Advances in the Synthesis of Ferrierite Zeolite
Abstract
:1. Introduction
2. The Routes for the Synthesis of Aluminosilicate FER Zeolite
3. Synthesis of Aluminosilicate FER Zeolite with the Use of the Different Organic Templates
4. Organotemplate-Free Synthesis of Aluminosilicate FER Zeolite
5. Morphology Control Strategies of Aluminosilicate FER Zeolite
6. Creation of Intracrystalline Mesopores in Aluminosilicate FER Zeolite
7. Synthesis of Heteroatomic FER Zeolite
8. Summary and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Davis, M.E. Ordered Porous Materials for Emerging Applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent Advances of Pore System Construction in Zeolite-Catalyzed Chemical Industry Processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Martínez, C.; Corma, A. Synthesis Strategies for Preparing Useful Small Pore Zeolites and Zeotypes for Gas Separations and Catalysis. Chem. Mater. 2014, 26, 246–258. [Google Scholar] [CrossRef]
- Xiao, F.-S.; Meng, X. (Eds.) Green chemistry and sustainable technology. In Zeolites in Sustainable Chemistry: Synthesis, Characterization and Catalytic Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Davis, M.E.; Lobo, R.F. Zeolite and Molecular Sieve Synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Chu, W.; Chen, F.; Guo, C.; Li, X.; Zhu, X.; Gao, Y.; Xie, S.; Liu, S.; Jiang, N.; Xu, L. Synthesis of FER Zeolite with Piperidine as Structure-Directing Agent and Its Catalytic Application. Chin. J. Catal. 2017, 38, 1880–1887. [Google Scholar] [CrossRef]
- Margarit, V.J.; Díaz-Rey, M.R.; Navarro, M.T.; Martínez, C.; Corma, A. Direct Synthesis of Nano-Ferrierite along the 10-Ring-Channel Direction Boosts Their Catalytic Behavior. Angew. Chem. 2018, 130, 3517–3521. [Google Scholar] [CrossRef]
- Lim, J.B.; Cha, S.H.; Hong, S.B. Direct N2O Decomposition over Iron-Substituted Small-Pore Zeolites with Different Pore Topologies. Appl. Catal. B Environ. 2019, 243, 750–759. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Sulikowski, B.; Janas, J.; Haber, J.; Kubacka, A.; Wloch, E.; Olejniczak, Z. The Synergetic Effect of Cobalt and Indium in Ferrierite Catalysts for Selective Catalytic Reduction of Nitric Oxide with Methane. Chem. Commun. 1998, 2755–2756. [Google Scholar] [CrossRef]
- Park, S.Y.; Shin, C.-H.; Bae, J.W. Selective Carbonylation of Dimethyl Ether to Methyl Acetate on Ferrierite. Catal. Commun. 2016, 75, 28–31. [Google Scholar] [CrossRef]
- Bolshakov, A.; van de Poll, R.; van Bergen-Brenkman, T.; Wiedemann, S.C.C.; Kosinov, N.; Hensen, E.J.M. Hierarchically Porous FER Zeolite Obtained via FAU Transformation for Fatty Acid Isomerization. Appl. Catal. B Environ. 2020, 263, 118356. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, H.-G.; Lee, K.-Y.; Cho, S.J. Improved Methanol-to-Olefin Reaction Selectivity and Catalyst Life by CeO2 Coating of Ferrierite Zeolite. Microporous Mesoporous. Mater. 2018, 256, 155–164. [Google Scholar] [CrossRef]
- Arora, S.S.; Shi, Z.; Bhan, A. Mechanistic Basis for Effects of High-Pressure H2 Cofeeds on Methanol-to-Hydrocarbons Catalysis over Zeolites. ACS Catal. 2019, 9, 6407–6414. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME Hydrogenation Reaction: New Evidences of a Superior Behaviour of FER-Based Hybrid Systems to Obtain High DME Yield. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Catizzone, E.; Daele, S.V.; Bianco, M.; Di Michele, A.; Aloise, A.; Migliori, M.; Valtchev, V.; Giordano, G. Catalytic Application of Ferrierite Nanocrystals in Vapour-Phase Dehydration of Methanol to Dimethyl Ether. Appl. Catal. B Environ. 2019, 243, 273–282. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Chu, W.; Zhao, D.; Chen, F.; Zhu, X.; Li, X.; Liu, S.; Xie, S.; Xu, L. Synthesis and Catalytic Application of FER Zeolites with Controllable Size. J. Mater. Chem. A 2019, 7, 7573–7580. [Google Scholar] [CrossRef]
- Xu, H.; Yu, Y.; Zhu, L.; Bian, C.; Zhai, H.; Tong, J.; Wu, H.; Shen, C. Preparation of Aluminosilicate Ferrierite Zeolite Nanosheets with Controllable Thickness in the Presence of a Sole Organic Structure Directing Agent. Molecules 2020, 25, 771. [Google Scholar] [CrossRef] [Green Version]
- Gies, H.; Gunawardane, R.P. One-Step Synthesis, Properties and Crystal Structure of Aluminium-Free Ferrierite. Zeolites 1987, 7, 442–445. [Google Scholar] [CrossRef]
- Plank, C.J.; Rosinski, E.J. Crystalline Zeolite and Method of Preparing Same. U.S. Patent 4016245, 5 April 1977. [Google Scholar]
- Zhang, H.; Guo, Q.; Ren, L.; Yang, C.; Zhu, L.; Meng, X.; Li, C.; Xiao, F.-S. Organotemplate-Free Synthesis of High-Silica Ferrierite Zeolite Induced by CDO-Structure Zeolite Building Units. J. Mater. Chem. 2011, 21, 9494. [Google Scholar] [CrossRef]
- Kuperman, A. Non-Aqueous Synthesis of Giant Crystals of Zeolites and Molecular Sieves. Nature 1993, 365, 239–242. [Google Scholar] [CrossRef]
- He, M.; Zhang, J.; Liu, R.; Sun, X.; Chen, B. The Distribution and Strength of Brönsted Acid Sites on the Multi-Aluminum Model of FER Zeolite: A Theoretical Study. Catalysts 2017, 7, 11. [Google Scholar] [CrossRef]
- Liu, B.; García-Pérez, E.; Dubbeldam, D.; Smit, B.; Calero, S. Understanding Aluminum Location and Non-Framework Ions Effects on Alkane Adsorption in Aluminosilicates: A Molecular Simulation Study. J. Phys. Chem. C 2007, 111, 10419–10426. [Google Scholar] [CrossRef]
- Hawkins, D.B. Zeolite Studies, I. Synthesis of Some Alkaline Earth Zeolites. Mater. Res. Bull. 1967, 2, 951–958. [Google Scholar] [CrossRef]
- Guo, G.; Long, Y.; Sun, Y. Synthesis of FER Type Zeolite with Tetrahydrofuran as the Template. Chem. Commun. 2000, 1893–1894. [Google Scholar] [CrossRef]
- Du, H.; Qiu, S.; Pang, W. Syntheses of Ferrierite and Mordenite Zeolites from Dried Gel in Alcoholic System. Chem. Res. Chin. Univ. 1995, 11, 364–366. [Google Scholar]
- Matsukata, M.; Nishiyama, N.; Ueyama, K. Crystallization of FER and MFI Zeolites by a Vapor-Phase Transport Method. Microporous Mater. 1996, 7, 109–117. [Google Scholar] [CrossRef]
- Pál-Borbély, G.; Beyer, H.K.; Kiyozumi, Y.; Mizukami, F. Synthesis and Characterization of a Ferrierite Made by Recrystallization of an Aluminium-Containing Hydrated Magadiite. Microporous Mesoporous Mater. 1998, 22, 57–68. [Google Scholar] [CrossRef]
- Pan, R.; Jia, M.; Li, Y.; Li, X.; Dou, T. In Situ Delamination of Ferrierite Zeolite and Its Performance in the Catalytic Cracking of C4 Hydrocarbons. Chin. J. Chem. Eng. 2014, 22, 1237–1242. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, X.; Wang, Y.; Chu, W.; Xie, S.; Yang, Z.; Liu, X.; Li, X.; Xu, L. Rapid Synthesis of Ferrierite Zeolite through Microwave Assisted Organic Template Free Route. Microporous Mesoporous Mater. 2019, 279, 220–227. [Google Scholar] [CrossRef]
- Kibby, C. Composition and Catalytic Properties of Synthetic Ferrierite. J. Catal. 1974, 35, 256–272. [Google Scholar] [CrossRef]
- Borade, R.B.; Clearfield, A. Synthesis of ZSM-35 Using Trimethylcetylammonium Hydroxide as a Template. Zeolites 1994, 14, 458–461. [Google Scholar] [CrossRef]
- Forbes, N.R.; Rees, L.V.C. The Synthesis of Ferrierite, ZSM-5, and Theta-1 in the Presence of Diethanolamine: Experimental. Zeolites 1995, 15, 444–451. [Google Scholar] [CrossRef]
- Xu, W.-Q.; Yin, Y.-G.; Suib, S.L.; Edwards, J.C.; OYoung, C.-L. n-Butene Skeletal Isomerization to Isobutylene on Shape Selective Catalysts: Ferrierite/ZSM-35. J. Phys. Chem. 1995, 99, 9443–9451. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, J.; Guo, J.; Sun, J.; Long, Y. High-Silica Ferrierite Zeolite Self-Transformed from Aluminosilicate Gel. ChemPhysChem 2006, 7, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Pál-Borbély, G.; Szegedi, Á.; Beyer, H.K. Solid-State Recrystallization of Aluminum-Containing Kanemite Varieties to Ferrierite. Microporous Mesoporous Mater. 2000, 35–36, 573–584. [Google Scholar] [CrossRef]
- Schreyeck, L.; Caullet, P.; Mougenel, J.C.; Guth, J.L.; Marler, B. PREFER: A New Layered (Alumino) Silicate Precursor of FER-Type Zeolite. Microporous Mater. 1996, 6, 259–271. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, J.; Xu, H.; Liu, Y.; Peng, H.; Wu, P. Selective Skeletal Isomerization of 1-Butene over FER-Type Zeolites Derived from PLS-3 Lamellar Precursors. Appl. Catal. A Gen. 2013, 455, 107–113. [Google Scholar] [CrossRef]
- Gálvez, P.; Bernardo-Maestro, B.; Vos, E.; Díaz, I.; López-Arbeloa, F.; Pérez-Pariente, J.; Gómez-Hortigüela, L. ICP-2: A New Hybrid Organo-Inorganic Ferrierite Precursor with Expanded Layers Stabilized by π–π Stacking Interactions. J. Phys. Chem. C 2017, 121, 24114–24127. [Google Scholar] [CrossRef] [Green Version]
- Roth, W.J.; Gil, B.; Makowski, W.; Sławek, A.; Grzybek, J.; Kubu, M.; Čejka, J. Interconversion of the CDO Layered Precursor ZSM-55 between FER and CDO Frameworks by Controlled Deswelling and Reassembly. Chem. Mater. 2016, 28, 3616–3619. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.-S. Green Routes for Synthesis of Zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Qi, G.; Guo, Q.; Pan, S.; Meng, X.; Xu, J.; Deng, F.; Fan, F.; Feng, Z.; et al. Sustainable Synthesis of Zeolites without Addition of Both Organotemplates and Solvents. J. Am. Chem. Soc. 2014, 136, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wu, Q.; Chen, F.; Xiao, F.-S. Solvent-Free Synthesis of Zeolite Catalysts. Sci. China Chem. 2015, 58, 6–13. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Wang, S.; Meng, X.; Xiao, F.-S. 110th Anniversary: Sustainable Synthesis of Zeolites: From Fundamental Research to Industrial Production. Ind. Eng. Chem. Res. 2019, 58, 11653–11658. [Google Scholar] [CrossRef]
- Chu, P.; Dwyer, F.G.; Vartuli, J.C. Crystallization Method Employing Microwave Radiation. U.S. Patent 4778666, 18 October 1988. [Google Scholar]
- Nasser, G.A.; Muraza, O.; Nishitoba, T.; Malaibari, Z.; Yamani, Z.H.; Al-Shammari, T.K.; Yokoi, T. Microwave-Assisted Hydrothermal Synthesis of CHA Zeolite for Methanol-to-Olefins Reaction. Ind. Eng. Chem. Res. 2019, 58, 60–68. [Google Scholar] [CrossRef]
- Shalmani, F.M.; Askari, S.; Halladj, R. Microwave Synthesis of SAPO Molecular Sieves. Rev. Chem. Eng. 2013, 29. [Google Scholar] [CrossRef]
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P.A. Synthesis of Inorganic Solids Using Microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar] [CrossRef]
- Chen, X.; Todorova, T.; Vimont, A.; Ruaux, V.; Qin, Z.; Gilson, J.-P.; Valtchev, V. In Situ and Post-Synthesis Control of Physicochemical Properties of FER-Type Crystals. Microporous Mesoporous Mater. 2014, 200, 334–342. [Google Scholar] [CrossRef]
- Ahedi, R.K.; Kotasthane, A.N. Studies in the Crystallization of Ferrierite (FER) Type Zeolites in Presence of Promoting Medium. J. Porous Mater. 1997, 9. [Google Scholar] [CrossRef]
- Ahedi, R.K.; Kotasthane, A.N.; Rao, B.S.; Manna, A.; Kulkarni, B.D. Synthesis of Ferrierite-Type Zeolite in the Presence of a Catalytic Amount of Pyrrolidine and Sodium Bis(2-Ethyhlhexyl) Sulfosuccinate. J. Colloid Interface Sci. 2001, 236, 47–51. [Google Scholar] [CrossRef]
- Khomane, R.B.; Kulkarni, B.D.; Ahedi, R.K. Synthesis and Characterization of Ferrierite-Type Zeolite in the Presence of Nonionic Surfactants. J. Colloid Interface Sci. 2001, 236, 208–213. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Zhang, Y.; Lv, T.; Zheng, J.; Gao, W.; Liu, X.; Cui, M.; Meng, C. Study on the Synthesis of MFI and FER in the Presence of N-Butylamine and the Property of n-Butylamine in a Confined Region of Zeolites. RSC Adv. 2016, 6, 114808–114817. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Mineva, T.; van Daele, S.; Valtchev, V.; Giordano, G. New Synthesis Routes and Catalytic Applications of Ferrierite Crystals. Part 1: 1,8-Diaminooctane as a New OSDA. Microporous Mesoporous Mater. 2020, 296, 109987. [Google Scholar] [CrossRef]
- Shen, K.; Huang, X.; Wang, J.; Chen, Z.; Yu, Y.; Tang, Z.; Liu, Y. Synthesis of FER/MFI Composite Zeolite Using Isopropylamine as a Structure-Directing Agent. Microporous Mesoporous Mater. 2020, 297, 110027. [Google Scholar] [CrossRef]
- Smith, W.J.; Dewing, J.; Dwyer, J. Zeolite Synthesis in the SiO2-Al2O3-Na2O-Pyridine-H2O System. J. Chem. Soc. Faraday Trans. 1989, 85, 3623–3628. [Google Scholar] [CrossRef]
- Asensi, M.A.; Martínez, A. Selective Isomerization of n-Butenes to Isobutene on High Si/Al Ratio Ferrierite in the Absence of Coke Deposits: Implications on the Reaction Mechanism. Appl. Catal. A Gen. 1999, 183, 155–165. [Google Scholar] [CrossRef]
- Jongkind, H.; Datema, K.P.; Nabuurs, S.; Seive, A.; Stork, W.H.J. Synthesis and Characterisation of Zeolites Using Saturated Cyclic Amines as Structure-Directing Agents. Microporous Mater. 1997, 10, 149–161. [Google Scholar] [CrossRef]
- Qian, B.; Guo, G.; Wang, X.; Zeng, Y.; Sun, Y.; Long, Y. Templating of High-Silica Zeolites by Tetrahydrofuran in the Na2O–SiO2–Al2O3–H2O System. Phys. Chem. Chem. Phys. 2001, 3, 4164–4169. [Google Scholar] [CrossRef]
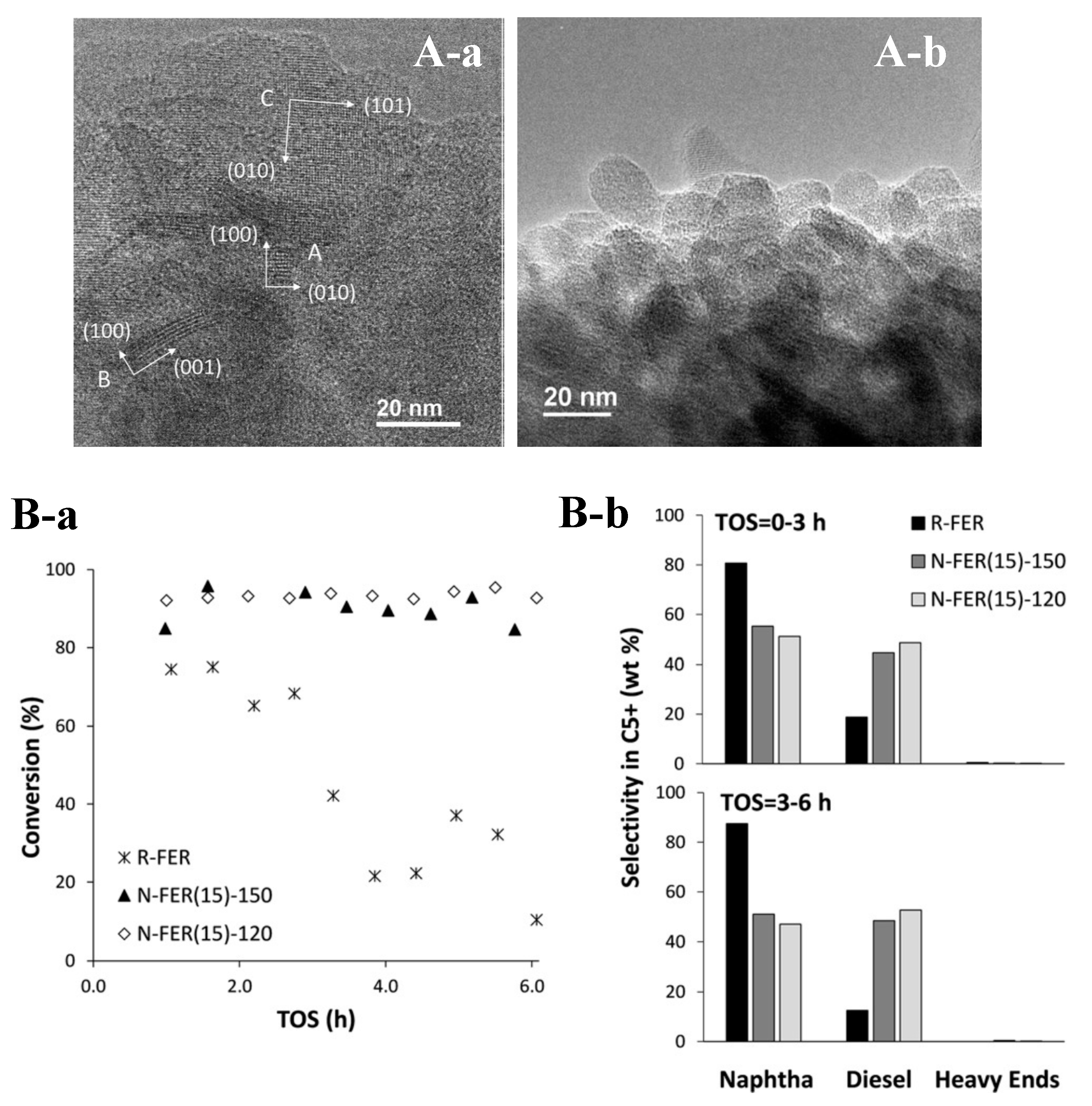
- Xu, H.; Chen, W.; Zhang, G.; Wei, P.; Wu, Q.; Zhu, L.; Meng, X.; Li, X.; Fei, J.; Han, S.; et al. Ultrathin Nanosheets of Aluminosilicate FER Zeolites Synthesized in the Presence of a Sole Small Organic Ammonium. J. Mater. Chem. A 2019, 7, 16671–16676. [Google Scholar] [CrossRef]
- Vinaches, P.; Bernardo-Gusmão, K.; Pergher, S. An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules 2017, 22, 1307. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Deimund, M.A.; Xie, D.; Davis, M.E. Synthesis of RTH-Type Zeolites Using a Diverse Library of Imidazolium Cations. Chem. Mater. 2015, 27, 3756–3762. [Google Scholar] [CrossRef]
- Candamano, S.; Frontera, P.; Kora´nyi, T.I.; Macario, A.; Crea, F.; Nagy, J.B. Characterization of (Fe, Al) FER Synthesized in Presence of Ethylene Glycol and Ethylene Diamine. Microporous Mesoporous Mater. 2010, 127, 9–16. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, M.; Zhang, Y.; Zheng, J.; Lv, T.; Gao, W.; Liu, X.; Meng, C. Study on the Synthesis of FER and SOD in the Presence of Ethylene Glycol and the Oxidation Transformation of Ethylene Glycol in a Confined Region of Zeolites. J. Alloys Compd. 2017, 696, 788–794. [Google Scholar] [CrossRef]
- Pinar, A.B.; Gomez-Hortiguela, L.; Perez-Pariente, J. Cooperative Structure Directing Role of the Cage-Forming Tetramethylammonium Cation and the Bulkier Benzylmethylpyrrolidinium in the Synthesis of Zeolite Ferrierite. Chem. Mater. 2007, 19, 5617–5626. [Google Scholar] [CrossRef]
- Kim, S.H.; Komarneni, S.; Heo, N.H. ZSM-5 and Ferrierite Single Crystals with Lower Si/Al Ratios: Synthesis and Single-Crystal Synchrotron X-ray Diffraction Studies. Microporous Mesoporous Mater. 2011, 143, 243–248. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Moliner, M.; Davis, M.E. Impact of Controlling the Site Distribution of Al Atoms on Catalytic Properties in Ferrierite-Type Zeolites. J. Phys. Chem. C 2011, 115, 1096–1102. [Google Scholar] [CrossRef]
- Almeida, R.K.S.; Gómez-Hortigüela, L.; Pinar, A.B.; Peréz-Pariente, J. Synthesis of Ferrierite by a New Combination of Co-Structure-Directing Agents: 1,6-Bis(n-methylpyrrolidinium)hexane and Tetramethylammonium. Microporous Mesoporous Mater. 2016, 232, 218–226. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Thogersen, J.R.; Corma, A. Efficient One-Pot Preparation of Cu-SSZ-13 Materials Using Cooperative OSDAs for Their Catalytic Application in the SCR of NOx. ChemCatChem 2013, 5, 3316–3323. [Google Scholar] [CrossRef]
- Rima, D.; Djamal, D.; Fatiha, D. Synthesis of High Silica Zeolites Using a Combination of Pyrrolidine and Tetramethylammonium as Template. Mater. Res. Express 2018, 6, 035017. [Google Scholar] [CrossRef]
- Jiang, J.; Jorda, J.L.; Yu, J.; Baumes, L.A.; Mugnaioli, E.; Diaz-Cabanas, M.J.; Kolb, U.; Corma, A. Synthesis and Structure Determination of the Hierarchical Meso-Microporous Zeolite ITQ-43. Science 2011, 333, 1131–1134. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable Single-Unit-Cell Nanosheets of Zeolite MFI as Active and Long-Lived Catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Zhu, X.; Hofmann, J.P.; Mezari, B.; Kosinov, N.; Wu, L.; Qian, Q.; Weckhuysen, B.M.; Asahina, S.; Ruiz-Martínez, J.; Hensen, E.J.M. Trimodal Porous Hierarchical SSZ-13 Zeolite with Improved Catalytic Performance in the Methanol-to-Olefins Reaction. ACS Catal. 2016, 6, 2163–2177. [Google Scholar] [CrossRef]
- Xue, T.; Liu, H.; Wang, Y.M. Synthesis of Hierarchical Ferrierite Using Piperidine and Tetramethylammonium Hydroxide as Cooperative Structure-Directing Agents. RSC Adv. 2015, 5, 12131–12138. [Google Scholar] [CrossRef]
- Ng, E.-P.; Chateigner, D.; Bein, T.; Valtchev, V.; Mintova, S. Capturing Ultrasmall EMT Zeolite from Template-Free Systems. Science 2012, 335, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, S.; Zones, S.I.; Iglesia, E. Synthesis of Zeolites via Interzeolite Transformations without Organic Structure-Directing Agents. Chem. Mater. 2015, 27, 2056–2066. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.-P.; Retoux, R.; Boullay, P.; Goupil, J.-M.; Valtchev, V.; Mintova, S. Template-Free Nanosized Faujasite-Type Zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Song, J.; Ren, L.; Ji, Y.; Li, J.; Xiao, F.-S. Organotemplate-Free and Fast Route for Synthesizing Beta Zeolite. Chem. Mater. 2008, 20, 4533–4535. [Google Scholar] [CrossRef]
- Rakoczy, R.A.; Breuninger, M.; Hunger, M.; Traa, Y.; Weitkamp, J. Template-Free Synthesis of Zeolite Ferrierite and Characterization of Its Acid Sites. Chem. Eng. Technol. 2002, 25, 273–275. [Google Scholar] [CrossRef]
- Suzuki, Y.; Wakihara, T.; Itabashi, K.; Ogura, M.; Okubo, T. Cooperative Effect of Sodium and Potassium Cations on Synthesis of Ferrierite. Top. Catal. 2009, 52, 67–74. [Google Scholar] [CrossRef]
- Itabashi, K.; Kamimura, Y.; Iyoki, K.; Shimojima, A.; Okubo, T. A Working Hypothesis for Broadening Framework Types of Zeolites in Seed-Assisted Synthesis without Organic Structure-Directing Agent. J. Am. Chem. Soc. 2012, 134, 11542–11549. [Google Scholar] [CrossRef]
- Jo, D.; Ryu, T.; Park, G.T.; Kim, P.S.; Kim, C.H.; Nam, I.-S.; Hong, S.B. Synthesis of High-Silica LTA and UFI Zeolites and NH3–SCR Performance of Their Copper-Exchanged Form. ACS Catal. 2016, 6, 2443–2447. [Google Scholar] [CrossRef]
- Boal, B.W.; Schmidt, J.E.; Deimund, M.A.; Deem, M.W.; Henling, L.M.; Brand, S.K.; Zones, S.I.; Davis, M.E. Facile Synthesis and Catalysis of Pure-Silica and Heteroatom LTA. Chem. Mater. 2015, 27, 7774–7779. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Oumi, Y.; Itabashi, K.; Lu, B.; Teranishi, T.; Sano, T. Direct Hydrothermal Synthesis and Stabilization of High-Silica Mordenite (Si:Al = 25) Using Tetraethylammonium and Fluoride Ions. J. Mater. Chem. 2003, 13, 1173–1179. [Google Scholar] [CrossRef]
- Sonoda, T.; Maruo, T.; Yamasaki, Y.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Synthesis of High-Silica AEI Zeolites with Enhanced Thermal Stability by Hydrothermal Conversion of FAU Zeolites, and Their Activity in the Selective Catalytic Reduction of NOx with NH3. J. Mater. Chem. A 2015, 3, 857–865. [Google Scholar] [CrossRef]
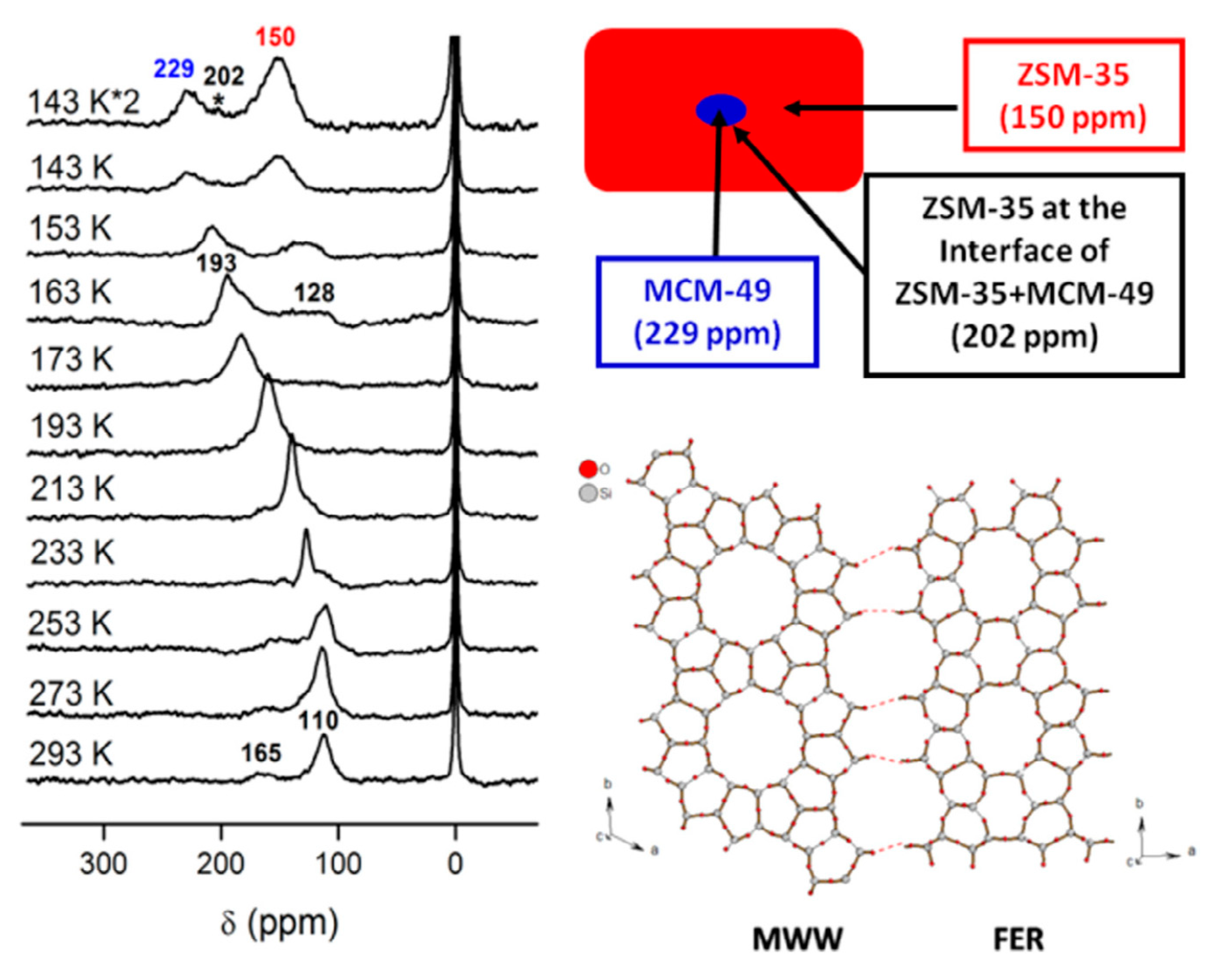
- Wang, L.; Tian, P.; Yuan, Y.; Yang, M.; Fan, D.; Zhou, H.; Zhu, W.; Xu, S.; Liu, Z. Seed-Assisted Synthesis of High Silica ZSM-35 through Interface-Induced Growth over MCM-49 Seeds. Microporous Mesoporous Mater. 2014, 196, 89–96. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI Zeolite Nanosheets of a Single-Unit-Cell Thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef]
- Li, R.; Linares, N.; Sutjianto, J.G.; Chawla, A.; Garcia-Martinez, J.; Rimer, J.D. Ultrasmall Zeolite L Crystals Prepared from Highly Interdispersed Alkali-Silicate Precursors. Angew. Chem. Int. Ed. 2018, 57, 11283–11288. [Google Scholar] [CrossRef] [Green Version]
- Valtchev, V.; Tosheva, L. Porous Nanosized Particles: Preparation, Properties, and Applications. Chem. Rev. 2013, 113, 6734–6760. [Google Scholar] [CrossRef]
- Park, W.; Yu, D.; Na, K.; Jelfs, K.E.; Slater, B.; Sakamoto, Y.; Ryoo, R. Hierarchically Structure-Directing Effect of Multi-Ammonium Surfactants for the Generation of MFI Zeolite Nanosheets. Chem. Mater. 2011, 23, 5131–5137. [Google Scholar] [CrossRef]
- Emdadi, L.; Wu, Y.; Zhu, G.; Chang, C.-C.; Fan, W.; Pham, T.; Lobo, R.F.; Liu, D. Dual Template Synthesis of Meso- and Microporous MFI Zeolite Nanosheet Assemblies with Tailored Activity in Catalytic Reactions. Chem. Mater. 2014, 26, 1345–1355. [Google Scholar] [CrossRef]
- Wuamprakhon, P.; Wattanakit, C.; Warakulwit, C.; Yutthalekha, T.; Wannapakdee, W.; Ittisanronnachai, S.; Limtrakul, J. Direct Synthesis of Hierarchical Ferrierite Nanosheet Assemblies via an Organosilane Template Approach and Determination of Their Catalytic Activity. Microporous Mesoporous Mater. 2016, 219, 1–9. [Google Scholar] [CrossRef]
- Lee, Y.; Park, M.B.; Kim, P.S.; Vicente, A.; Fernandez, C.; Nam, I.-S.; Hong, S.B. Synthesis and Catalytic Behavior of Ferrierite Zeolite Nanoneedles. ACS Catal. 2013, 3, 617–621. [Google Scholar] [CrossRef]
- Marthala, V.R.R.; Hunger, M.; Kettner, F.; Krautscheid, H.; Chmelik, C.; Kärger, J.; Weitkamp, J. Solvothermal Synthesis and Characterization of Large-Crystal All-Silica, Aluminum-, and Boron-Containing Ferrierite Zeolites. Chem. Mater. 2011, 23, 2521–2528. [Google Scholar] [CrossRef]
- Meng, X.; Nawaz, F.; Xiao, F.-S. Templating Route for Synthesizing Mesoporous Zeolites with Improved Catalytic Properties. Nano Today 2009, 4, 292–301. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; van der Made, A.; Yang, C.; Pan, S.; Wang, L.; Zhu, L.; Jin, Y.; et al. Highly Mesoporous Single-Crystalline Zeolite Beta Synthesized Using a Nonsurfactant Cationic Polymer as a Dual-Function Template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.N.; Wei, F.; Yang, J.Y.; Lin, N.; Lin, W.G.; Wang, Y.; Zhu, J.H. New Strategy to Synthesis of Hierarchical Mesoporous Zeolites. Chem. Mater. 2010, 22, 2442–2450. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Aloise, A.; Lamberti, R.; Giordano, G. Hierarchical Low Si/Al Ratio Ferrierite Zeolite by Sequential Postsynthesis Treatment: Catalytic Assessment in Dehydration Reaction of Methanol. J. Chem. 2019, 2019, 3084356. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Cacciaguerra, T.; Minoux, D.; Dath, J.-P.; Fajula, F.; Gérardin, C. Generation of Parallelepiped-Shaped Mesopores and Structure Transformation in Highly Stable Ferrierite Zeolite Crystals by Framework Desilication in NaOH Solution. Microporous Mesoporous Mater. 2018, 260, 132–145. [Google Scholar] [CrossRef]
- Khitev Yu, P.; Kolyagin Yu, G.; Ivanova, I.I.; Ponomareva, O.A.; Thibault-Starzyk, F.; Gilson, J.-P.; Fernandez, C.; Fajula, F. Synthesis and Catalytic Properties of Hierarchical Micro/Mesoporous Materials Based on FER Zeolite. Microporous Mesoporous Mater. 2011, 146, 201–207. [Google Scholar] [CrossRef]
- Pan, S.; Wu, Q.; Wang, X.; Chen, F.; Meng, X.; Xiao, F.-S. Mesoporous EU-1 Zeolite Synthesized in the Presence of Cationic Polymer. Microporous Mesoporous Mater. 2016, 235, 246–252. [Google Scholar] [CrossRef]
- Xu, H.; Lei, C.; Wu, Q.; Zhu, Q.; Meng, X.; Dai, D.; Maurer, S.; Parvulescu, A.-N.; Müller, U.; Xiao, F. Organosilane Surfactant-Assisted Synthesis of Mesoporous SSZ-39 Zeolite with Enhanced Catalytic Performance in the Methanol-to-Olefins Reaction. Front. Chem. Sci. Eng. 2020, 14, 267–274. [Google Scholar] [CrossRef]
- Wu, L.; Degirmenci, V.; Magusin, P.C.M.M.; Szyja, B.M.; Hensen, E.J.M. Dual Template Synthesis of a Highly Mesoporous SSZ-13 Zeolite with Improved Stability in the Methanol-to-Olefins Reaction. Chem. Commun. 2012, 48, 9492. [Google Scholar] [CrossRef] [PubMed]
- Sulikowski, B.; Klinowski, J. Synthesis of a Novel Gallosilicate with the Ferrierite Structure. J. Chem. Soc. Chem. Commun. 1989, 1289. [Google Scholar] [CrossRef]
- Borade, R.B.; Clearfield, A. Synthesis of an Iron Silicate with the Ferrierite Structure. Chem. Commun. 1996, 2267. [Google Scholar] [CrossRef]
- Shevade, S.; Ahedi, R.K.; Kotasthane, A.N. Synthesis and Characterization of Ferrisilicate Analogs of Ferrierite (Fe-FER) Zeolites. Catal. Lett. 1997, 49, 69–75. [Google Scholar] [CrossRef]
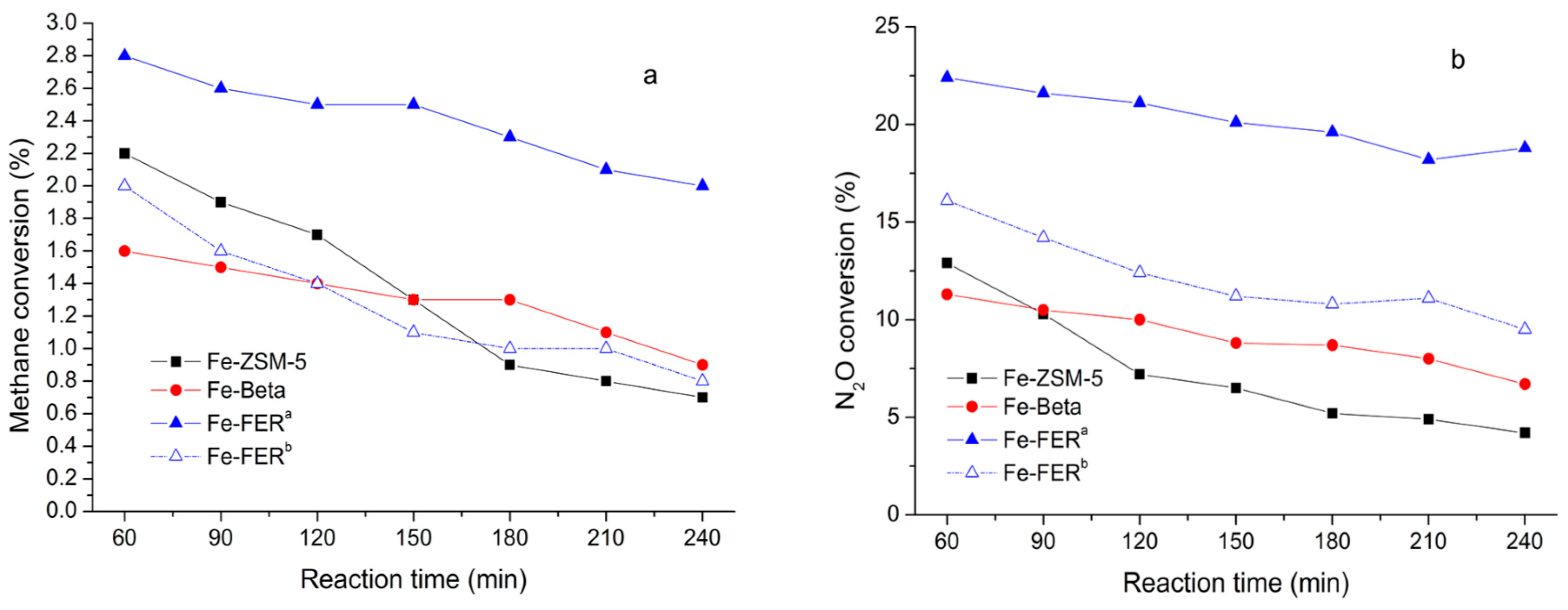
- Zhao, G.; Benhelal, E.; Adesina, A.; Kennedy, E.; Stockenhuber, M. Comparison of Direct, Selective Oxidation of Methane by N2O over Fe-ZSM-5, Fe-Beta, and Fe-FER Catalysts. J. Phys. Chem. C 2019, 123, 27436–27447. [Google Scholar] [CrossRef]
- Ahedi, R.K.; Kotasthane, A.N. Synthesis of FER Titanosilicates from a Non-Aqueous Alkali-Free Seeded System. J. Mater. Chem. 1998, 8, 1685–1686. [Google Scholar] [CrossRef]
- Shevade, S.S.; Rao, B.S. Synthesis of a Vanadium Analog of Siliceous FER. J. Mater. Chem. 1999, 9, 2459–2462. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Domine, M.E.; Fornés, V. Ti-Ferrierite and TiITQ-6: Synthesis and Catalytic Activity for the Epoxidation of Olefins with H2O2. Chem. Commun. 2000, 137–138. [Google Scholar] [CrossRef]
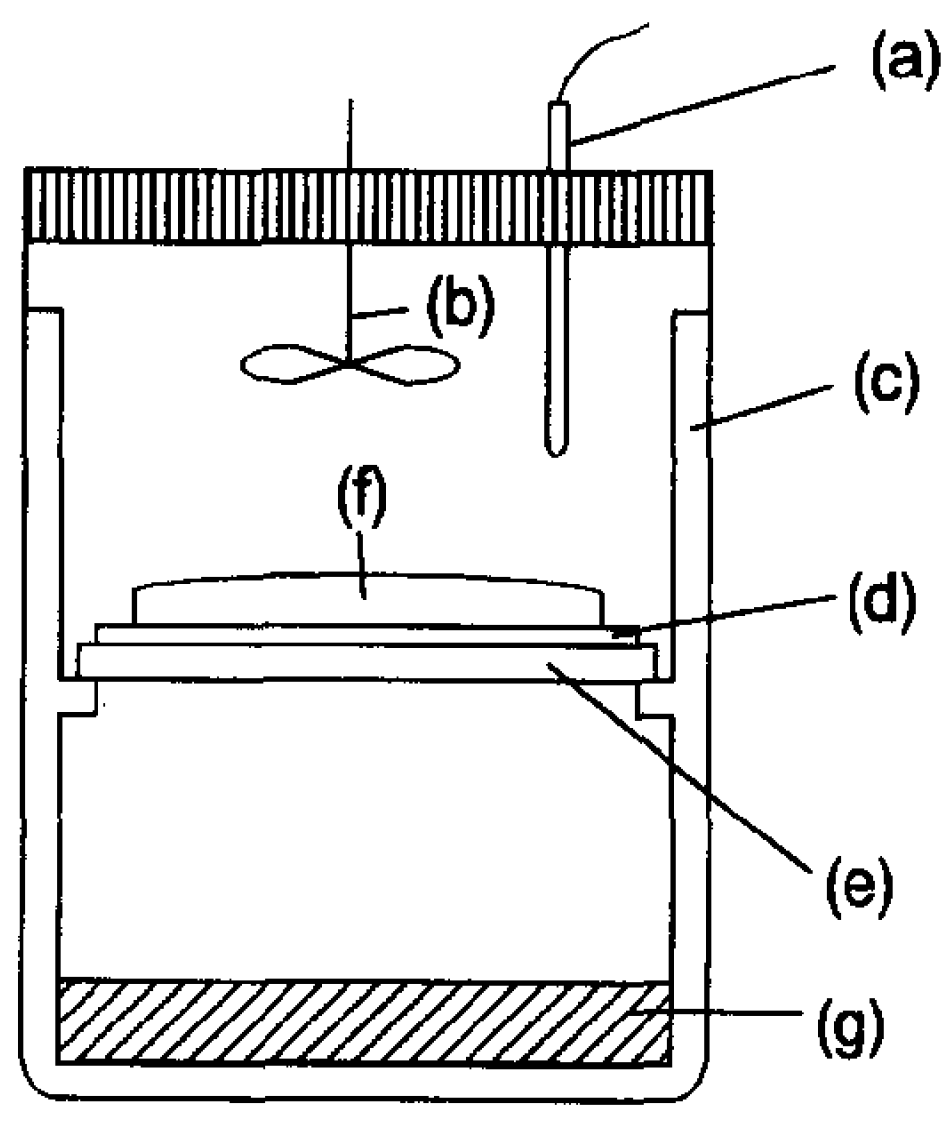
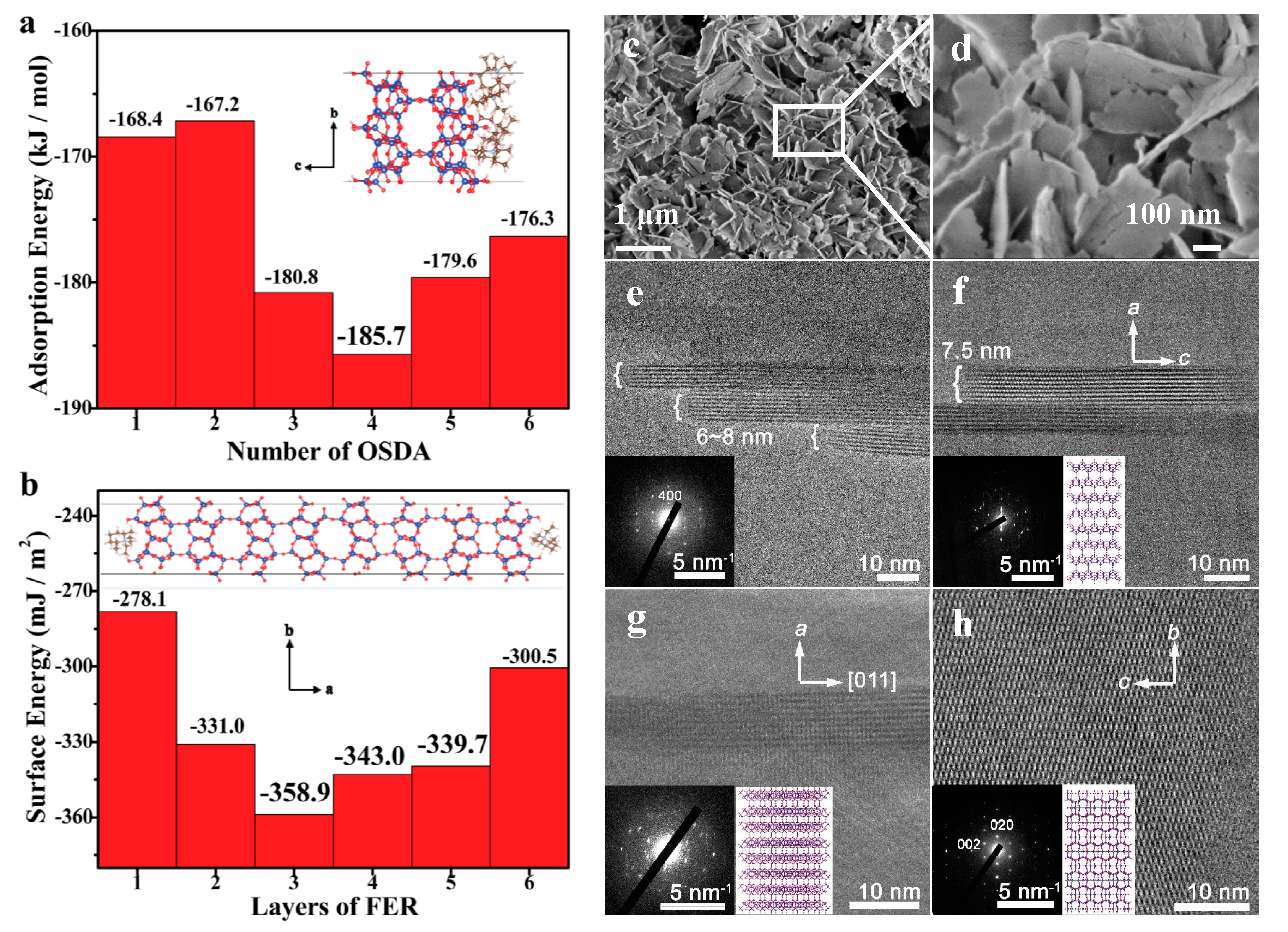
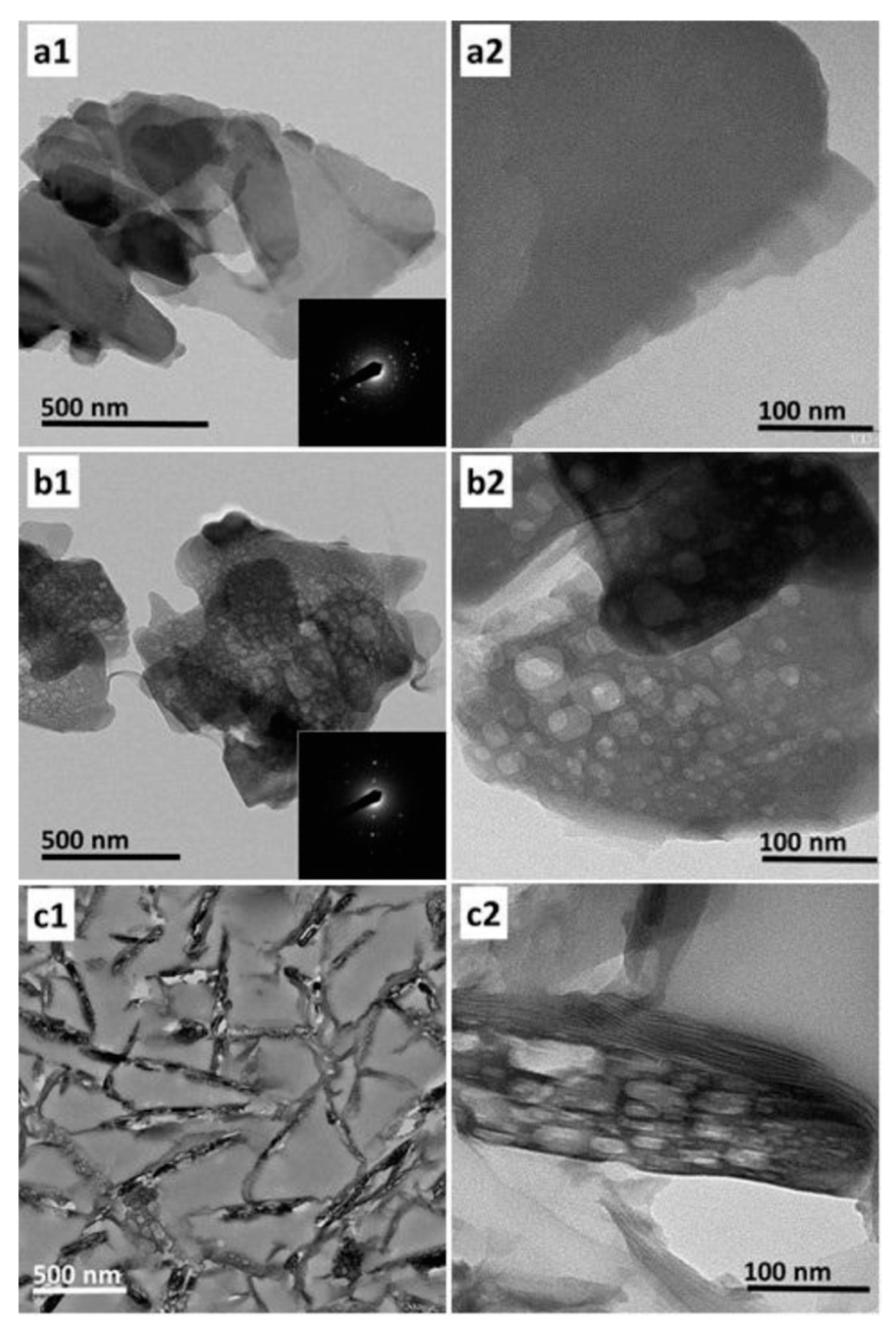
| Entry | Synthesis Routes | Features | Ref. |
|---|---|---|---|
| 1 | Hydrothermal synthesis | water as a solvent | [20,32,33,34,35] |
| 2 | Solvothermal synthesis | organic molecules as the solvents | [22,27] |
| 3 | Vapor-phase transport (VPT) | vapor containing a small amount of OSDA and water in the dry aluminosilicate gel | [28,36] |
| 4 | Transformation synthesis | recrystallization of zeolites with different topologies | [29,37] |
| 5 | Solid-transformation synthesis | low water content and relatively high OSDA content | [30] |
| 6 | Microwave-assisted synthesis | rapid synthesis with microwave as the energy source | [31] |
| 7 | Topotactic conversion | condensation of layered precursors | [38,39,40,41] |
| Entry | Organic Templates | Structures | Synthesis Method 1 | Ref |
|---|---|---|---|---|
| 1 | tetramethylammonium (TMA) |  | HT | [32] |
| 2 | ethylenediamine (EDA) |  | HT, VPT, SS | [30,34,50] |
| 3 | pyrrolidine (THP) | 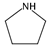 | HT | [35,50,51,52,53] |
| 4 | dibutylamine (DBA) | 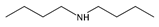 | ST | [27] |
| 5 | n-butylamine (n-BA) |  | HT | [54] |
| 6 | 1,8-diaminooctane (DAO) |  | HT | [55] |
| 7 | isopropylamine (IPA) |  | HT | [56] |
| 8 | pyridine (Py) | 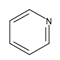 | HT | [57,58] |
| 9 | piperidine (Pi) | 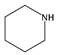 | HT, SS, | [6,29,37,59] |
| 10 | tetrahydrofuran (THF) |  | HT, VPT | [26,36,60] |
| 11 | N, N-diethyl-cis-2,6-dimethyl piperidinium (DMPi) | 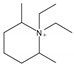 | HT | [18,61] |
| 12 | 1,3-dimethyimidazolium (DMI) |  | SS | [62,63] |
| 13 | ethylene glycol (EG) | 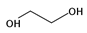 | HT | [64,65] |
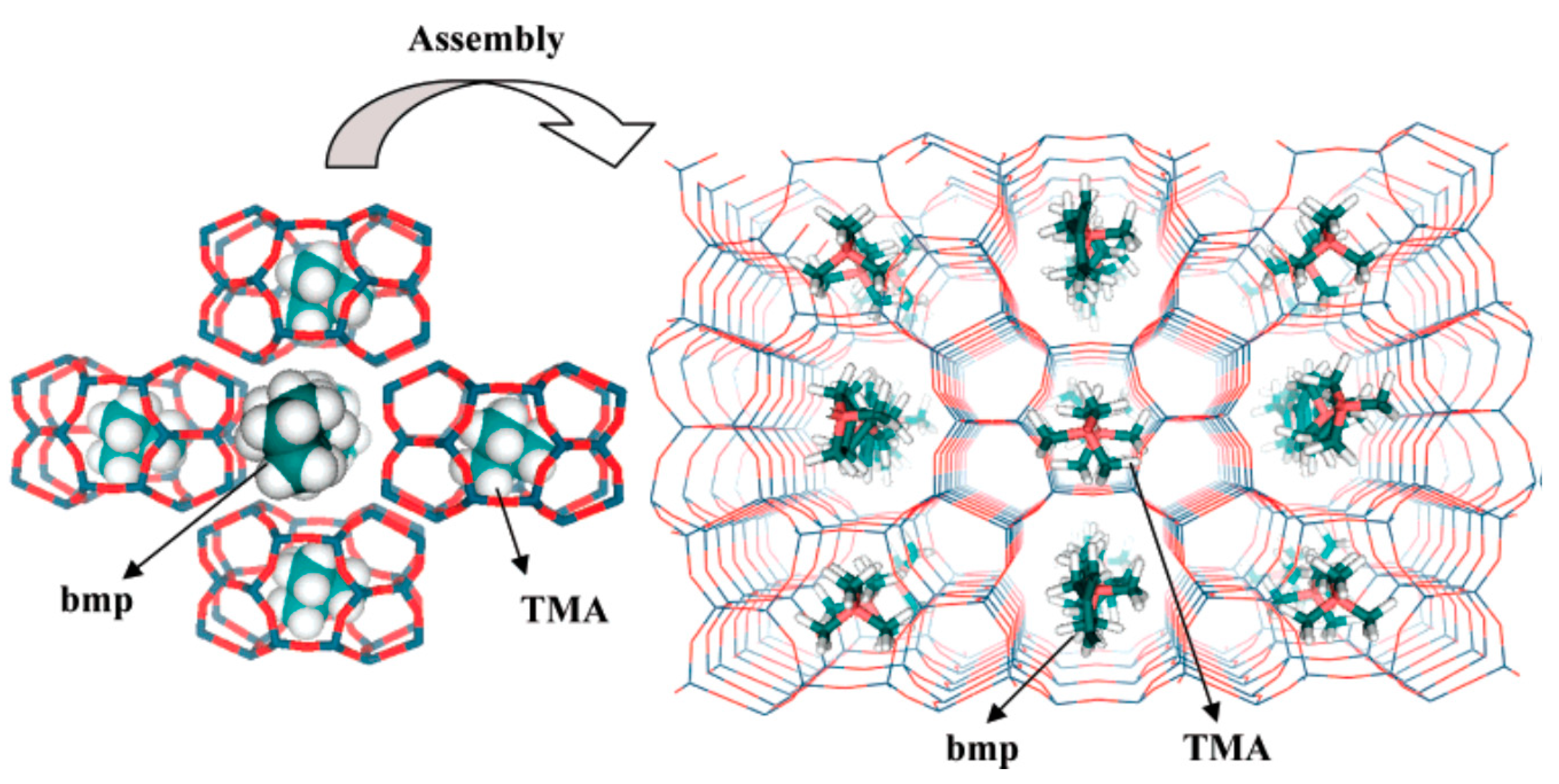
| 14 | benzylmethylpyrrolidinium (BMP) | 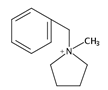 | HT (combined with TMA) | [66] |
| 15 | hexamethyleneimine (HMI) | 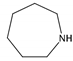 | HT (combined with TMA) | [68] |
| 16 | 1,4-diazabicyclo [2.2.2] octane (DAB) | 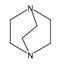 | HT (combined with TMA) | [68] |
| 17 | 1,6-bis (N-methylpyrrolidinium)hexane (MPH) |  | HT (combined with TMA) | [69] |
| Entry | Heteroatomic FER Zeolites | Si/Heteroatom Ratio | Ref. |
|---|---|---|---|
| 1 | B-FER | Not mentioned | [19] |
| 2 | Si, Ga-FER | Si/Ga = 6.6 | [107] |
| 3 | Fe-FER | Si/Fe = 16 | [8] |
| Si/Fe = 11 | [108] | ||
| Si/Fe = 15–100 | [109] | ||
| Si/Fe = 38 | [110] | ||
| 4 | Ti-FER | Si/Ti = 83–182 | [111] |
| Not mentioned | [113] | ||
| 5 | V-FER | Si/V = 120, 180 | [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Zhu, J.; Zhu, L.; Zhou, E.; Shen, C. Advances in the Synthesis of Ferrierite Zeolite. Molecules 2020, 25, 3722. https://doi.org/10.3390/molecules25163722
Xu H, Zhu J, Zhu L, Zhou E, Shen C. Advances in the Synthesis of Ferrierite Zeolite. Molecules. 2020; 25(16):3722. https://doi.org/10.3390/molecules25163722
Chicago/Turabian StyleXu, Hao, Jie Zhu, Longfeng Zhu, Enmu Zhou, and Chao Shen. 2020. "Advances in the Synthesis of Ferrierite Zeolite" Molecules 25, no. 16: 3722. https://doi.org/10.3390/molecules25163722








