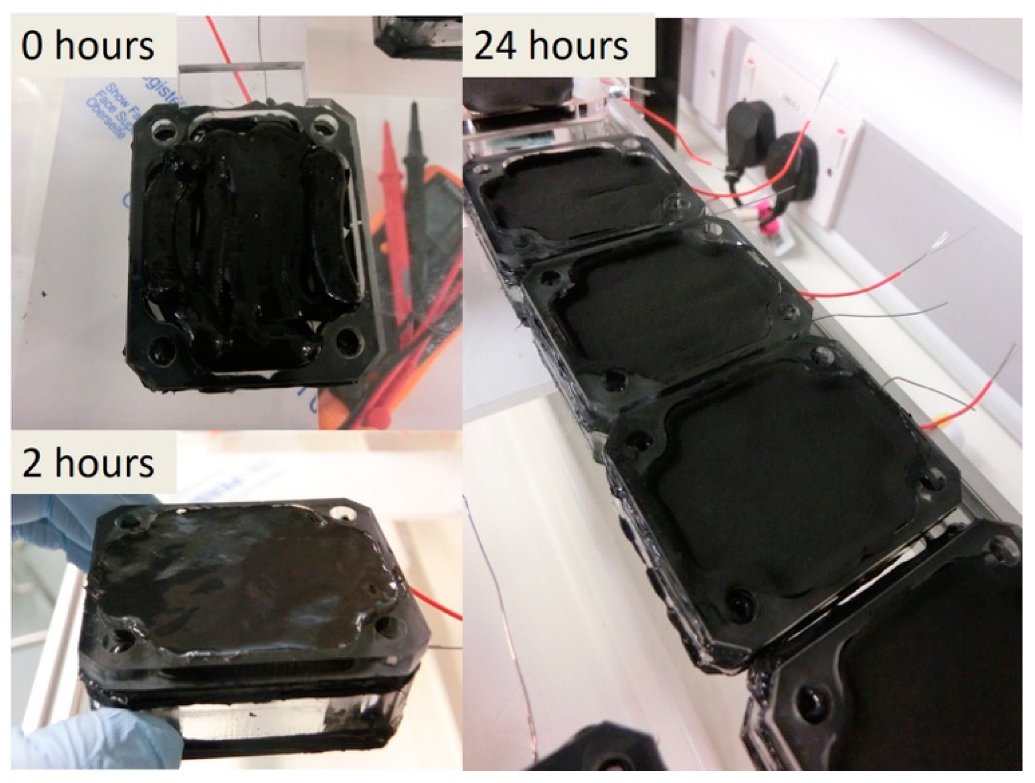
3.3. 3D-Printing Feasibility Using EVOBOT
4. Materials and Methods
4.1. MFC Architecture
4.2. Cathode Electrode Material
4.3. Operating Conditions
4.4. Polarisation Experiment
4.5. Electrochemical Analysis of the Cathode Electrode
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bennetto, H.P. Electricity generation by microorganisms. Biotechnol. Educ. 1990, 1, 163–168. [Google Scholar]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Potter, M. Electrical Effects Accompanying the Decomposition of Organic Compounds Published by: The Royal Society Electrical Effects accompanying the Decormposition of Organic. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1911, 84, 260–276. [Google Scholar]
- Petar, K. History of Additive Manufacturing. In 3D Printing and Its Impact on the Production of Fully Functional Components: Emerging Research and Opportunities; IGI Global: Hershey, PA, USA, 2017; pp. 1–24. ISBN 978-0-9913332-0-2. [Google Scholar]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Improved energy output levels from small-scale Microbial Fuel Cells. Bioelectrochemistry 2010, 78, 44–50. [Google Scholar] [CrossRef]
- Walters, P.; Lewis, A.; Stinchcombe, A.; Stephenson, R.; Ieropoulos, I. Artificial heartbeat: Design and fabrication of a biologically inspired pump. Bioinspir. Biomim. 2013, 8, 046012. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Thomson, A.R.; Schneider, K.; Cameron, P.J.; Ieropoulos, I. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens. Bioelectron. 2014, 62, 182–188. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Preen, R.J.; Bull, L.; Greenman, J.; Ieropoulos, I. 3D printed components of microbial fuel cells: Towards monolithic microbial fuel cell fabrication using additive layer manufacturing. Sustain. Energy Technol. Assess. 2017, 19, 94–101. [Google Scholar] [CrossRef]
- Philamore, H.; Rossiter, J.; Walters, P.; Winfield, J.; Ieropoulos, I. Cast and 3D printed ion exchange membranes for monolithic microbial fuel cell fabrication. J. Power Sources 2015, 289, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Calignano, F.; Tommasi, T.; Manfredi, D.; Chiolerio, A.; Koomey, J.G.; Berard, S.; Sanchez, M.; Wong, H.; Sun, M.; Cheng, S.; et al. Additive Manufacturing of a Microbial Fuel Cell—A detailed study. Sci. Rep. 2015, 5, 17373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strack, G. Additive manufacturing approaches for biological power generation. Curr. Opin. Electrochem. 2019, 17, 167–173. [Google Scholar] [CrossRef]
- Jannelli, E.; Di Trolio, P.; Flagiello, F.; Minutillo, M. Development and Performance analysis of Biowaste based Microbial Fuel Cells fabricated employing Additive Manufacturing technologies. Energy Procedia 2018, 148, 1135–1142. [Google Scholar] [CrossRef]
- EVOBLISS. Available online: https://blogit.itu.dk/evoblissproject/ (accessed on 30 May 2020).
- Faíña, A.; Nejatimoharrami, F.; Stoy, K.; Theodosiou, P.; Taylor, B.; Ieropoulos, I. EvoBot: An Open-Source, Modular Liquid Handling Robot for Nurturing Microbial Fuel Cells. In Proceedings of the Artificial Life Conference 2016, Cancún, Mexico, 4–8 July 2016; pp. 626–633. [Google Scholar]
- Theodosiou, P.; Faina, A.; Nejatimoharrami, F.; Stoy, K.; Greenman, J.; Melhuish, C.; Ieropoulos, I. EvoBot: Towards a Robot-Chemostat for Culturing and Maintaining Microbial Fuel Cells (MFCs). In Biomimetic and Biohybrid Systems; Springer: Cham, Switzerland, 2017; pp. 453–464. [Google Scholar]
- Theodosiou, P.; Greenman, J.; Ieropoulos, I. Towards monolithically printed Mfcs: Development of a 3d-printable membrane electrode assembly (mea). Int. J. Hydrogen Energy 2019, 44, 4450–4462. [Google Scholar] [CrossRef]
- Chambre, A. Effects of Carbon Filtration Type on Filter Efficiency and Efficacy: Granular Loose-Fill vs. Bonded Filters. Air Sci. 2014, 1, 1–5. [Google Scholar]
- Bigoni, F.; De Giorgio, F.; Soavi, F.; Arbizzani, C. Sodium Alginate: A Water-Processable Binder in High-Voltage Cathode Formulations. J. Electrochem. Soc. 2017, 164, 6171–6177. [Google Scholar] [CrossRef]
- Xu, J.; Chou, S.-L.; Gu, Q.; Liu, H.-K.; Dou, S.-X. The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Bigoni, F.; De Giorgio, F.; Soavi, F.; Arbizzani, C. New Formulations of High-Voltage Cathodes for Li-Ion Batteries with Water- Processable Binders. ECS Trans. 2016, 73, 249–257. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Theodosiou, P.; Taylor, B.; Greenman, J.; Melhuish, C. Gelatin as a promising printable feedstock for microbial fuel cells (MFC). Int. J. Hydrogen Energy 2017, 42, 1783–1790. [Google Scholar] [CrossRef]
- Jefferies, D. Activated Carbon Filters: Comparing loose-fill with bonded carbon filters. Filtr. Sep. 1995, 32, 385–387. [Google Scholar]
- Walter, X.A.; Greenman, J.; Ieropoulos, I. Binder materials for the cathodes applied to self-stratifying membraneless microbial fuel cell. Bioelectrochemistry 2018, 123, 119–124. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Simultaneous electricity generation and microbially-assisted electrosynthesis in ceramic MFCs. Bioelectrochemistry 2015, 104, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degrenne, N.; Buret, F.; Allard, B.; Bevilacqua, P. Electrical energy generation from a large number of microbial fuel cells operating at maximum power point electrical load. J. Power Sources 2012, 205, 188–193. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Ivanov, I.; Hatzell, M.C.; Yang, W.; Ahn, Y.; Logan, B.E. Reference and counter electrode positions affect electrochemical characterization of bioanodes in different bioelectrochemical systems. Biotechnol. Bioeng. 2014, 111, 1931–1939. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Pham, T.H.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds for the PTFE_FREE_AC are available from the authors. Hardware and software information and files for EVOBOT are available from https://www.thingiverse.com/thing:2776125. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosiou, P.; Greenman, J.; Ieropoulos, I.A. Developing 3D-Printable Cathode Electrode for Monolithically Printed Microbial Fuel Cells (MFCs). Molecules 2020, 25, 3635. https://doi.org/10.3390/molecules25163635
Theodosiou P, Greenman J, Ieropoulos IA. Developing 3D-Printable Cathode Electrode for Monolithically Printed Microbial Fuel Cells (MFCs). Molecules. 2020; 25(16):3635. https://doi.org/10.3390/molecules25163635
Chicago/Turabian StyleTheodosiou, Pavlina, John Greenman, and Ioannis A. Ieropoulos. 2020. "Developing 3D-Printable Cathode Electrode for Monolithically Printed Microbial Fuel Cells (MFCs)" Molecules 25, no. 16: 3635. https://doi.org/10.3390/molecules25163635





