The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose
Abstract
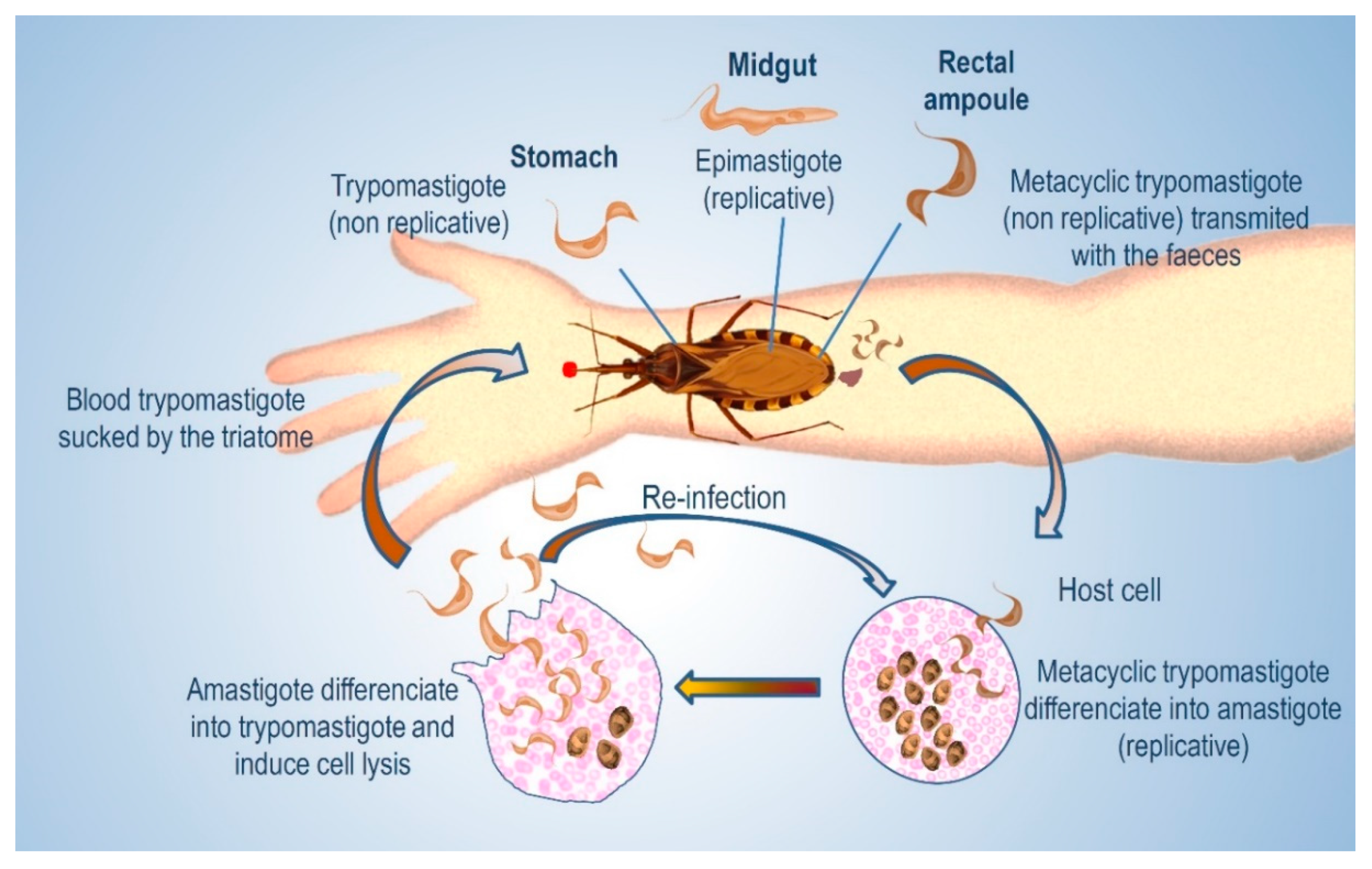
:1. Introduction
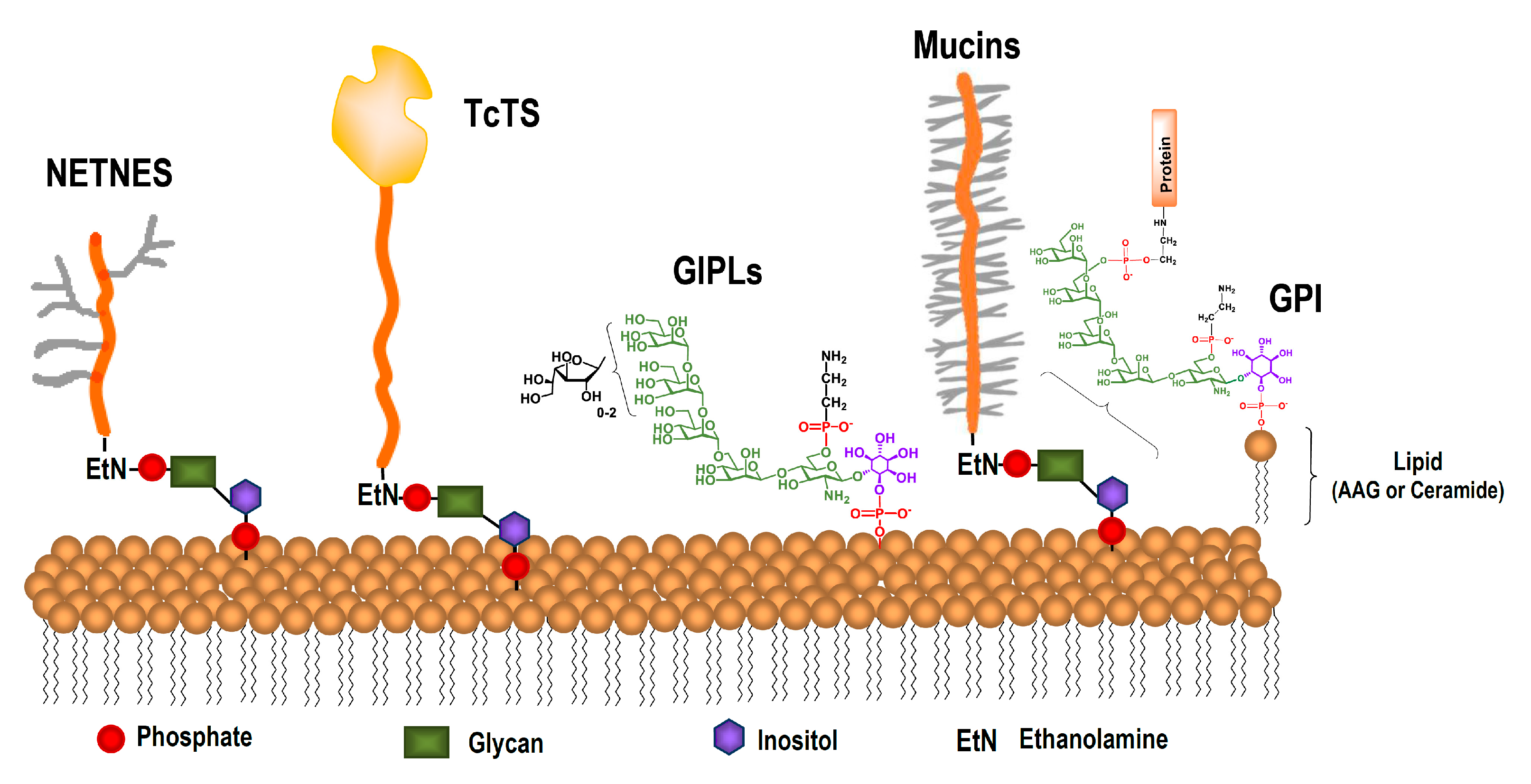
2. Cell-Surface Glycans in T. cruzi
3. The Glycan in Mucins of T. cruzi
4. Galactofuranose in Glycoconjugates of T. cruzi Epimastigotes
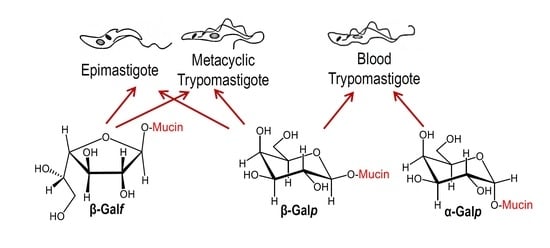
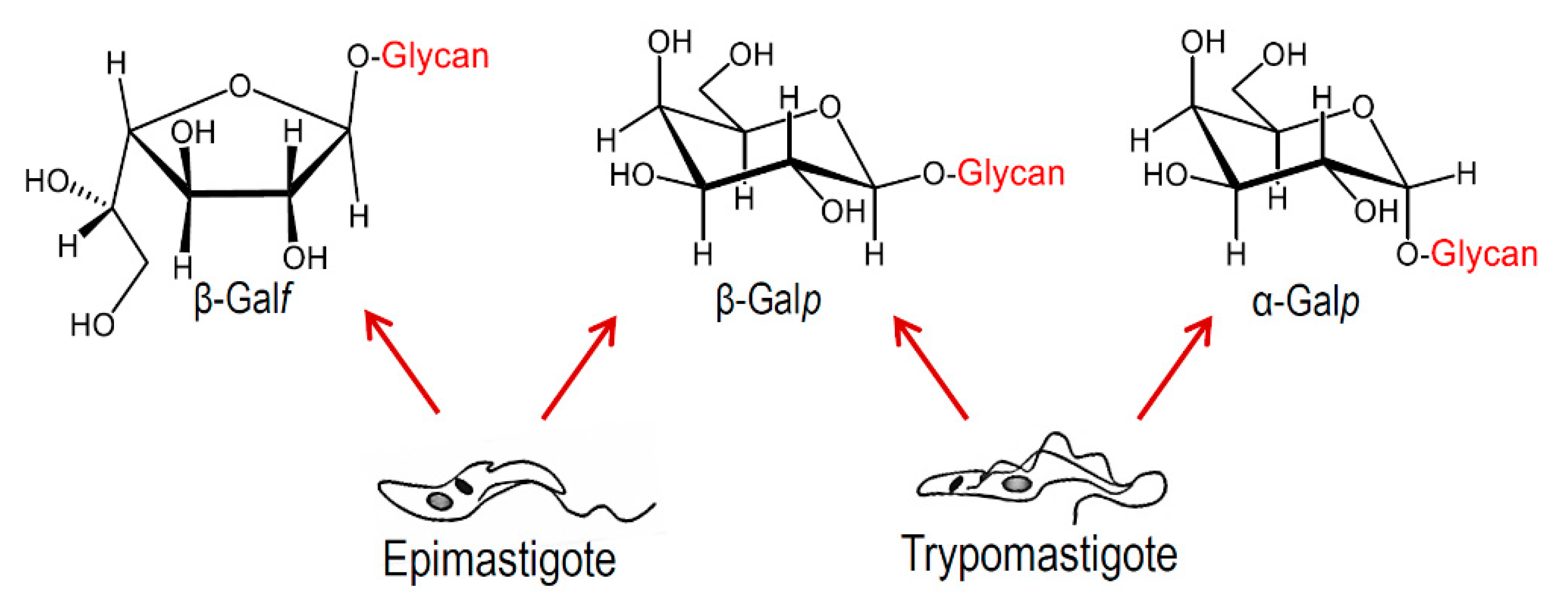
5. Structure of O-Glycans in Mucins of Epimastigotes and Metacyclic Trypomastigotes
6. Structure of Glycans in Mucins from Mammalian Cell-Derived Trypomastigotes. Immunogenicity of the α-Gal Epitope
7. Potential Role of Galectins in the Infection of T. cruzi
8. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Guarner, J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019, 36, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012, 12, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Kollien, A.; Schaub, G. The development of Trypanosoma cruzi in triatominae. Parasitol. Today 2000, 16, 381–387. [Google Scholar] [CrossRef]
- Gonçalves, C.S.; Ávila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors 2018, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Alba Soto, C.D.; González Cappa, S.M. Trypanosoma cruzi journey from the insect vector to the host cell. In Chagas Disease: A Clinical Approach; Altcheh, J.M., Freilij, H., Eds.; Springer International Publishing: Cham, Switerland, 2019; pp. 25–59. [Google Scholar] [CrossRef]
- Monteon, V. Trypanosoma cruzi: The early contact between insect-derived metacyclic trypomastigotes and the mammalian cells. Ann. Parasitol. 2019, 65, 193–204. [Google Scholar] [CrossRef]
- Benchimol, P.R.B. The oral transmission of Chagas’ disease: An acute form of infection responsible for regional outbreaks. Int. J. Cardiol. 2006, 112, 132–133. [Google Scholar] [CrossRef]
- Steindel, M.; Kramer Pacheco, L.; Scholl, D.; Soares, M.; de Moraes, M.H.; Eger, I.; Kosmann, C.; Sincero, T.C.M.; Stoco, P.H.; Murta, S.M.F.; et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn. Microbiol. Infect. Dis. 2008, 60, 25–32. [Google Scholar] [CrossRef]
- Butler, C.E.; Tyler, K.M. Membrane traffic and synaptic cross-talk during host cell entry by Trypanosoma cruzi. Cell. Microbiol. 2012, 14, 1345–1353. [Google Scholar] [CrossRef]
- Mattos, E.C.; Canuto, G.; Manchola, N.C.; Magalhães, R.D.M.; Crozier, T.W.M.; Lamont, D.J.; Tavares, M.F.M.; Colli, W.; Ferguson, M.A.J.; Alves, M.J.M. Reprogramming of Trypanosoma cruzi metabolism triggered by parasite interaction with the host cell extracellular matrix. PLoS Negl. Trop. Dis. 2019, 13, e0007103. [Google Scholar] [CrossRef]
- Barrias, E.S.; de Carvalho, T.M.; De Souza, W. Trypanosoma cruzi: Entry into Mammalian Host Cells and Parasitophorous Vacuole Formation. Front. Immunol. 2013, 4, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, M.F.; Nájera, C.A.; Meneghelli, I.; Bahia, D. The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival. Front. Cell Dev Biol 2020, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Freire-de-Lima, L.; Gentile, L.B.; da Fonseca, L.M.; da Costa, K.M.; Santos Lemos, J.; Jacques, L.R.; Morrot, A.; Freire-de-Lima, C.G.; Nunes, M.P.; Takiya, C.M.; et al. Role of Inactive and Active Trypanosoma cruzi Trans-sialidases on T Cell Homing and Secretion of Inflammatory Cytokines. Front. Microbiol. 2017, 8, 1307. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, L.M.; da Costa, K.M.; Chaves, V.d.S.; Freire-de-Lima, C.G.; Morrot, A.; Mendonça-Previato, L.; Previato, J.O.; Freire-de-Lima, L. Theft and Reception of Host Cell’s Sialic Acid: Dynamics of Trypanosoma cruzi Trans-sialidases and Mucin-Like Molecules on Chagas’ Disease Immunomodulation. Front. Immunol. 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, I.; Boiani Santurio, L.; Chidichimo, A.; Yu, H.; Chen, X.; Mucci, J.; Agüero, F.; Buscaglia, C.A. Molecular diversity of the Trypanosoma cruzi TcSMUG family of mucin genes and proteins. Biochem. J. 2011, 438, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Di Noia, J.M.; Buscaglia, C.A.; De Marchi, C.R.; Almeida, I.C.; Frasch, A.C.C. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas’ disease is due to a single parasite lineage. J. Exp. Med. 2002, 195, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Buscaglia, C.A.; Campo, V.A.; Di Noia, J.M.; Torrecilhas, A.C.; De Marchi, C.R.; Ferguson, M.A.; Frasch, A.C.; Almeida, I.C. The surface coat of the mammal-dwelling infective trypomastigote stage of Trypanosoma cruzi is formed by highly diverse immunogenic mucins. J. Biol. Chem. 2004, 279, 15860–15869. [Google Scholar] [CrossRef] [Green Version]
- Pollevick, G.D.; Di Noia, J.M.; Salto, M.L.; Lima, C.; Leguizamon, M.S.; de Lederkremer, R.M.; Frasch, A.C. Trypanosoma cruzi surface mucins with exposed variant epitopes. J. Biol. Chem. 2000, 275, 27671–27680. [Google Scholar] [CrossRef] [Green Version]
- Durante, I.M.; La Spina, P.E.; Carmona, S.J.; Agüero, F.; Buscaglia, C.A. High-resolution profiling of linear B-cell epitopes from mucin-associated surface proteins (MASPs) of Trypanosoma cruzi during human infections. PLoS Negl. Trop. Dis. 2017, 11, e0005986. [Google Scholar] [CrossRef]
- Bartholomeu, D.C.; Cerqueira, G.C.; Leao, A.C.; daRocha, W.D.; Pais, F.S.; Macedo, C.; Djikeng, A.; Teixeira, S.M.; El-Sayed, N.M. Genomic organization and expression profile of the mucin-associated surface protein (masp) family of the human pathogen Trypanosoma cruzi. Nucleic Acids Res. 2009, 37, 3407–3417. [Google Scholar] [CrossRef] [Green Version]
- Serna, C.; Lara, J.A.; Rodrigues, S.P.; Marques, A.F.; Almeida, I.C.; Maldonado, R.A. A synthetic peptide from Trypanosoma cruzi mucin-like associated surface protein as candidate for a vaccine against Chagas disease. Vaccine 2014, 32, 3525–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, M.; Castillo-Acosta, V.M.; Vidal, A.E.; González-Pacanowska, D. Overview of the role of kinetoplastid surface carbohydrates in infection and host cell invasion: Prospects for therapeutic intervention. Parasitology 2019, 146, 1743–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.P.; Fortes, B.; Silva-Filho, J.L.; Terra-Granado, E.; Santos, L.; Conde, L.; de Araujo Oliveira, I.; Freire-de-Lima, L.; Martins, M.V.; Pinheiro, A.A.; et al. Inhibitory effects of Trypanosoma cruzi sialoglycoproteins on CD4+ T cells are associated with increased susceptibility to infection. PLoS ONE 2013, 8, e77568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, M.A.; Corvo, L.; Soto, M.; Fresno, M.; Bonay, P. Interactions of human galectins with Trypanosoma cruzi: Binding profile correlate with genetic clustering of lineages. Glycobiology 2015, 25, 197–210. [Google Scholar] [CrossRef]
- Previato, J.O.; Gorin, P.A.; Mazurek, M.; Xavier, M.T.; Fournet, B.; Wieruszesk, J.M.; Mendonca-Previato, L. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J. Biol. Chem. 1990, 265, 2518–2526. [Google Scholar]
- De Lederkremer, R.M.; Lima, C.; Ramirez, M.I.; Ferguson, M.A.; Homans, S.W.; Thomas-Oates, J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi Epimastigotes. J. Biol. Chem. 1991, 266, 23670–23675. [Google Scholar]
- De Lederkremer, R.M.; Agusti, R. Chapter 7 glycobiology of Trypanosoma cruzi. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 2009; Volume 62, pp. 311–366. [Google Scholar] [CrossRef]
- Acosta-Serrano, A.; Almeida, I.C.; Freitas-Junior, L.H.; Yoshida, N.; Schenkman, S. The mucin-like glycoprotein super-family of Trypanosoma cruzi: Structure and biological roles. Mol. Biochem. Parasitol. 2001, 114, 143–150. [Google Scholar] [CrossRef]
- Pereira-Chioccola, V.L.; Acosta-Serrano, A.; Correia de Almeida, I.; Ferguson, M.A.; Souto-Padron, T.; Rodrigues, M.M.; Travassos, L.R.; Schenkman, S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 2000, 113, 1299. [Google Scholar]
- Golgher, D.B.; Colli, W.; Souto-Padrón, T.; Zingales, B. Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1993, 60, 249–264. [Google Scholar] [CrossRef]
- Buscaglia, C.A.; Campo, V.A.; Frasch, A.C.; Di Noia, J.M. Trypanosoma cruzi surface mucins: Host-dependent coat diversity. Nat. Rev. Microbiol. 2006, 4, 229–236. [Google Scholar] [CrossRef]
- Schenkman, S.; Eichinger, D.; Pereira, M.E.; Nussenzweig, V. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 1994, 48, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.E.; de Lederkremer, R.M. Trans-sialidase and mucins of Trypanosoma cruzi: An important interplay for the parasite. Carbohydr. Res. 2011, 346, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Macrae, J.I.; Acosta-Serrano, A.; Morrice, N.A.; Mehlert, A.; Ferguson, M.A. Structural characterization of NETNES, a novel glycoconjugate in Trypanosoma cruzi epimastigotes. J. Biol. Chem. 2005, 280, 12201–12211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dc-Rubin, S.S.; Schenkman, S. Trypanosoma cruzi trans-sialidase as a multifunctional enzyme in Chagas’ disease. Cell. Microbiol. 2012, 14, 1522–1530. [Google Scholar] [CrossRef]
- Freire-de-Lima, L.; Fonseca, L.M.; Oeltmann, T.; Mendonça-Previato, L.; Previato, J.O. The trans-sialidase, the major Trypanosoma cruzi virulence factor: Three decades of studies. Glycobiology 2015, 25, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Campetella, O.; Buscaglia, C.A.; Mucci, J.; Leguizamón, M.S. Parasite-host glycan interactions during Trypanosoma cruzi infection: Trans-Sialidase rides the show. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165692. [Google Scholar] [CrossRef]
- Cremona, M.L.; Sánchez, D.O.; Frasch, A.C.; Campetella, O. A single tyrosine differentiates active and inactive Trypanosoma cruzi trans-sialidases. Gene 1995, 160, 123–128. [Google Scholar] [CrossRef]
- Pinzón Martín, S.; Seeberger, P.H.; Varón Silva, D. Corrigendum: Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators during Infection and Targets for Diagnostics and Vaccines. Front. Chem. 2019, 7, 846. [Google Scholar] [CrossRef] [Green Version]
- Previato, J.O.; Sola-Penna, M.; Agrellos, O.A.; Jones, C.; Oeltmann, T.; Travassos, L.R.; Mendonca-Previato, L. Biosynthesis of O-N-acetylglucosamine-linked glycans in Trypanosoma cruzi. Characterization of the novel uridine diphospho-N-acetylglucosamine:polypeptide N-acetylglucosaminyltransferase-catalyzing formation of N-acetylglucosamine α1→O-threonine. J. Biol. Chem. 1998, 273, 14982–14988. [Google Scholar] [CrossRef] [Green Version]
- Mendonca-Previato, L.; Penha, L.; Garcez, T.C.; Jones, C.; Previato, J.O. Addition of alpha-O-GlcNAc to threonine residues define the post-translational modification of mucin-like molecules in Trypanosoma cruzi. Glycoconj. J. 2013, 30, 659–766. [Google Scholar] [CrossRef] [Green Version]
- Roper, J.R.; Ferguson, M.A. Cloning and characterisation of the UDP-glucose 4′-epimerase of Trypanosoma cruzi. Mol. Biochem. Parasitol. 2003, 132, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Singh, D.; van der Wel, H.; Sassi, S.O.; Johnson, J.M.; Feasley, C.L.; Koeller, C.M.; Previato, J.O.; Mendonça-Previato, L.; West, C.M. Molecular analysis of a UDP-GlcNAc:polypeptide α-N-acetylglucosaminyltransferase implicated in the initiation of mucin-type O-glycosylation in Trypanosoma cruzi. Glycobiology 2009, 19, 918–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiribao, M.L.; Libisch, M.G.; Osinaga, E.; Parodi-Talice, A.; Robello, C. Cloning, localization and differential expression of the Trypanosoma cruzi TcOGNT-2 glycosyl transferase. Gene 2012, 498, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, A.R.; da Silveira, E.X.; Jones, C.; Wait, R.; Previato, J.O.; Mendonça-Previato, L. Structure of O-glycosidically linked oligosaccharides from glycoproteins of Trypanosoma cruzi CL-Brener strain: Evidence for the presence of O-linked sialyl-oligosaccharides. Glycobiology 2001, 11, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Serrano, A.; Schenkman, S.; Yoshida, N.; Mehlert, A.; Richardson, J.M.; Ferguson, M.A.J. The Lipid Structure of the Glycosylphosphatidylinositol-anchored Mucin-like Sialic Acid Acceptors of Trypanosoma cruzi Changes during Parasite Differentiation from Epimastigotes to Infective Metacyclic Trypomastigote Forms. J. Biol. Chem. 1995, 270, 27244–27253. [Google Scholar] [CrossRef] [Green Version]
- Previato, J.O.; Jones, C.; Gonçalves, L.P.; Wait, R.; Travassos, L.R.; Mendonça-Previato, L. O-glycosidically linked N-acetylglucosamine-bound oligosaccharides from glycoproteins of Trypanosoma cruzi. Biochem. J. 1994, 301, 151–159. [Google Scholar] [CrossRef]
- Agrellos, O.A.; Jones, C.; Todeschini, A.R.; Previato, J.O.; Mendonça-Previato, L. A novel sialylated and galactofuranose-containing O-linked glycan, Neu5Ac(α2→3)Galp(β1→6)Galf(β1→4)GlcNAc, is expressed on the sialoglycoprotein of Trypanosoma cruzi Dm28c. Mol. Biochem. Parasitol. 2003, 126, 93–96. [Google Scholar] [CrossRef]
- Todeschini, A.R.; de Almeida, E.G.; Agrellos, O.A.; Jones, C.; Previato, J.O.; Mendonça-Previato, L. ±-N-acetylglucosamine-linked O-glycans of sialoglycoproteins (Tc-mucins) from Trypanosoma cruzi Colombiana strain. Mem. Inst. Oswaldo Cruz 2009, 104, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.; Todeschini, A.R.; Agrellos, O.A.; Previato, J.O.; Mendonça-Previato, L. Heterogeneity in the biosynthesis of mucin O-glycans from Trypanosoma cruzi tulahuen strain with the expression of novel galactofuranosyl-containing oligosaccharides. Biochemistry 2004, 43, 11889–11897. [Google Scholar] [CrossRef]
- Salto, M.L.; Gallo-Rodriguez, C.; Lima, C.; de Lederkremer, R.M. Separation of Galfβ1→XGlcNAc and Galpβ1→XGlcNAc (X = 3, 4, and 6) as the Alditols by High-pH Anion-Exchange Chromatography and Thin-Layer Chromatography: Characterization of Mucins from Trypanosoma cruzi. Anal. Biochem. 2000, 279, 79–84. [Google Scholar] [CrossRef]
- Tetaud, E.; Barrett, M.P.; Bringaud, F.; Baltz, T. Kinetoplastid glucose transporters. Biochem. J. 1997, 325, 569–580. [Google Scholar] [CrossRef] [Green Version]
- MacRae, J.I.; Obado, S.O.; Turnock, D.C.; Roper, J.R.; Kierans, M.; Kelly, J.M.; Ferguson, M.A.J. The suppression of galactose metabolism in Trypanosoma cruzi epimastigotes causes changes in cell surface molecular architecture and cell morphology. Mol. Biochem. Parasitol. 2006, 147, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mortara, R.A.; da Silva, S.; Araguth, M.F.; Blanco, S.A.; Yoshida, N. Polymorphism of the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi metacyclic trypomastigotes. Infect. Immun. 1992, 60, 4673–4678. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.W.; Hong, K.-s.; Robbins, E.S.; Nussenzweig, V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp. Parasitol. 1987, 64, 474–484. [Google Scholar] [CrossRef]
- Ley, V.; Andrews, N.W.; Robbins, E.S.; Nussenzweig, V. Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells. J. Exp. Med. 1988, 168, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharfstein, J.; Morrot, A. A role for extracellular amastigotes in the immunopathology of Chagas disease. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. S1), 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florentino, P.T.V.; Real, F.; Orikaza, C.M.; da Cunha, J.P.C.; Vitorino, F.N.L.; Cordero, E.M.; Sobreira, T.J.P.; Mortara, R.A. A Carbohydrate Moiety of Secreted Stage-Specific Glycoprotein 4 Participates in Host Cell Invasion by Trypanosoma cruzi Extracellular Amastigotes. Front. Microbiol. 2018, 9, 693. [Google Scholar] [CrossRef]
- Marino, C.; Lederkremer, R. Galactose configurations in nature with emphasis on the biosynthesis of galactofuranose in glycans. In Galactose: Structure and Function in Biology and Medicine; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 107–133. [Google Scholar]
- De Lederkremer, R.M.; Colli, W. Galactofuranose-containing glycoconjugates in trypanosomatids. Glycobiology 1995, 5, 547–552. [Google Scholar] [CrossRef] [PubMed]
- McConville, M.J.; Thomas-Oates, J.E.; Ferguson, M.A.; Homans, S.W. Structure of the lipophosphoglycan from Leishmania major. J. Biol. Chem. 1990, 265, 19611–19623. [Google Scholar]
- Späth, G.F.; Epstein, L.; Leader, B.; Singer, S.M.; Avila, H.A.; Turco, S.J.; Beverley, S.M. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA 2000, 97, 9258–9263. [Google Scholar] [CrossRef] [Green Version]
- Jankute, M.; Cox, J.A.; Harrison, J.; Besra, G.S. Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol. 2015, 69, 405–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latge, J.P. Galactofuranose containing molecules in Aspergillus fumigatus. Med. Mycol. 2009, 47 (Suppl. S1), S104–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, C.; Rinflerch, A.; de Lederkremer, R.M. Galactofuranose antigens, a target for diagnosis of fungal infections in humans. Future Sci. OA 2017, 3, FSO199. [Google Scholar] [CrossRef] [PubMed]
- Seničar, M.; Lafite, P.; Eliseeva, S.V.; Petoud, S.; Landemarre, L.; Daniellou, R. Galactofuranose-Related Enzymes: Challenges and Hopes. Int. J. Mol. Sci. 2020, 21, 3465. [Google Scholar] [CrossRef]
- Stevenson, G.; Neal, B.; Liu, D.; Hobbs, M.; Packer, N.H.; Batley, M.; Redmond, J.W.; Lindquist, L.; Reeves, P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 1994, 176, 4144–4156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassau, P.M.; Martin, S.L.; Brown, R.E.; Weston, A.; Monsey, D.; McNeil, M.R.; Duncan, K. Galactofuranose biosynthesis in Escherichia coli K-12: Identification and cloning of UDP-galactopyranose mutase. J. Bacteriol. 1996, 178, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Tanner, J.J.; Boechi, L.; Andrew McCammon, J.; Sobrado, P. Structure, mechanism, and dynamics of UDP-galactopyranose mutase. Arch. Biochem. Biophys. 2014, 544, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Kizjakina, K.; Tanner, J.J.; Sobrado, P. Targeting UDP-galactopyranose mutases from eukaryotic human pathogens. Curr. Pharm. Des. 2013, 19, 2561–2573. [Google Scholar] [CrossRef] [Green Version]
- Beverley, S.M.; Owens, K.L.; Showalter, M.; Griffith, C.L.; Doering, T.L.; Jones, V.C.; McNeil, M.R. Eukaryotic UDP-galactopyranose mutase (GLF gene) in microbial and metazoal pathogens. Eukaryot. Cell 2005, 4, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Dhatwalia, R.; Singh, H.; Oppenheimer, M.; Sobrado, P.; Tanner, J.J. Crystal Structures of Trypanosoma cruzi UDP-Galactopyranose Mutase Implicate Flexibility of the Histidine Loop in Enzyme Activation. Biochemistry 2012, 51, 4968–4979. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, N.M.; Myler, P.J.; Bartholomeu, D.C.; Nilsson, D.; Aggarwal, G.; Tran, A.N.; Ghedin, E.; Worthey, E.A.; Delcher, A.L.; Blandin, G.; et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 2005, 309, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoco, P.H.; Aresi, C.; Lückemeyer, D.D.; Sperandio, M.M.; Sincero, T.C.M.; Steindel, M.; Miletti, L.C.; Grisard, E.C. Trypanosoma rangeli expresses a β-galactofuranosyl transferase. Exp. Parasitol. 2012, 130, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lederkremer, R.M.; Alves, M.J.M.; Fonseca, G.C.; Colli, W. A lipopeptidophosphoglycan from Trypanosoma cruzi (epimastigota): Isolation, purification and carbohydate composition. Biochim. Biophys. Acta Gen. Subj. 1976, 444, 85–96. [Google Scholar] [CrossRef]
- Carreira, J.C.; Jones, C.; Wait, R.; Previato, J.O.; Mendonça-Previato, L. Structural variation in the glycoinositolphospholipids of different strains of Trypanos. Cruzi. Glycoconj. J. 1996, 13, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Miletti, L.C.; Mariño, K.; Marino, C.; Colli, W.; Alves, M.J.; de Lederkremer, R.M. Evidence for exo beta-d-galactofuranosidase in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2003, 127, 85–88. [Google Scholar] [CrossRef]
- De Lederkremer, R.M.; Bertello, L.E. Glycoinositolphospholipids, free and as anchors of proteins, in Trypanosoma cruzi. Curr. Pharm. Des. 2001, 7, 1165–1179. [Google Scholar] [CrossRef]
- Previato, J.O.; Wait, R.; Jones, C.; DosReis, G.A.; Todeschini, A.R.; Heise, N.; Previato, L.M. Glycoinositolphospholipid from Trypanosoma cruzi: Structure, biosynthesis and immunobiology. Adv. Parasitol. 2004, 56, 1–41. [Google Scholar] [CrossRef]
- Previato, J.O.; Jones, C.; Xavier, M.T.; Wait, R.; Travassos, L.R.; Parodi, A.J.; Mendonça-Previato, L. Structural characterization of the major glycosylphosphatidylinositol membrane-anchored glycoprotein from epimastigote forms of Trypanosoma cruzi Y-strain. J. Biol. Chem. 1995, 270, 7241–7250. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Mortara, R.A.; Araguth, M.F.; Gonzalez, J.C.; Russo, M. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect. Immun 1989, 57, 1663–1667. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N. Molecular basis of mammalian cell invasion by Trypanosoma cruzi. Anais da Academia Brasileira de Ciencias 2006, 78, 87–111. [Google Scholar] [CrossRef] [Green Version]
- Demeu, L.M.K.; Soares, R.J.; Miranda, J.S.; Pacheco-Lugo, L.A.; Oliveira, K.G.; Cortez Plaza, C.A.; Billiald, P.; Ferreira de Moura, J.; Yoshida, N.; Alvarenga, L.M.; et al. Engineering a single-chain antibody against Trypanosoma cruzi metacyclic trypomastigotes to block cell invasion. PLoS ONE 2019, 14, e0223773. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.; Garcez, T.C.; Penha, L.; Freire, D.E.L.L.; Maes, E.; Costa, K.M.; Mendonça-Previato, L.; Previato, J.O. Expanding the knowledge of the chemical structure of glycoconjugates from Trypanosoma cruzi TcI genotype. Contribution to taxonomic studies. An. Acad. Bras. Ciênc. 2016, 88, 1519–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo-Rodriguez, C.; Varela, O.; Lederkremer, R.M. First synthesis of beta-d-Galf(1-4)GlcNAc, a structural unit attached O-Glycosidically in glycoproteins of Trypanosoma cruzi. J. Org. Chem. 1996, 61, 1886–1889. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, V.M.; Kashiwagi, G.A.; de Lederkremer, R.M.; Gallo-Rodriguez, C. Synthesis of trisaccharides containing internal galactofuranose O-linked in Trypanosoma cruzi mucins. Carbohydr. Res. 2010, 345, 385–396. [Google Scholar] [CrossRef]
- Gallo-Rodriguez, C.; Varela, O.; de Lederkremer, R.M. One-pot synthesis of β-d-Galf(1→4)[β-d-Galp(1→6)]-d-GlcNAc, a ‘core’ trisaccharide linked O-glycosidically in glycoproteins of Trypanosoma cruzi. Carbohydr. Res. 1997, 305, 163–170. [Google Scholar] [CrossRef]
- Gallo-Rodriguez, C.; Gil-Libarona, M.A.; Mendoza, V.M.; de Lederkremer, R.M. Synthesis of β-d-Galp-(1→3)-β-d-Galp-(1→6)-[β-d-Galf-(1→4)]-d-GlcNAc, a tetrasaccharide component of mucins of Trypanosoma cruzi. Tetrahedron 2002, 58, 9373–9380. [Google Scholar] [CrossRef]
- Mendoza, V.M.; Agusti, R.; Gallo-Rodriguez, C.; de Lederkremer, R.M. Synthesis of the O-linked pentasaccharide in glycoproteins of Trypanosoma cruzi and selective sialylation by recombinant trans-sialidase. Carbohydr. Res. 2006, 341, 1488–1497. [Google Scholar] [CrossRef]
- Kashiwagi, G.A.; Mendoza, V.M.; de Lederkremer, R.M.; Gallo-Rodriguez, C. Synthesis of the O-linked hexasaccharide containing β-d-Galf-(1→2)-β-d-Galf in Trypanosoma cruzi mucins. Org. Biomol. Chem. 2012, 10, 6322–6332. [Google Scholar] [CrossRef]
- Agustí, R.; Giorgi, M.E.; Mendoza, V.M.; Kashiwagi, G.A.; de Lederkremer, R.M.; Gallo-Rodriguez, C. Synthesis of the O-linked hexasaccharide containing β-d-Galp-(1→2)-d-Galf in Trypanosoma cruzi mucins. Differences on sialylation by trans-sialidase of the two constituent hexasaccharides. Bioorganic Med. Chem. 2015, 23, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, G.A.; Cori, C.R.; de Lederkremer, R.M.; Gallo-Rodriguez, C. Synthesis of the hexasaccharide from Trypanosoma cruzi mucins with the Galp(1→2)Galf unit constructed with a superarmed thiogalactopyranosyl donor. Carbohydr. Res. 2019, 482, 107734. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, N.F.S.; Gonzalez, M.S.; Gomes, J.E.; de Souza, W.; Garcia, E.S.; Azambuja, P.; Nohara, L.L.; Almeida, I.C.; Zingales, B.; Colli, W. Trypanosoma cruzi: Involvement of glycoinositolphospholipids in the attachment to the luminal midgut surface of Rhodnius prolixus. Exp. Parasitol. 2007, 116, 120–128. [Google Scholar] [CrossRef] [PubMed]
- De los Milagros Cámara, M.; Balouz, V.; Centeno Cameán, C.; Cori, C.R.; Kashiwagi, G.A.; Gil, S.A.; Macchiaverna, N.P.; Cardinal, M.V.; Guaimas, F.; Lobo, M.M.; et al. Trypanosoma cruzi surface mucins are involved in the attachment to the Triatoma infestans rectal ampoule. PLoS Negl. Trop. Dis. 2019, 13, e0007418. [Google Scholar] [CrossRef] [PubMed]
- De Godoy, L.M.; Marchini, F.K.; Pavoni, D.P.; Rampazzo Rde, C.; Probst, C.M.; Goldenberg, S.; Krieger, M.A. Quantitative proteomics of Trypanosoma cruzi during metacyclogenesis. Proteomics 2012, 12, 2694–2703. [Google Scholar] [CrossRef]
- Almeida, I.C.; Ferguson, M.A.; Schenkman, S.; Travassos, L.R. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas’ disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 1994, 304, 793–802. [Google Scholar] [CrossRef]
- Lantos, A.B.; Carlevaro, G.; Araoz, B.; Ruiz Diaz, P.; Camara, M.D.L.M.; Buscaglia, C.A.; Bossi, M.; Yu, H.; Chen, X.; Bertozzi, C.R.; et al. Sialic acid glycobiology unveils Trypanosoma cruzi trypomastigote membrane physiology. PLoS Pathog. 2016, 12, e1005559. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.P.; Torrecilhas, A.C.; Assis, R.R.; Rocha, M.N.; Moura e Castro, F.A.; Freitas, G.F.; Murta, S.M.; Santos, S.L.; Marques, A.F.; Almeida, I.C.; et al. Intraspecies variation in Trypanosoma cruzi GPI-mucins: Biological activities and differential expression of α-galactosyl residues. Am. J. Trop Med. Hyg. 2012, 87, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Galili, U.; Clark, M.R.; Shohet, S.B.; Buehler, J.; Macher, B.A. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1–3Gal epitope in primates. Proc. Natl. Acad. Sci. USA 1987, 84, 1369–1373. [Google Scholar] [CrossRef] [Green Version]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef]
- Ashmus, R.A.; Schocker, N.S.; Cordero-Mendoza, Y.; Marques, A.F.; Monroy, E.Y.; Pardo, A.; Izquierdo, L.; Gállego, M.; Gascon, J.; Almeida, I.C.; et al. Potential use of synthetic α-galactosyl-containing glycotopes of the parasite Trypanosoma cruzi as diagnostic antigens for Chagas disease. Org. Biomol. Chem. 2013, 11, 5579–5583. [Google Scholar] [CrossRef]
- Ortega-Rodriguez, U.; Portillo, S.; Ashmus, R.A.; Duran, J.A.; Schocker, N.S.; Iniguez, E.; Montoya, A.L.; Zepeda, B.G.; Olivas, J.J.; Karimi, N.H.; et al. Purification of glycosylphosphatidylinositol-anchored mucins from Trypanosoma cruzi trypomastigotes and synthesis of α-Gal-containing neoglycoproteins: Application as biomarkers for reliable diagnosis and early assessment of chemotherapeutic outcomes of chagas disease. In T. cruzi Infection; Humana Press: New York, NY, USA, 2019; Volume 1955, pp. 287–308. [Google Scholar] [CrossRef]
- Brito, C.R.; McKay, C.S.; Azevedo, M.A.; Santos, L.C.; Venuto, A.P.; Nunes, D.F.; D’Ávila, D.A.; Rodrigues da Cunha, G.M.; Almeida, I.C.; Gazzinelli, R.T.; et al. Virus-like particle display of the α-Gal epitope for the diagnostic assessment of chagas disease. ACS Infect. Dis. 2016, 2, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, M.-J.; Thomas, M.-C.; Bustamante, J.; Almeida, I.C.D.; Lopez, M.-C.; Gascon, J. Biomarkers of therapeutic responses in chronic Chagas disease: State of the art and future perspectives. Mem. Inst. Oswaldo Cruz 2015, 110, 422–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schocker, N.S.; Portillo, S.; Brito, C.R.; Marques, A.F.; Almeida, I.C.; Michael, K. Synthesis of Galα(1,3)Galβ(1,4)GlcNAcα-, Galβ(1,4)GlcNAcα- and GlcNAc-containing neoglycoproteins and their immunological evaluation in the context of Chagas disease. Glycobiology 2016, 26, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, R.; Giorgi, M.E.; Melgarejo, L.T.; Ducrey, I.; Balouz, V.; González-Salas, D.; Cámara, M.L.M.; Buscaglia, C.A.; de Lederkremer, R.M.; Marino, C. Synthesis and characterization of α-d-Galp-(1→3)-β-d-Galp epitope-containing neoglycoconjugates for chagas disease serodiagnosis. Carbohydr. Res. 2019, 478, 58–67. [Google Scholar] [CrossRef]
- Portillo, S.; Zepeda, B.G.; Iniguez, E.; Olivas, J.J.; Karimi, N.H.; Moreira, O.C.; Marques, A.F.; Michael, K.; Maldonado, R.A.; Almeida, I.C. A prophylactic α-Gal-based glycovaccine effectively protects against murine acute Chagas disease. Npj Vaccines 2019, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.S.; Austin, V.; Schocker, N.S.; Montoya, A.L.; Anderson, M.S.; Ashmus, R.A.; Mesri, M.; Al-Salem, W.; Almeida, I.C.; Michael, K.; et al. Anti-α-Gal antibodies detected by novel neoglycoproteins as a diagnostic tool for old world cutaneous leishmaniasis caused by Leishmania major. Parasitology 2018, 145, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, E.; Schocker, N.S.; Subramaniam, K.; Portillo, S.; Montoya, A.L.; Al-Salem, W.S.; Torres, C.L.; Rodriguez, F.; Moreira, O.C.; Acosta-Serrano, A.; et al. An α-Gal-containing neoglycoprotein-based vaccine partially protects against murine cutaneous leishmaniasis caused by Leishmania major. PLoS Negl. Trop. Dis. 2017, 11, e0006039. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.P.V.; Santos, L.C.B.; Brito, C.R.N.; Valencia, E.; Junqueira, C.; Filho, A.A.P.; Sant’Anna, M.R.V.; Gontijo, N.F.; Bartholomeu, D.C.; Fujiwara, R.T.; et al. Virus-like particle display of the α-Gal carbohydrate for vaccination against leishmania infection. ACS Cent. Sci. 2017, 3, 1026–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, R.; Reese, R.T. Terminal galactose residues and the antigenicity of Plasmodium falciparum glycoproteins. Mol. Biochem. Parasitol. 1986, 19, 91–101. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; de la Fuente, J. Immunity to α-Gal: The opportunity for malaria and tuberculosis control. Front. Immunol. 2017, 8, 1733. [Google Scholar] [CrossRef] [Green Version]
- Rabinovich, G.A.; Gruppi, A. Galectins as immunoregulators during infectious processes: From microbial invasion to the resolution of the disease. Parasite Immunol. 2005, 27, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.N.; Ochieng, J.; Villalta, F. Novel mechanism that Trypanosoma cruzi uses to adhere to the extracellular matrix mediated by human galectin-3. FEBS Lett. 2000, 470, 305–308. [Google Scholar] [CrossRef]
- Turner, C.W.; Lima, M.F.; Villalta, F. Trypanosoma cruzi uses a 45-kDa mucin for adhesion to mammalian cells. Biochem. Biophys. Res. Commun. 2002, 290, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kleshchenko, Y.Y.; Moody, T.N.; Furtak, V.A.; Ochieng, J.; Lima, M.F.; Villalta, F. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect. Immun. 2004, 72, 6717–6721. [Google Scholar] [CrossRef] [Green Version]
- Vray, B.; Camby, I.; Vercruysse, V.; Mijatovic, T.; Bovin, N.V.; Ricciardi-Castagnoli, P.; Kaltner, H.; Salmon, I.; Gabius, H.-J.; Kiss, R. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology 2004, 14, 647–657. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Chain, M.; de Medeiros Paiva, C.A.; de Oliveira Maciel, I.; Neto, A.N.; Castro, V.F.; Oliveira, C.P.; dos Santos Mendonça, B.; de Moraes, G.N.; dos Reis, S.A.; de Carvalho, M.A.; et al. Galectin-3 mediates survival and apoptosis pathways during Trypanosoma cruzi–host cell interplay. Exp. Parasitol. 2020, 216, 107932. [Google Scholar] [CrossRef]
- Poncini, C.V.; Ilarregui, J.M.; Batalla, E.I.; Engels, S.; Cerliani, J.P.; Cucher, M.A.; van Kooyk, Y.; González-Cappa, S.M.; Rabinovich, G.A. Infection imparts a regulatory program in dendritic cells and T cells via Galectin-1–dependent mechanisms. J. Immunol. 2015, 195, 3311. [Google Scholar] [CrossRef] [Green Version]
- Benatar, A.F.; García, G.A.; Bua, J.; Cerliani, J.P.; Postan, M.; Tasso, L.M.; Scaglione, J.; Stupirski, J.C.; Toscano, M.A.; Rabinovich, G.A.; et al. Galectin-1 prevents infection and damage induced by Trypanosoma cruzi on cardiac cells. PLoS Negl. Trop. Dis. 2015, 9, e0004148. [Google Scholar] [CrossRef]
- Frasch, A.C. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol. Today Pers. Ed. 2000, 16, 282–286. [Google Scholar] [CrossRef]
- Yoshida, N.; Dorta, M.L.; Ferreira, A.T.; Oshiro, M.E.; Mortara, R.A.; Acosta-Serrano, A.; Favoreto Júnior, S. Removal of sialic acid from mucin-like surface molecules of Trypanosoma cruzi metacyclic trypomastigotes enhances parasite-host cell interaction. Mol. Biochem. Parasitol. 1997, 84, 57–67. [Google Scholar] [CrossRef]
- Agustí, R.; Giorgi, M.E.; Mendoza, V.M.; Gallo-Rodriguez, C.; de Lederkremer, R.M. Comparative rates of sialylation by recombinant trans-sialidase and inhibitor properties of synthetic oligosaccharides from Trypanosoma cruzi mucins-containing galactofuranose and galactopyranose. Bioorg. Med. Chem. 2007, 15, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, S.; Ferguson, M.A.J.; Heise, N.; Cardoso de Almeida, M.L.; Mortara, R.A.; Yoshida, N. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1993, 59, 293–303. [Google Scholar] [CrossRef]
- Watanabe Costa, R.; da Silveira, J.F.; Bahia, D. Interactions between Trypanosoma cruzi secreted proteins and host cell signaling pathways. Front. Microbiol. 2016, 7, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Structure | Strain (Ref) | Chemical Synthesis (Ref) |
|---|---|---|
 | G [47,48] Dm28c [49] Colombiana [50] Tulahuen [51] | [86] |
 | Tulahuen [51] | [87] |
 | Tulahuen [51] | [87] |
 | G [47,48] Dn28c [49] Colombiana [50] Tulahuen [51] | [88] |
 | G [47,48] Dn28c [49] Colombiana [50] Tulahuen [51] | [89] |
 | G [47,48] Dn28c [49] Colombiana [50] Tulahuen [51] | [90] |
 | Tulahuen [51] | - |
 | Dm28c [49] Tulahuen [51] | [91] |
 | G [47,48] Dm28c [49] Colombiana [50] Tulahuen [51] | [92,93] |
 | Dm28c [49] | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgi, M.E.; Lederkremer, R.M.d. The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose. Molecules 2020, 25, 3913. https://doi.org/10.3390/molecules25173913
Giorgi ME, Lederkremer RMd. The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose. Molecules. 2020; 25(17):3913. https://doi.org/10.3390/molecules25173913
Chicago/Turabian StyleGiorgi, María Eugenia, and Rosa M. de Lederkremer. 2020. "The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose" Molecules 25, no. 17: 3913. https://doi.org/10.3390/molecules25173913