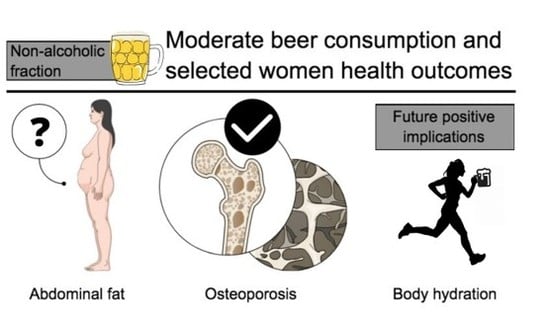
Effects of the Non-Alcoholic Fraction of Beer on Abdominal Fat, Osteoporosis, and Body Hydration in Women
Abstract
:1. Introduction
2. Beer Consumption Related to Health and Disease in Women
2.1. Beer, Abdominal Fat, and Weight Gain
2.2. Beer and Osteoporosis
2.3. Beer and Body Hydration
3. Implications and Future Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buiatti, S. Beer Composition: An Overview. In Beer in Health and Disease Prevention; Elsevier: London, UK, 2009; pp. 213–225. ISBN 9780123738912. [Google Scholar]
- Colen, L.; Swinnen, J. Economic growth, globalisation and beer consumption. J. Agric. Econ. 2016, 67, 186–207. [Google Scholar] [CrossRef]
- Handbook of Brewing, 2nd ed.; Stewart, G.G.; Priest, F.G. (Eds.) CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429116179. [Google Scholar]
- Tucker, K.L.; Jugdaohsingh, R.; Powell, J.J.; Qiao, N.; Hannan, M.T.; Sripanyakorn, S.; Cupples, L.A.; Kiel, D.P. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am. J. Clin. Nutr. 2009, 89, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, M. Consumption of beer and colorectal cancer incidence: A meta-analysis of observational studies. Cancer Causes Control 2015, 26, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Parent, M.E.; Siemiatycki, J. Consumption of alcoholic beverages and risk of lung cancer: Results from two case-control studies in Montreal, Canada. Cancer Causes Control 2006, 17, 469–480. [Google Scholar] [CrossRef]
- Chao, C. Associations between beer, wine, and liquor consumption and lung cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2436–2447. [Google Scholar] [CrossRef] [Green Version]
- Demoury, C.; Karakiewicz, P.; Parent, M.-E. Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada. Cancer Epidemiol. 2016, 45, 11–17. [Google Scholar] [CrossRef]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, K.; Lewis, L.B.; Nolen, J.D.L.; Kinney, G.L.; Sathya, B.; He, J. Alcohol consumption and risk of stroke: A meta-analysis. J. Am. Med. Assoc. 2003, 289, 579–588. [Google Scholar] [CrossRef]
- Mukamal, K.J. Alcohol, beer, and ischemic stroke. In Beer in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 623–634. ISBN 9780123738912. [Google Scholar]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: A meta-analysis. Eur. J. Epidemiol. 2011, 26, 833–850. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Rotondo, S.; Iacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef] [Green Version]
- Stachenfeld, N.S. Hormonal changes during menopause and the impact on fluid regulation. Reprod. Sci. 2014, 21, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, H.S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bainbridge, K.; Sowers, M.; Lin, X.; Harlow, S. Risk factors for low bone mineral density and the 6-year rate of bone loss among premenopausal and perimenopausal women. Osteoporos. Int. 2004, 15, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Sripanyakorn, S.; Jugdaohsingh, R.; Mander, A.; Davidson, S.L.; Thompson, R.P.H.; Powell, J.J. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J. Bone Miner. Res. 2009, 24, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [Green Version]
- Proestos, C.; Komaitis, M. Antioxidant Capacity of Hops; Elsevier Inc.: Amsterdam, The Netherlands, 2008; ISBN 9780123738912. [Google Scholar]
- Feick, P.; Gerloff, A.; Singer, M.V. The effect of beer and its non-alcoholic constituents on the exocrine and endocrine pancreas as well as on gastrointestinal hormones. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 587–601. ISBN 9780123738912. [Google Scholar]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—A review. Food Rev. Int. 2010, 26, 1–84. [Google Scholar] [CrossRef]
- Intelmann, D.; Haseleu, G.; Dunkel, A.; Lagemann, A.; Stephan, A.; Hofmann, T. Comprehensive sensomics analysis of hop-derived bitter compounds during storage of beer. J. Agric. Food Chem. 2011, 59, 1939–1953. [Google Scholar] [CrossRef]
- Rivero, D.; Pérez-Magariño, S.; González-Sanjosé, M.L.; Valls-Belles, V.; Codoñer, P.; Muñiz, P. Inhibition of induced DNA oxidative damage by beers: Correlation with the content of polyphenols and melanoidins. J. Agric. Food Chem. 2005, 53, 3637–3642. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; de la Torre, R. Beer phenolic composition of simple phenols, prenylated flavonoids and alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Česlová, L.; Holčapek, M.; Fidler, M.; Drštičková, J.; Lísa, M. Characterization of prenylflavonoids and hop bitter acids in various classes of Czech beers and hop extracts using high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar] [PubMed]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef] [Green Version]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, I.M.; Pohjanvirta, R. Estrogenic activities of food supplements and beers as assessed by a yeast bioreporter assay. J. Diet. Suppl. 2018, 15, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wannamethee, S.G. Beer and Adiposity; Elsevier Inc.: Amsterdam, The Netherlands, 2009; ISBN 9780123738912. [Google Scholar]
- Zugravu, C.-A.; Pătrașcu, D.; Otelea, M. Central obesity and beer consumption. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2019, 43, 110–124. [Google Scholar] [CrossRef]
- Ferreira, M.G.; Valente, J.G.; Gonçalves-Silva, R.M.V.; Sichieri, R. Alcohol consumption and abdominal fat in blood donors. Rev. Saude Publica 2008, 42, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Dorn, J.M.; Hovey, K.; Muti, P.; Freudenheim, J.L.; Russell, M.; Nochajski, T.H.; Trevisan, M. Alcohol drinking patterns differentially affect central adiposity as measured by abdominal height in women and men. J. Nutr. 2003, 133, 2655–2662. [Google Scholar] [CrossRef] [Green Version]
- Schütze, M.; Schulz, M.; Steffen, A.; Bergmann, M.M.; Kroke, A.; Lissner, L.; Boeing, H. Beer consumption and the “beer belly”: Scientific basis or common belief? Eur. J. Clin. Nutr. 2009, 63, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- Dallongeville, J.; Marécaux, N.; Ducimetière, P.; Ferrières, J.; Arveiler, D.; Bingham, A.; Ruidavets, J.; Simon, C.; Amouyel, P. Influence of alcohol consumption and various beverages on waist girth and waist-to-hip ratio in a sample of French men and women. Int. J. Obes. 1998, 22, 1178–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.-J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.B.; Carreau, A.-M.; Weisnagel, S.J.; Bergeron, J.; Labrie, F.; Lemieux, S.; Tchernof, A. Increased body fat mass explains the positive association between circulating estradiol and insulin resistance in postmenopausal women. Am. J. Physiol. Metab. 2018, 314, E448–E456. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Christensen, R.; Bartels, E.M.; Kok, F.J.; Sierksma, A.; Raben, A.; Astrup, A. Is beer consumption related to measures of abdominal and general obesity? A systematic review and meta-analysis. Nutr. Rev. 2013, 71, 67–87. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Schütze, M.; Steffen, A.; Boeing, H.; Halkjaer, J.; Tjonneland, A.; Travier, N.; Agudo, A.; Slimani, N.; Rinaldi, S.; et al. The association of lifetime alcohol use with measures of abdominal and general adiposity in a large-scale European cohort. Eur. J. Clin. Nutr. 2011, 65, 1079–1087. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Cabral, H.J.; Heeren, T.C.; Vasan, R.S.; Curtis Ellison, R. Alcohol consumption and the prevalence of the metabolic syndrome in the U.S.: A cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2954–2959. [Google Scholar] [CrossRef] [Green Version]
- Lapidus, L.; Bengtsson, C.; Hällström, T.; Björntorp, P. Obesity, adipose tissue distribution and health in women-Results from a population study in Gothenburg, Sweden. Appetite 1989, 13, 25–35. [Google Scholar] [CrossRef]
- Slattery, M.L.; McDonald, A.; Bild, D.E.; Caan, B.J.; Hilner, J.E.; Jacobs, D.R.; Liu, K. Associations of body fat and its distribution with dietary intake, physical activity, alcohol, and smoking in blacks and whites. Am. J. Clin. Nutr. 1992, 55, 943–949. [Google Scholar] [CrossRef]
- Kahn, H.S.; Tatham, L.M.; Heath, C.W. Contrasting factors associated with abdominal and peripheral weight gain among adult women. Int. J. Obes. 1997, 21, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Rosmond, R.; Björntorp, P. Psychosocial and socio-economic factors in women and their relationship to obesity and regional body fat distribution. Int. J. Obes. 1999, 23, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Machado, P.A.N.; Sichieri, R. Relação cintura-quadril e fatores de dieta em adultos. Rev. Saude Publica 2002, 36, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadstrup, E.; Petersen, L.; Sørensen, T.; Grønbaek, M. Waist circumference in relation to history of amount and type of alcohol: Results from the Copenhagen City Heart Study. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobak, M.; Skodova, Z.; Marmot, M. Beer and obesity: A cross-sectional study. Eur. J. Clin. Nutr. 2003, 57, 1250–1253. [Google Scholar] [CrossRef] [Green Version]
- Halkjær, J.; Sørensen, T.I.; Tjønneland, A.; Togo, P.; Holst, C.; Heitmann, B.L. Food and drinking patterns as predictors of 6-year BMI-adjusted changes in waist circumference. Br. J. Nutr. 2004, 92, 735–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halkjær, J.; Tjønneland, A.; Thomsen, B.L.; Overvad, K.; Sørensen, T.I.A. Intake of macronutrients as predictors of 5-y changes in waist circumference. Am. J. Clin. Nutr. 2006, 84, 789–797. [Google Scholar] [CrossRef]
- Deschamps, V.; Alamowitch, C.; Borys, J. Boissons alcooliques, poids et paramètres d’adiposité chez 520 adultes issus de l’étude Fleurbaix Laventie Ville Santé. Cah. Nutr. Diététique 2004, 39, 262–268. [Google Scholar] [CrossRef]
- Lukasiewicz, E.; Mennen, L.I.; Bertrais, S.; Arnault, N.; Preziosi, P.; Galan, P.; Hercberg, S. Alcohol intake in relation to body mass index and waist-to-hip ratio: The importance of type of alcoholic beverage. Public Health Nutr. 2005, 8, 315–320. [Google Scholar] [CrossRef]
- Krachler, B.; Eliasson, M.; Stenlund, H.; Johansson, I.; Hallmans, G.; Lindahl, B. Reported food intake and distribution of body fat: A repeated cross-sectional study. Nutr. J. 2006, 5, 1–11. [Google Scholar] [CrossRef]
- Tolstrup, J.S.; Halkjær, J.; Heitmann, B.L.; Tjønneland, A.M.; Overvad, K.; Sørensen, T.I.A.; Grønbæk, M.N. Alcohol drinking frequency in relation to subsequent changes in waist circumference. Am. J. Clin. Nutr. 2008, 87, 957–963. [Google Scholar] [CrossRef]
- Molina-Hidalgo, C.; De-Lao, A.; Jurado-Fasoli, L.; Amaro-Gahete, F.J.; Castillo, M.J. Beer or ethanol effects on the body composition response to high-intensity interval training. The BEER-HIIT study. Nutrients 2019, 11, 909. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Morimoto-Kobayashi, Y.; Koizumi, K.; Takahashi, C.; Nakajima, S.; Kitao, S.; Taniguchi, Y.; Katayama, M.; Ogawa, Y. Secretion of a gastrointestinal hormone, cholecystokinin, by hop-derived bitter components activates sympathetic nerves in brown adipose tissue. J. Nutr. Biochem. 2019, 64, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Morimoto-Kobayashi, Y.; Ohara, K.; Ashigai, H.; Kanaya, T.; Koizumi, K.; Manabe, F.; Kaneko, Y.; Taniguchi, Y.; Katayama, M.; Kowatari, Y.; et al. Matured hop extract reduces body fat in healthy overweight humans: A randomized, double-blind, placebo-controlled parallel group study. Nutr. J. 2015, 15, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayabe, T.; Ohya, R.; Kondo, K.; Ano, Y. Iso-α-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 2018, 8, 4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, Y.; Hosono, M.; Oyamada, C.; Odai, H.; Oikawa, S.; Kondo, K. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARα activations in C57BL/6 mice. Br. J. Nutr. 2005, 93, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Obara, K.; Mizutani, M.; Hitomi, Y.; Yajima, H.; Kondo, K. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin. Nutr. 2009, 28, 278–284. [Google Scholar] [CrossRef]
- Vanhoenacker, G.; De Keukeleire, D.; Sandra, P. Analysis of iso-α-acids and reduced iso-α-acids in beer by direct injection and liquid chromatography with ultraviolet absorbance detection or with mass spectrometry. J. Chromatogr. A 2004, 1035, 53–61. [Google Scholar] [CrossRef]
- Morimoto-Kobayashi, Y.; Ohara, K.; Takahashi, C.; Kitao, S.; Wang, G.; Taniguchi, Y.; Katayama, M.; Nagai, K. Matured hop bittering components induce thermogenesis in brown adipose tissue via sympathetic nerve activity. PLoS ONE 2015, 10, e131042. [Google Scholar] [CrossRef] [Green Version]
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R bitter taste receptors mediate the psychophysical responses to bitter compounds of hops (Humulus lupulus L.) and beer. Chemosens. Percept. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Kok, B.P.; Galmozzi, A.; Littlejohn, N.K.; Albert, V.; Godio, C.; Kim, W.; Kim, S.M.; Bland, J.S.; Grayson, N.; Fang, M.; et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab. 2018, 16, 76–87. [Google Scholar] [CrossRef]
- Kidd, M.; Modlin, I.M.; Gustafsson, B.I.; Drozdov, I.; Hauso, O.; Pfragner, R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, 260–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.V.; Rozengurt, N.; Yang, M.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2392–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyofuji, A.; Yui, K.; Takahashi, K.; Osada, K. Effects of xanthohumol-rich hop extract on the differentiation of preadipocytes. J. Oleo Sci. 2014, 63, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, C.L.; Elias, V.D.; Hay, J.J.; Choi, J.; Reed, R.L.; Stevens, J.F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch. Biochem. Biophys. 2016, 599, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bobe, G.; Revel, J.S.; Rodrigues, R.R.; Sharpton, T.J.; Fantacone, M.L.; Raslan, K.; Miranda, C.L.; Lowry, M.B.; Blakemore, P.R.; et al. Improvements in metabolic syndrome by xanthohumol derivatives are linked to altered gut microbiota and bile acid metabolism. Mol. Nutr. Food Res. 2020, 64, 1900789. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fukizawa, S.; Nonaka, Y. Hop-derived prenylflavonoid isoxanthohumol suppresses insulin resistance by changing the intestinal microbiota and suppressing chronic inflammation in high fat diet-fed mice. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, P.; Rauner, M.; Sipos, W.; Kerschan-Schindl, K. Osteoporosis: An age-related and gender-specific disease—A mini-review. Gerontology 2009, 55, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Biino, G.; Casula, L.; De Terlizzi, F.; Adamo, M.; Vaccargiu, S.; Francavilla, M.; Loi, D.; Casti, A.; Atzori, M.; Pirastu, M. Epidemiology of osteoporosis in an isolated sardinian population by using quantitative ultrasound. Am. J. Epidemiol. 2011, 174, 432–439. [Google Scholar] [CrossRef]
- Price, C.T.; Koval, K.J.; Langford, J.R. Silicon: A review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int. J. Endocrinol. 2013, 2013, 316783. [Google Scholar] [CrossRef] [Green Version]
- Fairweather-Tait, S.J.; Skinner, J.; Guile, G.R.; Cassidy, A.; Spector, T.D.; MacGregor, A.J. Diet and bone mineral density study in postmenopausal women from the twinsUK registry shows a negative association with a traditional english dietary pattern and a positive association with wine. Am. J. Clin. Nutr. 2011, 94, 1371–1375. [Google Scholar] [CrossRef] [Green Version]
- Marrone, J.A.; Maddalozzo, G.F.; Branscum, A.J.; Hardin, K.; Cialdella-Kam, L.; Philbrick, K.A.; Breggia, A.C.; Rosen, C.J.; Turner, R.T.; Iwaniec, U.T. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause 2012, 19, 974–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liel, Y.; Shany, S.; Smirnoff, P.; Schwartz, B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression bioresponse in the rat duodenal mucosa. Endocrinology 1999, 140, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L. Osteoporosis prevention and nutrition. Curr. Osteoporos. Rep. 2009, 7, 111. [Google Scholar] [CrossRef]
- Sommer, I.; Erkkilä, A.T.; Järvinen, R.; Mursu, J.; Sirola, J.; Jurvelin, J.S.; Kröger, H.; Tuppurainen, M. Alcohol consumption and bone mineral density in elderly women. Public Health Nutr. 2013, 16, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLernon, D.J.; Powell, J.J.; Jugdaohsingh, R.; Macdonald, H.M. Do lifestyle choices explain the effect of alcohol on bone mineral density in women around menopause? Am. J. Clin. Nutr. 2012, 95, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Winzenberg, T.; Quinn, S.; Giles, G.; Jones, G. Beverage-specific alcohol intake and bone loss in older men and women: A longitudinal study. Eur. J. Clin. Nutr. 2011, 65, 526–532. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Robbins, J.A.; Cauley, J.A.; Kern, L.M.; Siscovick, D.S. Alcohol consumption, bone density, and hip fracture among older adults: The cardiovascular health study. Osteoporos. Int. 2007, 18, 593–602. [Google Scholar] [CrossRef]
- Pedrera-Zamorano, J.D.; Lavado-Garcia, J.M.; Roncero-Martin, R.; Calderon-Garcia, J.F.; Rodriguez-Dominguez, T.; Canal-Macias, M.L. Effect of beer drinking on ultrasound bone mass in women. Nutrition 2009, 25, 1057–1063. [Google Scholar] [CrossRef]
- Kubo, J.T.; Stefanick, M.L.; Robbins, J.; Wactawski-Wende, J.; Cullen, M.R.; Freiberg, M.; Desai, M. Preference for wine is associated with lower hip fracture incidence in post-menopausal women. BMC Womens Health 2013, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.M.; Kunins, H.V.; Jackson, J.L.; Nahvi, S.; Chaudhry, A.; Harris, K.A.; Malik, R.; Arnsten, J.H. Association between alcohol consumption and both osteoporotic fracture and bone density. Am. J. Med. 2008, 121, 406–418. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.A.; Folsom, A.R.; Kushi, L.H.; Sellers, T.A. Association of fractures with caffeine and alcohol in postmenopausal women: The Iowa Women’s Health Study. Public Health Nutr. 2000, 3, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; MacDonald, H.M. Associations between dietary flavonoid intakes and bone health in a scottish population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.E.; Johnsen, S.A.; Monroe, D.G.; Spelsberg, T.C.; Westendorf, J.J. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005, 96, 387–399. [Google Scholar] [CrossRef]
- Xia, T.S.; Lin, L.Y.; Zhang, Q.Y.; Jiang, Y.P.; Li, C.H.; Liu, X.Y.; Qin, L.P.; Xin, H.L. Humulus lupulus, L. extract prevents ovariectomy-induced osteoporosis in mice and regulates activities of osteoblasts and osteoclasts. Chin. J. Integr. Med. 2019, 1–8. [Google Scholar] [CrossRef]
- Tobe, H.; Muraki, Y.; Kitamura, K.; Komiyama, O.; Sato, Y.; Sugioka, T.; Maruyama, H.B.; Matsuda, E.; Nagai, M. Bone resorption inhibitors from hop extract. Biosci. Biotechnol. Biochem. 1997, 61, 158–159. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am. J. Clin. Nutr. 2017, 106, ajcn151464. [Google Scholar] [CrossRef] [Green Version]
- Prouillet, C.; Mazière, J.-C.; Mazière, C.; Wattel, A.; Brazier, M.; Kamel, S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharmacol. 2004, 67, 1307–1313. [Google Scholar] [CrossRef]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Choi, Y.H.; Lee, K.Y.; Jeong, H.G. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem. Biophys. Res. Commun. 2011, 409, 82–89. [Google Scholar] [CrossRef]
- Luo, D.; Kang, L.; Ma, Y.; Chen, H.; Kuang, H.; Huang, Q.; He, M.; Peng, W. Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7. Food Sci. Nutr. 2014, 2, 341–350. [Google Scholar] [CrossRef]
- Dong, M.; Jiao, G.; Liu, H.; Wu, W.; Li, S.; Wang, Q.; Xu, D.; Li, X.; Liu, H.; Chen, Y. Biological silicon stimulates collagen type 1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol. Trace Elem. Res. 2016, 173, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Czubara, A.; Pasternak, K. Silicon in medicine and therapy. J. Elem. 2011, 16, 489–497. [Google Scholar] [CrossRef]
- Suh, K.S.; Rhee, S.Y.; Kim, Y.S.; Lee, Y.S.; Choi, E.M. Xanthohumol modulates the expression of osteoclast-specific genes during osteoclastogenesis in RAW264.7 cells. Food Chem. Toxicol. 2013, 62, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Ho, S.C.; Lam, S.S.H.; Ho, S.S.S.; Woo, J.L.F. Soy isoflavones have a favorable effect on bone loss in chinese postmenopausal women with lower bone mass: A double-blind, randomized, controlled trial. J. Clin. Endocrinol. Metab. 2003, 88, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Giersch, G.E.W.; Charkoudian, N.; Stearns, R.L.; Casa, D.J. Fluid balance and hydration considerations for women: Review and ruture directions. Sport. Med. 2020, 50, 253–261. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; DiPietro, L.; Palter, S.F.; Nadel, E.R. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, 187–195. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; Splenser, A.E.; Calzone, W.L.; Taylor, M.P.; Keefe, D.L. Selected contribution: Sex differences in osmotic regulation of AVP and renal sodium handling. J. Appl. Physiol. 2001, 91, 1893–1901. [Google Scholar] [CrossRef]
- González-SanJosé, M.L.; Rodríguez, P.M.; Valls-Bellés, V. Beer and its role in human health. In Fermented Foods in Health and Disease Prevention; Elsevie: Amsterdam, The Netherlands, 2017; pp. 365–384. ISBN 9780128023099. [Google Scholar]
- Jiménez-Pavón, D.; Cervantes-Borunda, M.S.; Díaz, L.E.; Marcos, A.; Castillo, M.J. Effects of a moderate intake of beer on markers of hydration after exercise in the heat: A crossover study. J. Int. Soc. Sports Nutr. 2015, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Orrù, S.; Imperlini, E.; Nigro, E.; Alfieri, A.; Cevenini, A.; Polito, R.; Daniele, A.; Buono, P.; Mancini, A. Role of functional beverages on sport performance and recovery. Nutrients 2018, 10, 1470. [Google Scholar] [CrossRef] [Green Version]
- Hobson, R.M.; Maughan, R.J. Hydration status and the diuretic action of a small dose of alcohol. Alcohol Alcohol. 2010, 45, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Polhuis, K.C.M.M.; Wijnen, A.H.C.; Sierksma, A.; Calame, W.; Tieland, M. The diuretic action of weak and strong alcoholic beverages in elderly men: A randomized diet-controlled crossover trial. Nutrients 2017, 9, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirreffs, S.M.; Maughan, R.J. The effect of alcohol on athletic performance. Curr. Sports Med. Rep. 2006, 5, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.J.; Mündel, T.; Stannard, S.R. A low dose of alcohol does not impact skeletal muscle performance after exercise-induced muscle damage. Eur. J. Appl. Physiol. 2011, 111, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Maughan, R.J. Restoration of fluid balance after exercise-induced dehydration: Effects of alcohol consumption. J. Appl. Physiol. 1997, 83, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Flores-Salamanca, R.; Aragón-Vargas, L.F. Postexercise rehydration with beer impairs fluid retention, reaction time, and balance. Appl. Physiol. Nutr. Metab. 2014, 39, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Castro-Sepulveda, M.; Johannsen, N.; Astudillo, S.; Jorquera, C.; Álvarez, C.; Zbinden-Foncea, H.; Ramírez-Campillo, R. Effects of beer, non-alcoholic beer and water consumption before exercise on fluid and electrolyte homeostasis in athletes. Nutrients 2016, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Wijnen, A.H.C.; Steennis, J.; Catoire, M.; Wardenaar, F.C.; Mensink, M. Post-exercise rehydration: Effect of consumption of beer with varying alcohol content on fluid balance after mild dehydration. Front. Nutr. 2016, 3, 45. [Google Scholar] [CrossRef]
- Desbrow, B.; Murray, D.; Leveritt, M. Beer as a sports drink? Manipulating beer’s ingredients to replace lost fluid. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 593–600. [Google Scholar] [CrossRef]
- Desbrow, B.; Cecchin, D.; Jones, A.; Grant, G.; Irwin, C.; Leveritt, M. Manipulations to the alcohol and sodium content of beer for postexercise rehydration. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 262–270. [Google Scholar] [CrossRef]
| Bioactive Compound | Avarege Level (mg/330 mL) |
|---|---|
| Phytoestrogens Xanthohumol 6-Prenylnaringenin 8-Prenylnaringenin Isoxanthohumol | 4.653 × 10−3 8.547 × 10−3 3.432 × 10−3 0.132 |
| Bitter acids α+β acids Iso-α-humulones Minerals Silicon Sodium Potassium | 0.891 a 9.207 a 6.336 14.883 116.589 |
| Authors Year [Ref] | Type of Study | Study Population | Key Finding |
|---|---|---|---|
| Lapidus et al., 1989 [43] | Cross-sectional | 1462 women 38–60 years-old | No correlation was found between WHR and beer consumption. |
| Slattery et al., 1992 [44] | Cross-sectional | 1447 black women 1284 white women 18–30 years-old | Higher beer consumption was associated with a higher WHR among white and black women. |
| Kahn et al., 1997 [45] | Prospective observational | 44080 women 40–54 years-old | OR of abdominal weight gain was positively associated in women drinking >0 to <5 days per week and no associated in women drinking <5 days per week versus non-drinkers |
| Dallongeville et al., 1998 [37] | Cross-sectional | 11730 women 35–64 years-old | Beer & cider consumption was associated with a higher WHR. |
| Rosmond & Bjorntorp 1999 [46] | Cross-sectional | 1137 women 40 years-old | Beer consumption was negatively correlated to WHR. |
| Machado & Sichieri 2002 | Cross-sectional | 1396 women 20–60 years-old | No trend association for OR for WHR >0.80 across beer consumption categories was found. |
| Vadstrup et al., 2003 [48] | Prospective observational | 3970 women 20–83 years-old | Positive trend association was found for WC at follow-up across beer intake categories. |
| Bobak et al., 2003 [49] | Cross-sectional | 1098 women 25–64 years-old | Beer intake was not associated with an increase in WHR. |
| Dorn et al., 2003 [35] | Cross-sectional | 1322 women 53.3 ± 9.4 years-old | No trend association was found between sagittal abdominal diameter and beer consumption. |
| Halkjaer et al., 2004 [50] | Prospective observational | 1131 women 30–60 years-old | Women consuming >4 drinks of beer per week have higher WC, while no significance increase in WC was found in the group drinking 1–3 drinks of beer per week compared to non-drinkers. |
| Deschamps et al., 2004 [52] | Cross-sectional | 284 women 42.4 ± 4.6 years-old | Women drinking >1 glass of beer per day have a higher WRC than abstainers and those who drink <1 glass of beer per day. No trend association was found for WC. |
| Lukasiewicz et al., 2005 [53] | Cross-sectional | 1268 women 47.7 ± 6.6 years-old | No trend association was found between beer consumption and WHC. |
| Halkjaer et al., 2006 | Prospective observational | 22570 women 55 (50–64) years-old | No trend association was found between ΔWC and beer consumption. |
| Krachler et al., 2006 [54] | Cross-sectional | 3087 women 25–64 years-old | Increased beer consumption was not significantly associated to WC. |
| Tolstrup et al., 2008 [55] | Prospective observational | 1610 women 50–65 years-old | Negative association was found for OR of WC across beer intake frequency categories among women who preferred beer. |
| Schütze et al. [36] 2009 | Cross-sectional | 2749 women 35–65 years-old | Positive trend association for ΔWC and ΔWHR was found across beer consumption categories. |
| Schütze et al., 2009 [36] | Prospective observational | 12749 women 35–65 years-old | No trend association for WC was found across beer consumption categories. |
| Bergmann et al., 2011 [41] | Cross-sectional | 158796 women 52.9 ± 9 years-old | Positive association was found for OR of WC and WHR for women drinking <6 versus ≤ 6 g per day of alcohol from beer. |
| Zugravu et al., 2019 [33] | Cross-sectional | 784 women >18 years-old | No linear trend association was found between beer consumption and WC or WHR. |
| Authors Year [Ref] | Type of Study | Study Population | Key Finding |
|---|---|---|---|
| Pedrera-Zamorano et al., 2009 [86] | Cross-sectional | 1697 women (710 premenopausal; 176 perimenopausal and 811 postmenopausal) 48.8 ± 12.59 years-old | Light or moderate consumption of beer was associated to higher bone mass in women independently on their gonadal status. |
| Fairweather-Tait et al., 2011 [76] | Cross-sectional | 2464 postmenopausal women twins 56.3 ± 11.9 years-old | Beer consumption was not associated with higher BMD. |
| Yin et al., 2011 [82] | Cross-sectional | 428 women 62.6 ± 7.2 years-old | Low alcohol beer consumption frequency was positively associated with BMD at lumbar spine. |
| Yin et al., 2011 [82] | Prospective observational | 428 women 62.6 ± 7.2 years-old | No association between beer consumption frequency and BMD at hip was found. |
| McLenon et al., 2012 [81] | Prospective observational | 3173 women 50–62 years-old | Moderate beer consumption had a positive significant effect on lumbar spine BMD after adjustment for lifestyle. |
| Kubo et al., 2013 [85] | Prospective observational | 115,655 postmenopausal women 50–79 years-old | No association was observed between ≥ 1 servings of beer per week and risk of hip fracture. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trius-Soler, M.; Vilas-Franquesa, A.; Tresserra-Rimbau, A.; Sasot, G.; Storniolo, C.E.; Estruch, R.; Lamuela-Raventós, R.M. Effects of the Non-Alcoholic Fraction of Beer on Abdominal Fat, Osteoporosis, and Body Hydration in Women. Molecules 2020, 25, 3910. https://doi.org/10.3390/molecules25173910
Trius-Soler M, Vilas-Franquesa A, Tresserra-Rimbau A, Sasot G, Storniolo CE, Estruch R, Lamuela-Raventós RM. Effects of the Non-Alcoholic Fraction of Beer on Abdominal Fat, Osteoporosis, and Body Hydration in Women. Molecules. 2020; 25(17):3910. https://doi.org/10.3390/molecules25173910
Chicago/Turabian StyleTrius-Soler, Marta, Arnau Vilas-Franquesa, Anna Tresserra-Rimbau, Gemma Sasot, Carolina E. Storniolo, Ramon Estruch, and Rosa M. Lamuela-Raventós. 2020. "Effects of the Non-Alcoholic Fraction of Beer on Abdominal Fat, Osteoporosis, and Body Hydration in Women" Molecules 25, no. 17: 3910. https://doi.org/10.3390/molecules25173910