Sponges and Sponge-Like Materials in Sample Preparation: A Journey from Past to Present and into the Future
Abstract
:1. Introduction
2. The Beginning of the Trend
3. The Revival of the Trend
3.1. Recent Uses of Polyurethane Foams
3.2. Development of Carbon-Based Foams
3.3. Combinations of Carbon-Based Foams with Metals
3.4. Development of Functionalized Melamine Sponges
3.5. Use of Natural Sponges
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, Y.; Chen, L.; Li, J.; Liu, D.; Chen, L. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. TrAC-Trends Anal. Chem. 2014, 59, 26–41. [Google Scholar] [CrossRef]
- Kuhtinskaja, M.; Koel, M. Smart Materials and Green Analytical Chemistry. In Handbook of Smart Materials in Analytical Chemistry; Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 503–530. [Google Scholar]
- Chatzimitakos, T.; Stalikas, C. Carbon-based nanomaterials functionalized with ionic liquids for microextraction in sample preparation. Separations 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Chow, A. X-ray fluorescence spectrometric determination of germanium after extraction with polyurethane foam. Anal. Chim. Acta 1990, 238, 423–426. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Medeiros, J.A.; Nóbrega, A.W.; Mantovano, J.L.; Rocha, V.P.A. Direct determination of gallium on polyurethane foam by X-ray fluorescence. Talanta 1995, 42, 45–47. [Google Scholar] [CrossRef]
- Santiago De Jesus, D.; Souza De Carvalho, M.; Spínola Costa, A.C.; Costa Ferreira, S.L. Quantitative separation of zinc traces from cadmium matrices by solid-phase extraction with polyurethane foam. Talanta 1998, 46, 1525–1530. [Google Scholar] [CrossRef]
- Lemos, V.A.; Santos, M.S.; Santos, E.S.; Santos, M.J.S.; dos Santos, W.N.L.; Souza, A.S.; de Jesus, D.S.; das Virgens, C.F.; Carvalho, M.S.; Oleszczuk, N.; et al. Application of polyurethane foam as a sorbent for trace metal pre-concentration-A review. Spectrochim. Acta-Part B At. Spectrosc. 2007, 62, 4–12. [Google Scholar] [CrossRef]
- Braun, T.; Navratil, J.D.; Farag, A.B. Polyurethane Foam Sorbents in Separation Science; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Soriano, S.; Cassella, R.J. Solid-phase extraction of Cu(II) using polyurethane foam and eriochrome black T as ligand for its determination in waters by flame atomic absorption spectrometry. J. Braz. Chem. Soc. 2013, 24, 1172–1179. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Melamine sponge decorated with copper sheets as a material with outstanding properties for microextraction of sulfonamides prior to their determination by high-performance liquid chromatography. J. Chromatogr. A 2018, 1554, 28–36. [Google Scholar] [CrossRef]
- García-Valverde, M.T.; Chatzimitakos, T.; Lucena, R.; Cárdenas, S.; Stalikas, C.D. Melamine sponge functionalized with urea-formaldehyde co-oligomers as a sorbent for the solid-phase extraction of hydrophobic analytes. Molecules 2018, 23, 2595. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Basheer, C.; Mansha, M. Membrane protected micro-solid-phase extraction of organochlorine pesticides in milk samples using zinc oxide incorporated carbon foam as sorbent. J. Chromatogr. A 2016, 1475, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, M.; Harner, T. Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 2002, 36, 4142–4151. [Google Scholar] [CrossRef]
- Strandberg, B.; Julander, A.; Sjöström, M.; Lewné, M.; Koca Akdeva, H.; Bigert, C. Evaluation of polyurethane foam passive air sampler (PUF) as a tool for occupational PAH measurements. Chemosphere 2018, 190, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Estellano, V.H.; Pozo, K.; Harner, T.; Corsolini, S.; Focardi, S. Using PUF disk passive samplers to simultaneously measure air concentrations of persistent organic pollutants (POPs) across the Tuscany region, Italy. Atmos. Pollut. Res. 2012, 3, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Jaward, F.M.; Farrar, N.J.; Harner, T.; Sweetman, A.J.; Jones, K.C. Passive air sampling of polycyclic aromatic hydrocarbons and polychlorinated naphthalenes across Europe. Environ. Toxicol. Chem. 2004, 23, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Motelay-Massei, A.; Harner, T.; Shoeib, M.; Diamond, M.; Stern, G.; Rosenberg, B. Using passive air samplers to assess urban-rural trends for persistent organic pollutants and polycyclic aromatic hydrocarbons. 2. Seasonal trends for PAHs, PCBs, and organochlorine pesticides. Environ. Sci. Technol. 2005, 39, 5763–5773. [Google Scholar] [CrossRef]
- Santiago, E.C.; Cayetano, M.G. Polycyclic aromatic hydrocarbons in ambient air in the Philippines derived from passive sampler with polyurethane foam disk. Atmos. Environ. 2007, 41, 4138–4147. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Absorption by polyurethane foams; new method of separation. J. Chem. Soc. A Inorg. Phys. Theor. Chem. 1970, 1082–1085. [Google Scholar] [CrossRef]
- Cassella, R.J. On-line solid phase extraction with polyurethane foam: Trace level spectrophotometric determination of iron in natural waters and biological materials. J. Environ. Monit. 2002, 4, 522–527. [Google Scholar] [CrossRef]
- de Almeida, G.N.; de Sousa, L.M.; Pereira Netto, A.D.; Cassella, R.J. Characterization of solid-phase extraction of Fe(III) by unloaded polyurethane foam as thiocyanate complex. J. Colloid Interface Sci. 2007, 315, 63–69. [Google Scholar] [CrossRef]
- Sant’Ana, O.D.; Jesuino, L.S.; Cassella, R.J.; Carvalho, M.S.; Santelli, R.E. Solid Phase Extraction of Cu(II) as Diethyldithiocarbamate (DDTC) Complex by Polyurethane Foam. J. Braz. Chem. Soc. 2003, 14, 728–733. [Google Scholar] [CrossRef]
- De Jesus, D.S.; Das Graças Korn, M.; Ferreira, S.L.C.; Carvalho, M.S. Separation method to overcome the interference of aluminum on zinc determination by inductively coupled plasma atomic emission spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 389–394. [Google Scholar] [CrossRef]
- De Carvalho, M.S.; Domingues, M.D.L.F.; Mantovano, J.L.; Da Cunha, J.W.S.D. Preconcentration method for the determination of thorium in natural water by wavelength dispersive X-ray fluorescence spectrometry. J. Radioanal. Nucl. Chem. 2002, 253, 253–256. [Google Scholar] [CrossRef]
- Trokhimenko, O.M.; Sukhan, V.V.; Nabivanets, B.I.; Ishchenko, V.B. Sorption preconcentration of thallium(I) on polyurethane foam modified with molybdophosphate. J. Anal. Chem. 2000, 55, 626–629. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Maria de Lourdes, F.D.; Mantovano, J.L.; Filho, E.Q.S. Uranium determination at ppb levels by X-ray fluorescence after its preconcentration on polyurethane foam. Spectrochim. Acta Part B At. Spectrosc. 1998, 53, 1945–1949. [Google Scholar] [CrossRef]
- Costa Ferreira, S.L.; Costa Dos Santos, H.; Santiago De Jesus, D. Molybdenum determination in iron matrices by ICP-AES after separation and preconcentration using polyurethane foam. Fresenius. J. Anal. Chem. 2001, 369, 187–190. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Dos Santos, H.C.; Campos, R.C. The determination of molybdenum in water and biological samples by graphite furnace atomic spectrometry after polyurethane foam column separation and preconcentration. Talanta 2003, 61, 789–795. [Google Scholar] [CrossRef]
- Abbas, M.N.D. Solid phase spectrophotometric determination of traces of arsenate and phosphate in water using polyurethane foam sorbent. Anal. Lett. 2003, 36, 1231–1244. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; De Jesus, D.S.; Cassella, R.J.; Costa, A.C.S.; De Carvalho, M.S.; Santelli, R.E. An on-line solid phase extraction system using polyurethane foam for the spectrophotometric determination of nickel in silicates and alloys. Anal. Chim. Acta 1999, 378, 287–292. [Google Scholar] [CrossRef]
- Xue, D.; Wang, H.; Liu, Y.; Shen, P.; Sun, J. Cytosine-functionalized polyurethane foam and its use as a sorbent for the determination of gold in geological samples. Anal. Methods 2016, 8, 29–39. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Almehdi, M. Qualitative, semi-quantitative and spectrophotometric determination of ruthenium(III) by solid-phase extraction with 3-hydroxy-2-methyl-1,4-naphthoquinone-4-oxime-loaded polyurethane foam columns. J. Chromatogr. A 1995, 697, 185–190. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.S.; Mostafa, G.A.E.; Hassanien, M.M. A new selective chromogenic reagent for the spectrophotometric determination of thallium(I) and (III) and its separation using flotation and the solid-phase extraction on polyurethane foam. Anal. Sci. 2003, 19, 1269–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpadjan, S.; Vuchkova, L.; Kostadinova, E. Sorption of arsenic, bismuth, mercury, antimony, selenium and tin on dithiocarbamate loaded polyurethane foam as a preconcentration method for their determination in water samples by simultaneous inductively coupled plasma atomic emission spectrometry and electrothermal atomic absorption spectrometry. Analyst 1997, 122, 243–246. [Google Scholar] [CrossRef]
- Maddalena, R.L.; McKone, T.E.; Kado, N.Y. Simple and rapid extraction of polycyclic aromatic hydrocarbons collected on polyurethane foam adsorbent. Atmos. Environ. 1998, 32, 2497–2503. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Gurariy, E.Y.; Nosov, R.E.; Zolotov, Y.A. Solid-phase extraction of polycyclic aromatic hydrocarbons from aqueous samples using polyurethane foams in connection with solid-matrix spectrofluorimetry. Anal. Lett. 2001, 34, 425–438. [Google Scholar] [CrossRef]
- Azevedo Lemos, V.; Alves De Jesus, A.; Moreira Gama, E.; Teixeira David, G.; Terumi Yamaki, R. On-line solid phase extraction and determination of copper in food samples using polyurethane foam loaded with Me-BTANC. Anal. Lett. 2005, 38, 683–696. [Google Scholar] [CrossRef]
- Maloney, M.P.; Moody, G.J.; Thomas, J.D.R. Extraction of metals from aqueous solution with polyurethane foam. Analyst 1980, 105, 1087–1097. [Google Scholar] [CrossRef]
- Hasany, S.M.; Saeed, M.M.; Ahmed, M. Adsorption isotherms and thermodynamic profile of Co(II)-SCN complex uptake on polyurethane foam. Sep. Sci. Technol. 2000, 35, 379–394. [Google Scholar] [CrossRef]
- de Raat, W.K.; Schulting, F.L.; Burghardt, E.; de Meijere, F.A. Application of polyurethane foam for sampling volatile mutagens from ambient air. Sci. Total Environ. 1987, 63, 175–189. [Google Scholar] [CrossRef]
- Vuchkova, L.; Arpadjan, S. Behaviour of the dithiocarbamate complexes of arsenic, antimony, bismuth, mercury, lead, tin and selenium in methanol with a hydride generator. Talanta 1996, 43, 479–486. [Google Scholar] [CrossRef]
- Zaranski, M.T.; Patton, G.W.; McConnell, L.L.; Bidleman, T.F.; Mulik, J.D. Collection of Nonpolar Organic Compounds from Ambient Air Using Polyurethane Foam-Granular Adsorbent Sandwich Cartridges. Anal. Chem. 1991, 63, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- McConnell, L.L.; Bidleman, T.F. Collection of two-ring aromatic hydrocarbons, chlorinated phenols, guaiacols, and benzenes from ambient air using polyurethane foam/Tenax-GC cartridges. Chemosphere 1998, 37, 885–898. [Google Scholar] [CrossRef]
- Alexandrova, A.; Arpadjan, S. Column solid phase extraction as preconcentration method for trace element determination in oxalic acid by atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 1995, 307, 71–77. [Google Scholar] [CrossRef]
- De Jesus, D.S.; Cassella, R.J.; Ferreira, S.L.C.; Costa, A.C.S.; De Carvalho, M.S.; Santelli, R.E. Polyurethane foam as a sorbent for continuous flow analysis: Preconcentration and spectrophotometric determination of zinc in biological materials. Anal. Chim. Acta 1998, 366, 263–269. [Google Scholar] [CrossRef]
- Lee, D.W.; Halmann, M. Selective Separation of Nickel(ll) by Dimethylglyoxime-Treated Polyurethane Foam. Anal. Chem. 1976, 48, 2214–2217. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Aldhaheri, S.M. Preconcentration and separation of acaricides by polyether based polyurethane foam. Anal. Chim. Acta 1996, 320, 277–287. [Google Scholar] [CrossRef]
- Hamon, R.F.; Chow, A. Extraction of cobalt from thiocyanate solutions with polyurethane foam. Talanta 1984, 31, 963–973. [Google Scholar] [CrossRef]
- Cassella, R.J.; Santelli, R.E.; Branco, A.G.; Lemos, V.A.; Ferreira, S.L.C.; De Carvalho, M.S. Selectivity enhancement in spectrophotometry: On-line interference suppression using polyurethane foam minicolumn for aluminum determination with Methyl Thymol Blue. Analyst 1999, 124, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Farag, A.B.; El-Shahawi, M.S. Removal of organic pollutants from aqueous solution. V. Comparative study of the extraction, recovery and chromatographic separation of some organic insectisides using unloaded polyurethane foam columns. J. Chromatogr. A 1991, 552, 371–379. [Google Scholar] [CrossRef]
- Khan, A.S.; Chow, A. Determination of arsenic by polyurethane foam extraction and X-ray fluorescence. Talanta 1984, 31, 304–306. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Krieger, M.S.; Miller, D.J. Supercritical Carbon Dioxide Extraction of Polychlorinated Biphenyls, Polycyclic Aromatic Hydrocarbons, Heteroatom-Containing Polycyclic Aromatic Hydrocarbons, and n-Alkanes from Polyurethane Foam Sorbents. Anal. Chem. 1989, 61, 736–740. [Google Scholar] [CrossRef]
- Hasany, S.M.; Saeed, M.M.; Ahmed, M. Sorption of traces of silver ions onto polyurethane foam from acidic solution. Talanta 2001, 54, 89–98. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Goncharova, L.V.; Zhigulev, A.V.; Nosov, R.E.; Kuzmin, N.M.; Zolotov, Y.A. Sorption-photometric determination of ascorbic acid using molybdosilicic heteropolyacid and polyurethane foam after microwave irradiation. Anal. Chim. Acta 1998, 373, 131–138. [Google Scholar] [CrossRef]
- Braun, T.; Abbas, M.N. Unloaded polyurethane foams as solid extractants for some metal thiocyanate complexes from aqueous solution. Anal. Chim. Acta 1982, 134, 321–326. [Google Scholar] [CrossRef]
- Makki, M.S.T.; Abdel-Rahman, R.M.; Alfooty, K.O.; El-Shahawi, M.S. Thiazolidinone steroids impregnated polyurethane foams as a solid phase extractant for the extraction and preconcentration of cadmium(II) from industrial wastewater. E-J. Chem. 2011, 8, 887–895. [Google Scholar] [CrossRef]
- El-sharief, F.M.; Asweisi, A.A.; Bader, N. Separation of Some Metal Ions Using β-Naphthol Modified Polyurethane Foam. Asian J. Nanosci. Mater. 2019. [Google Scholar] [CrossRef]
- El-Shahat, M.F.; Burham, N.; Azeem, S.M.A. Flow injection analysis-solid phase extraction (FIA-SPE) method for preconcentration and determination of trace amounts of penicillins using methylene blue grafted polyurethane foam. J. Hazard. Mater. 2010, 177, 1054–1060. [Google Scholar] [CrossRef]
- Maggira, M.; Deliyanni, E.A.; Samanidou, V.F. Synthesis of graphene oxide based sponges and their study as sorbents for sample preparation of cow milk prior to HPLC determination of sulfonamides. Molecules 2019, 24, 2086. [Google Scholar] [CrossRef] [Green Version]
- Ghani, M.; Maya, F.; Cerdà, V. Automated solid-phase extraction of organic pollutants using melamine-formaldehyde polymer-derived carbon foams. RSC Adv. 2016, 6, 48558–48565. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, M.; Yan, H.; Yuan, Y.; Tian, J. A new graphene oxide/polypyrrole foam material with pipette-tip solid-phase extraction for determination of three auxins in papaya juice. J. Chromatogr. A 2014, 1368, 37–43. [Google Scholar] [CrossRef]
- Qi, M.; Tu, C.; Li, Z.; Wang, W.; Chen, J.; Wang, A.J. Determination of Sulfonamide Residues in Honey and Milk by HPLC Coupled with Novel Graphene Oxide/Polypyrrole Foam Material-Pipette Tip Solid Phase Extraction. Food Anal. Methods 2018, 11, 2885–2896. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Du, T.; Kou, H.; Du, X.; Lu, X. Determination of six benzotriazole ultraviolet filters in water and cosmetic samples by graphene sponge-based solid-phase extraction followed by high-performance liquid chromatography. Anal. Bioanal. Chem. 2018, 410, 6955–6962. [Google Scholar] [CrossRef] [PubMed]
- Jillani, S.M.S.; Sajid, M.; Alhooshani, K. Evaluation of carbon foam as an adsorbent in stir-bar supported micro-solid-phase extraction coupled with gas chromatography–mass spectrometry for the determination of polyaromatic hydrocarbons in wastewater samples. Microchem. J. 2019, 144, 361–368. [Google Scholar] [CrossRef]
- Li, S.; Jia, M.; Guo, H.; Hou, X. Development and application of metal organic framework/chitosan foams based on ultrasound-assisted solid-phase extraction coupling to UPLC-MS/MS for the determination of five parabens in water. Anal. Bioanal. Chem. 2018, 410, 6619–6632. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Du, T.; Kou, H.; Du, X.; Lu, X. Application of ZIF-8-graphene oxide sponge to a solid phase extraction method for the analysis of sex hormones in milk and milk products by high-performance liquid chromatography. New J. Chem. 2019, 43, 2783–2789. [Google Scholar] [CrossRef]
- Sun, T.; Wang, M.; Wang, D.; Du, Z. Solid-phase microextraction based on nickel-foam@polydopamine followed by ion mobility spectrometry for on-site detection of Sudan dyes in tomato sauce and hot-pot sample. Talanta 2020, 207, 120244. [Google Scholar] [CrossRef]
- Hou, X.; Lu, X.; Niu, P.; Tang, S.; Wang, L.; Guo, Y. β-Cyclodextrin-modified three-dimensional graphene oxide-wrapped melamine foam for the solid-phase extraction of flavonoids. J. Sep. Sci. 2018, 41, 2207–2213. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Ma, Y.; Ji, W.; Chen, T.; Ma, X.; Xu, H. Functionalized melamine sponge based on β-cyclodextrin-graphene oxide as solid-phase extraction material for rapidly pre-enrichment of malachite green in seafood. Microchem. J. 2019, 150, 104167. [Google Scholar] [CrossRef]
- Qin, Z.; Jiang, Y.; Piao, H.; Li, J.; Tao, S.; Ma, P.; Wang, X.; Song, D.; Sun, Y. MIL-101(Cr)/MWCNTs-functionalized melamine sponges for solid-phase extraction of triazines from corn samples, and their subsequent determination by HPLC-MS/MS. Talanta 2020, 211, 120676. [Google Scholar] [CrossRef]
- Qi, L.; Gong, J. Facile in-situ polymerization of polyaniline-functionalized melamine sponge preparation for mass spectrometric monitoring of perfluorooctanoic acid and perfluorooctane sulfonate from biological samples. J. Chromatogr. A 2020, 1616, 460777. [Google Scholar] [CrossRef]
- Bagheri, H.; Zeinali, S.; Baktash, M.Y. A single–step synthesized supehydrophobic melamine formaldehyde foam for trace determination of volatile organic pollutants. J. Chromatogr. A 2017, 1525, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Frizzarin, R.M.; Maya, F.; Cerdà, V. In-syringe extraction using dissolvable layered double hydroxide-polymer sponges templated from hierarchically porous coordination polymers. J. Chromatogr. A 2016, 1453, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, P.; Huang, G.; Yan, X.; Li, X.F. Silica Monolith Nested in Sponge (SiMNS): A Composite Monolith as a New Solid Phase Extraction Material for Environmental Analysis. Anal. Chem. 2019, 91, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, A.; Soylak, M. Sea sponge as a low cost biosorbent for solid phase extraction of some heavy metal ions and determination by flame atomic absorption spectrometry. J. AOAC Int. 2014, 97, 1689–1695. [Google Scholar] [CrossRef]
- Karatepe, A.; Akalin, C.; Soylak, M. Solid-phase extraction of some food dyes on sea sponge column and determination by UV–vis spectrophotometer. Desalin. Water Treat. 2016, 57, 25822–25829. [Google Scholar] [CrossRef]
- Dai, L.; Sun, Z.; Zhou, P. Modification of luffa sponge for enrichment of phosphopeptides. Int. J. Mol. Sci. 2020, 21, 101. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.A.D.S.; Oliveira, J.D.A.N.; Siqueira, L.M.C.; Alves, V.N. Selective extraction and determination of chromium concentration using luffa cylindrica fibers as sorbent and detection by FAAS. J. Chem. 2019, 2019, 1679419. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, C.; Huang, X.; Jia, C.; Cao, Y.; Hu, L.; Lu, R.; Zhang, S.; Gao, H.; Zhou, W.; et al. Ionic liquid-modified luffa sponge fibers for dispersive solid-phase extraction of benzoylurea insecticides from water and tea beverage samples. New J. Chem. 2018, 42, 8791–8799. [Google Scholar] [CrossRef]

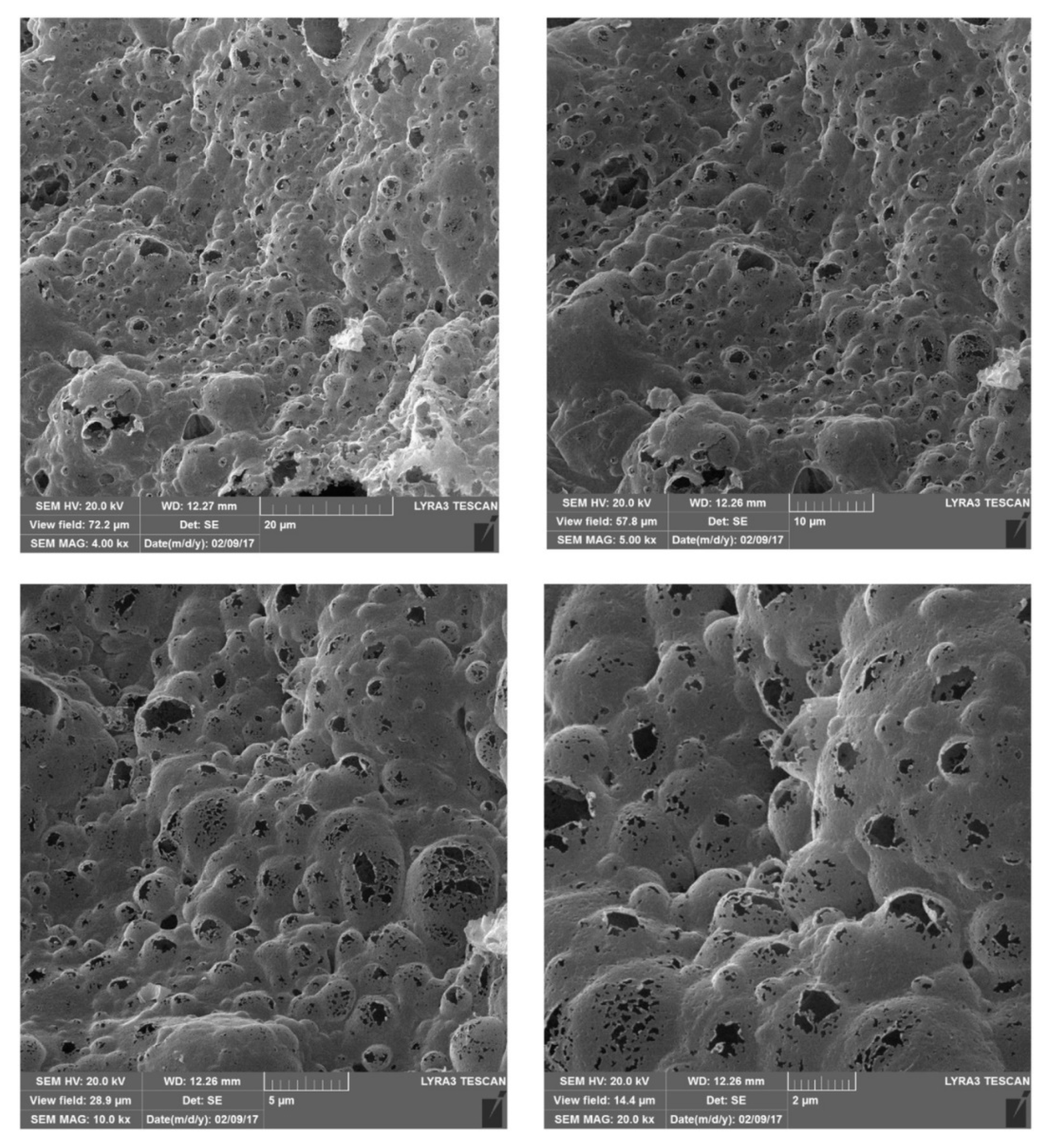

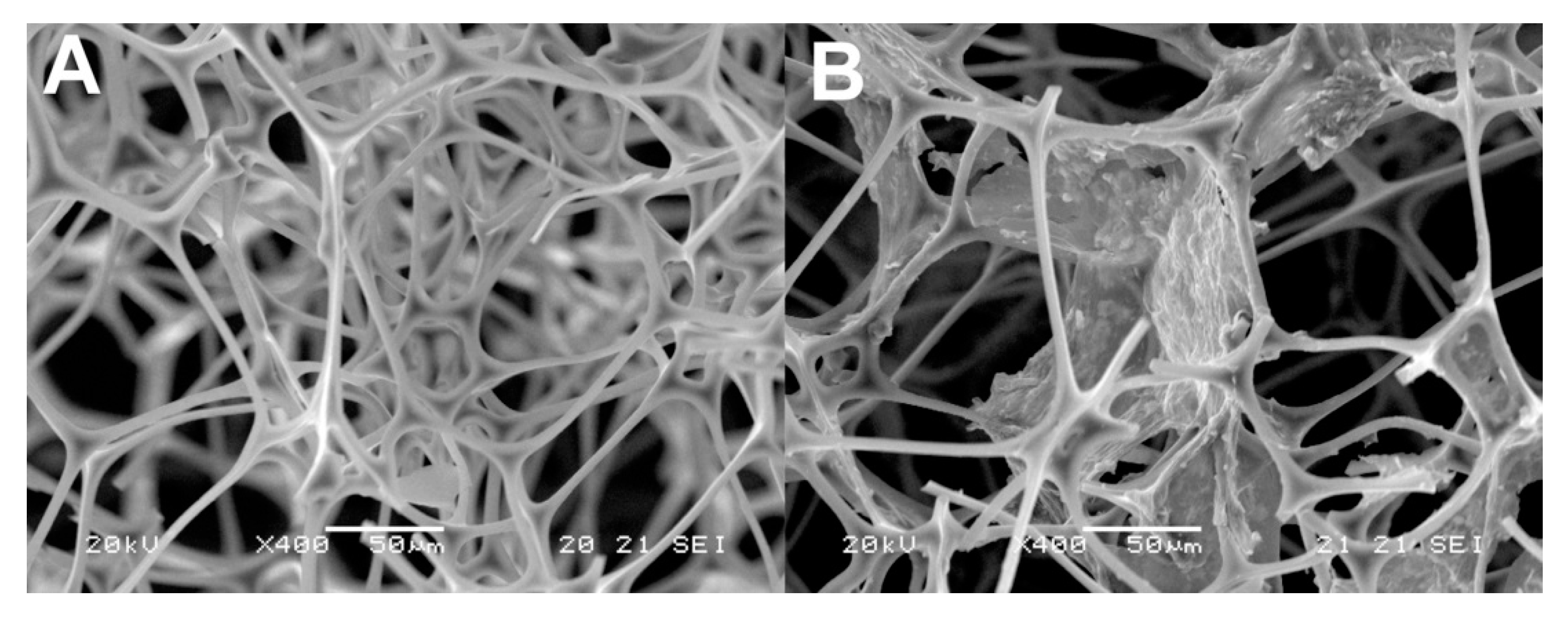

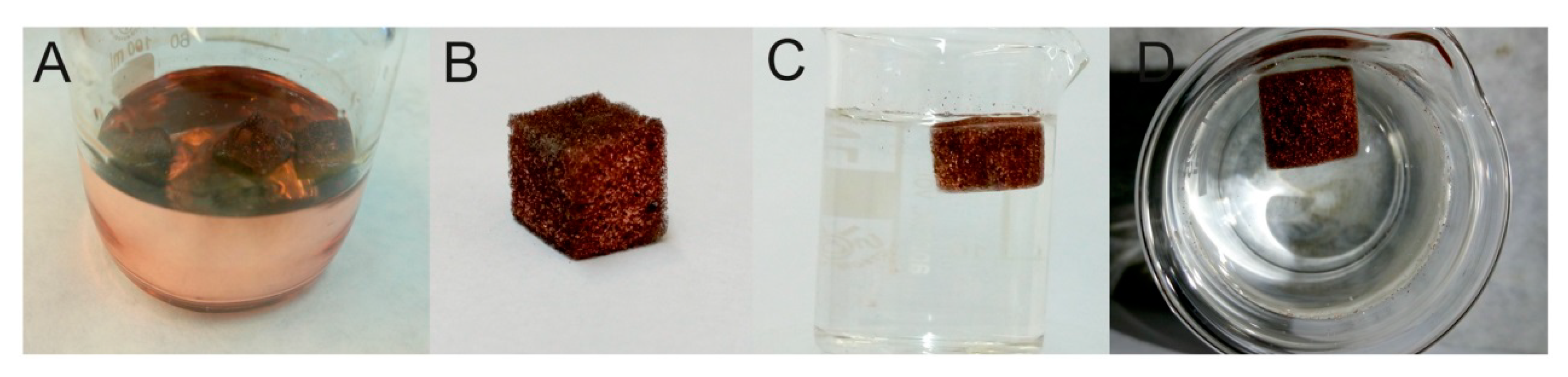
| Analyte | Ligand | Sample | Extraction Time (min) | Analytical Technique | LOD (Limit of Detection) μg·L−1 | Reference |
|---|---|---|---|---|---|---|
| Ge | Molybdate | Water | 60 | XRF | 70 | [4] |
| Zn2+ | Thiocyanate | Cadmium-rich matrices | 10 | SP | 20 | [6] |
| I, Hg, Au, Fe, Sb, Th, Mo, Re, U, benzene, chloroform, phenol | - | Water | 30 | SP | - | [20] |
| Fe3+ | Thiocyanate | Water and rice flour | 50 | SP | 450 | [21] |
| Fe3+ | Thiocyanate | Water | 5 | SP | - | [22] |
| Cu2+ | Diethyl dithiocarbamate | Water | 40 | ETAAS | - | [23] |
| Zn2+ | Thiocyanate | Aluminum matrices | 10 | ICP-AES | 0.02 | [24] |
| Th | 2-Ethylhexylphosphonic acid | Water | 30 | XRF | 4.0 | [25] |
| U | Salicylate | Water | 50 | XRF | 5.5 | [27] |
| Mo | Thiocyanate | Steel and pure iron | 10 | ICP-AES | 0.9 | [28] |
| Mo | Thiocyanate | Water, peach, apple, and citrus leaves | - | ETAAS | 0.08 | [29] |
| AsO43− and PO43− | Molybdate | Water | - | SP | 5.4 and 1.66 | [30] |
| Ni | Thiocyanate | Silicates and alloys | - | SP | 77 | [31] |
| Ru3+ | 3-Hydroxy-2-methyl-l,4-naphthoquinonexime | Water | 3–5 | SP | 20 | [33] |
| TI1+ and TI3+ | 9, 10 Phenathaquinone monomethyl thlio semicarbazone | Water and lead solutions | 30 | SP | 2.76 | [34] |
| As, Bi, Hg, Sb, Se, Sn, | Dithiocarbamate | Water | - | ETAAS | 0.06–0.3 | [35] |
| PAHs | - | Diesel exhaust | GC/MS | - | [36] | |
| PAHs | - | Water | 30 | Solid-matrix spectrofluorimetry | 0.02 | [37] |
| Cu2+ | - | Dried shrimp | SP | 1.2 | [38] | |
| Co, Fe, Zn, Cd, Ni, Hg | Thiocyanates (for Co, Fe, Zn), 1-(2-pyridylazo)-2-naphthol (for Ni, Hg, Cd) | Water | 180 | SP | - | [39] |
| Co2+ | thiocyanate | Water | 5 | SP | - | [40] |
| PAHs | - | ambient air | 960 | GC/MS | - | [41] |
| As, Bi, Pb, Sb, Sn, Se, Hg | Dithiocarbamate | Waste Water and seawater | 60 | ICP-AES | 0.03–30 | [42] |
| chlorobenzenes | ambient air | 1320 | GC/MS | - | [43] | |
| Two-ring aromatic hydrocarbons, chlorinated phenols, guaiacols, and benzenes | - | Ambient air | 720 | GC/electron capture detection | 200–500 | [44] |
| Cd, Co, Cu, Hg, Ni, and Pb | Hexamethylene ammonium hexamethylene dithiocarbamate | Oxalic acid | - | AAS or ICP-AES | 0.1–0.3 | [45] |
| Zn | Thiocyanate | Water | 5 | SP | 0.9 | [46] |
| Ni2+ | Dimethylglyoxime | Water | 15 | SP | 0.5 | [47] |
| acaricides | - | Water | 10 | SP | - | [48] |
| Co2+ | Thiocyanate | Water | 2 | Gamma-spectrometer | - | [49] |
| Al | Thiocyanate | Rhyolite, syenite, andesite, basalt, iron ore | 30 | SP | 30 | [50] |
| Dimethote, azodrine, lannate | - | Water | 10 | SP | - | [51] |
| As | - | Water | 60 | XRF | 36 | [52] |
| Polychlorinated biphenyls, PAHs, and n-alkanes | - | Diesel exhaust, cigarette smoke, and roofing tar volatiles | <20 | GC/MS | - | [53] |
| Ag | - | Water | 20 | SP | - | [54] |
| Ascorbic acid | Molybdosilicic heteropolyacid | Fruit juices and pharmaceutical preparations | 6.5 | SP | 0.6–40 | [55] |
| Zn, Hg, In | Thiocyanate | Water | 30 | [56] |
| Analyte | Functionalization Agent | Sample | Extraction Time (min) | Analytical Technique | LOD (Limit of Detection) μg·L−1 | Reference |
|---|---|---|---|---|---|---|
| Cu2+ | Eriochrome Black T | Water | 30 | Flame atomic absorption spectrometry | 20–100 | [9] |
| Cd2+ | 3-Sulfonamoyl-phenyl-spiro[4-oxo-thiazolidin-2,2′steroid] | Industrial wastewater | 20 | Flame atomic absorption spectrometry | 30–100 | [57] |
| Fe3+, Cu2+, Cr3+, Co2+, and Mn2+ | β-Naphthol | Water | Flame atomic absorption spectroscopy | [58] | ||
| Penicillin G, amoxicillin, and ampicillin | Methylene blue | Pharmaceuticals and milk | - | Flow injection analysis/solid-phase extraction | 12, 15, and 19 | [59] |
| Sulfathiazole, sulfamethizole, sulfadiazine, and sulfanilamide | Graphene oxide | Cow milk | 15 | HPLC-UV | 50 | [60] |
| Au3+ | Cytosine | Geological samples | - | Inductively coupled plasma optical emission spectrometry | 0.006 | [32] |
| Precursors for Carbon Foam | Analyte | Sample | Analytical Technique | LOD (μg·L−1) | Recoveries (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|
| Carbonization of melamine sponges | bisphenol A, 4-tert-octylphenol, and 4-n-nonylphenol | Well water, rainwater, and wastewater | Sequential injection analysis | 0.02–0.04 | >89.0 | 2.8–6.3 | [61] |
| Graphene oxide/polypyrrole | Sulfathiazole, sulfapyridine, sulfamethizole, sulfadoxine, sulfisoxazole, sulfamethoxazole, and sulfadimethoxine | Honey and milk | HPLC-UV | 0.00104–0.00150 | 62.3–109.0 | >11.2 | [63] |
| Graphene oxide/polypyrrole | Indole-3-butyric acid, indole-3-propionic acid, and 1-naphthaleneacetic acid | Papaya juice | HPLC-UV | 0.0012–0.0017 | 89.4–105.6 | <3 | [62] |
| Graphene oxide | Organic UV filters (UV-P, UV-234, UV-326, UV-327, UV-328, and UV-329) | Water and cosmetic products | HPLC-UV | 0.02–0.08 | 89–105 | <8.1 | [64] |
| Zinc nitrate and sucrose | Naphthalene, biphenyl, acenaphthene, fluorene, and phenanthrene | Wastewater | GC/MS | 0.29–8.4 | 91.8–102 | 3.8–10.9 | [65] |
| Sorbent | Analyte | Sample | Analytical Technique | LOD (μg·L−1) | Recoveries (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|
| Zinc oxide-incorporated carbon foam | Organochlorine pesticides | Milk | GC/MS | 0.19–1.64 | 85.1–100.7 | 2.3–10.2 | [13] |
| Metal organic framework/chitosan foams | Parabens | Water | UPLC–MS/MS | 0.09–0.45 | 78.75–102.1 | <7.4 | [66] |
| Zeolitic imidazolate framework-8@graphene oxide sponge | Sex hormones | Milk and milk products | HPLC | 520–2110 | 83.8–108.4 | <0.39 | [67] |
| Nickel foam functionalized with polydopamine | Sudan dyes | Tomato sauce and hotpot sample | Ion mobility spectrometry | 0.005–0.25 | 81%–91.3 | <15.5 | [68] |
| Functionalization Moieties | Analyte | Sample | Analytical Technique | LOD (μg·L−1) | Recoveries (%) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|
| β-Cyclodextrin/graphene oxide | Flavonoids | Lycium barbarum | HPLC | 0.5–2 | 77.9–102.6 | 3.5–6.8 | [69] |
| Polyvinylidene difluoride-MIL-101(Cr)/MWCNTs- | Triazines | Corn | HPLC–MS/MS | 0.01–0.04 | 90.3–116.5 | 1.08–12.32 | [71] |
| Graphene | Sulfonamides | Milk, eggs, and lake water | HPLC-UV | 0.03–0.44 | 90–108 | <10.1 | [10] |
| Copper sheets | Sulfonamides | Milk and lake water | HPLC-UV | 0.075–0.35 and 0.009–0.019 | 88–97 and 89–102 | 6.8–9.9 | [11] |
| Urea–formaldehyde co-oligomers | Fenbufen, flurbiprofen, benzophenone-8, butylparaben, cumylphenol, 4-octylphenol, chlorpyrifos, trifluralin, deltamethrin, tonalide | Lake water | HPLC-UV | 0.01–0.33 | 92–100 | 5.6–8.4 | [12] |
| β-Cyclodextrin and graphene oxide | Malachite green | Crayfish and squid | HPLC-Vis | 0.21 | 88.6–100.8 | - | [70] |
| Polyaniline | Perfluorooctanoic acid and perfluorooctane sulfonate | Human serum and urine | HPLC/MS | - | 79–91 | 5.5–8.2 | [72] |
| Trichloromethylsilane | Benzene, toluene, ethylbenzene, m-xylene, and o-xylene | Hookah, gulf water, and petrochemical wastewater | GC/MS | 0.005–0.0010 | 91–105 | <13 | [73] |
| Ni–Co layered double hydroxides | Gallic acid, p-hydroxybenzoic acid, and caffeic acid | Fruit juices | HPLC-UV | 0.15–0.35 | 89.7–95.3 | <10 | [74] |
| Silica monolith | Dipeptides (Tyr–Gly, Phe–Gly, Tyr–Val, Tyr–Ala, 3-I-Tyr–Ala, 3,5-dI-Tyr–Ala) | Water | HPLC–MS/MS | 0.00002−0.0013 | 100 | 2–3 | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimitakos, T.G.; Stalikas, C.D. Sponges and Sponge-Like Materials in Sample Preparation: A Journey from Past to Present and into the Future. Molecules 2020, 25, 3673. https://doi.org/10.3390/molecules25163673
Chatzimitakos TG, Stalikas CD. Sponges and Sponge-Like Materials in Sample Preparation: A Journey from Past to Present and into the Future. Molecules. 2020; 25(16):3673. https://doi.org/10.3390/molecules25163673
Chicago/Turabian StyleChatzimitakos, Theodoros G., and Constantine D. Stalikas. 2020. "Sponges and Sponge-Like Materials in Sample Preparation: A Journey from Past to Present and into the Future" Molecules 25, no. 16: 3673. https://doi.org/10.3390/molecules25163673







