NBO/NRT Two-State Theory of Bond-Shift Spectral Excitation
Abstract
:1. Introduction
- (1)
- Natural bond orbital (NBO) [10,11] donor-acceptor interaction that transfers two electrons from a Lewis-type (L; formally occupied) NBO of the parent natural Lewis-structure (NLS) bonding pattern (e.g., amide nitrogen lone pair, nN) to a non-Lewis (NL; formally vacant) NBO (e.g., adjacent carbonyl pi-antibond, π*CO);
- (2)
- Robinson-type “curly arrow” depiction [12] of vicinal π-type delocalization, leading to πCO → πCN bond shift, with concomitant nn → nO lone pair shift and charge transfer;
- (3)
- Resonance-structural depiction of the secondary bonding pattern that results from the e-pair bond shift in the parent NLS.
2. NBO/NRT Deletion Tools for Describing Resonance Delocalization
3. Diabatic Two-State Model of Resonance Mixing
4. Chemical Applications
4.1. Methods
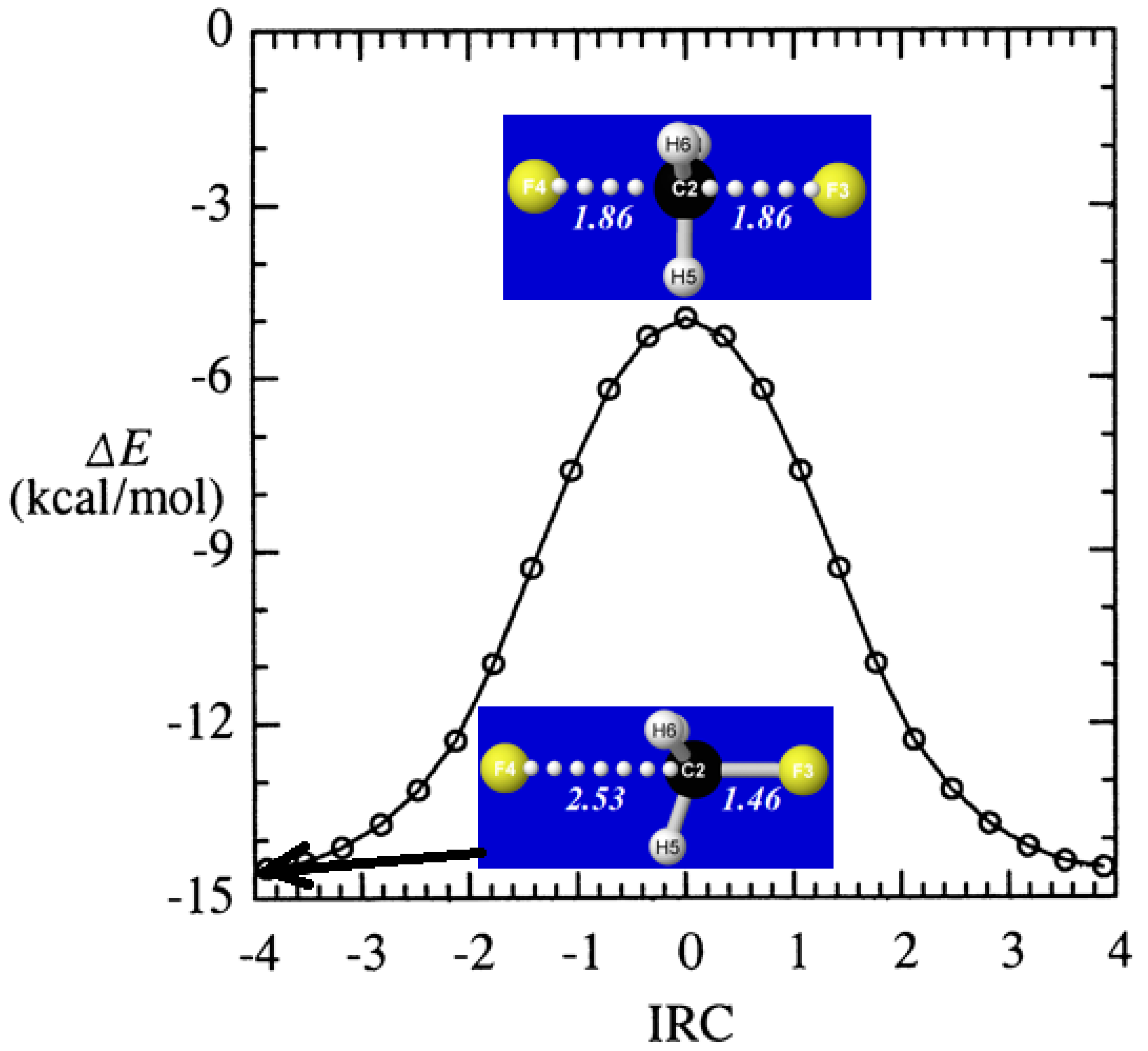
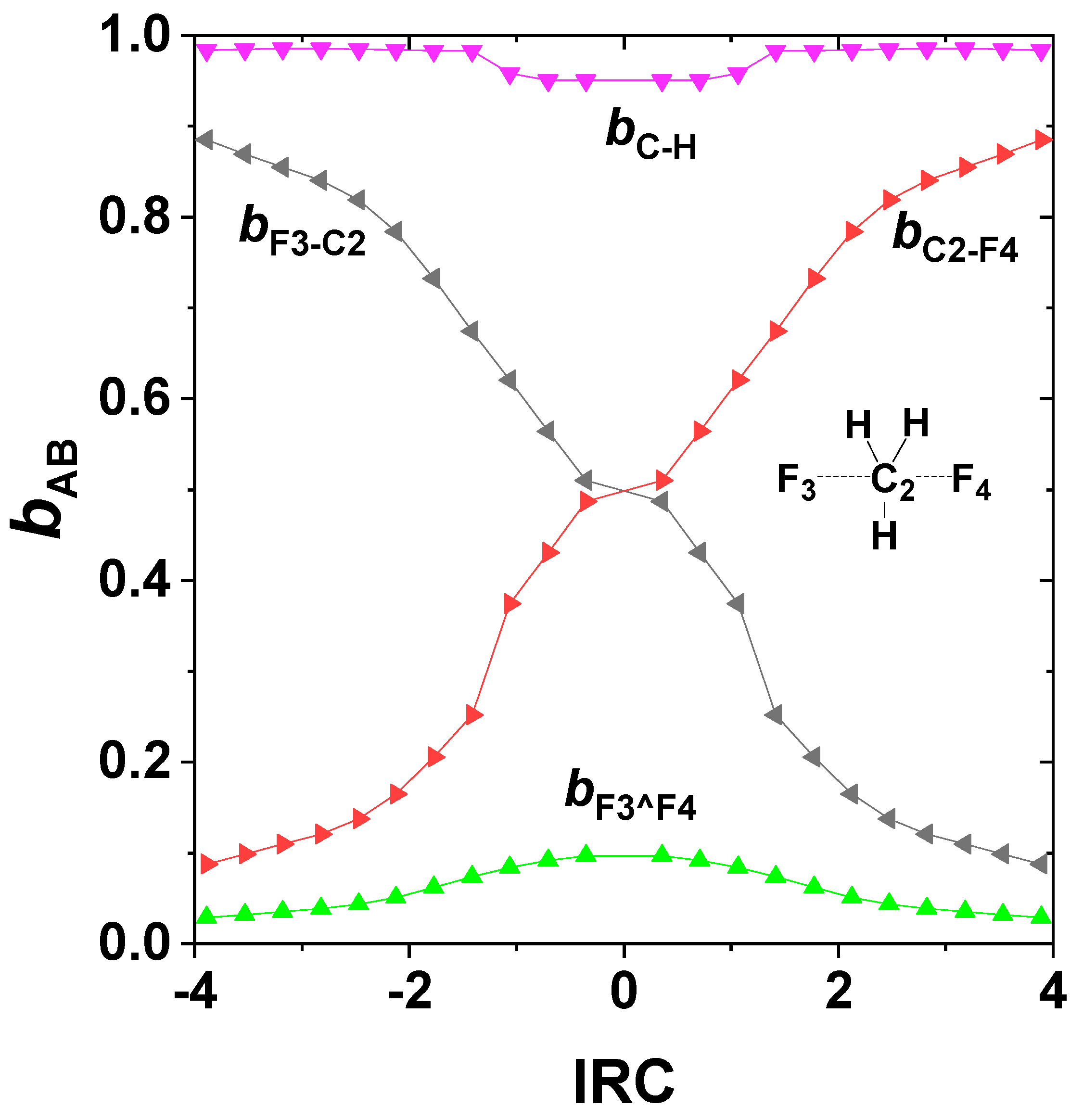
4.2. SN2 Fluoride Exchange Reaction
4.3. Polyene Bond Shifts of Cyanine Dyes
5. Concluding Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mulliken, R.S. Spectroscopy, molecular orbitals, and chemical bonding (Nobel Prize lecture, 1966). Science 1967, 157, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.N.; Kasha, M. Phosphorescence and the triplet state. J. Am. Chem. Soc. 1944, 66, 2100–2116. [Google Scholar] [CrossRef]
- Lewis, G.N.; Calvin, M.; Kasha, M. Photomagnetism. Determination of the paramagnetic susceptibility of a dye in its phosphorescent state. J. Chem. Phys. 1949, 17, 804–812. [Google Scholar] [CrossRef]
- Kasha, M. The triplet state: An example of G. N. Lewis’ research style. J. Chem. Educ. 1984, 61, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, H.E. Some theoretical aspects of organic photochemistry. Acc. Chem. Res. 1982, 10, 312–317. [Google Scholar] [CrossRef]
- Zimmerman, H.E.; Alabugin, I.V. Energy distribution and redistribution and chemical reactivity. Mechanistic and exploratory organic photochemistry. J. Am. Chem. Soc. 2000, 122, 952–953. [Google Scholar] [CrossRef]
- Kutateladze, A.G. Computational Methods in Photochemistry: Molecular and Supramolecular Photochemistry; CRC Press: Boca Raton, FL, USA, 2005; Volume 13. [Google Scholar]
- Olivucci, M. (Ed.) Computational Photochemistry; Elsevier Science: Amsterdam, The Netherlands, 2005; Volume 16. [Google Scholar]
- Albini, A. Photochemistry: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2016; p. 41ff. [Google Scholar]
- Weinhold, F.; Landis, C.R.; Glendening, E.D. What is NBO analysis and how is it useful? Int. Rev. Phys. Chem. 2016, 35, 399–440. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective; Cambridge U. Press: Cambridge, UK, 2005. [Google Scholar]
- Rzepa, H. The First Ever Curly Arrows. Available online: https://www.ch.imperial.ac.uk/rzepa/blog/?p=7234 (accessed on 2 September 2020).
- Glendening, E.D.; Weinhold, F. Natural resonance theory. I. General formulation. J. Comput. Chem. 1998, 19, 610–627. [Google Scholar] [CrossRef]
- Glendening, E.D.; Wright, S.J.; Weinhold, F. Efficient optimization of natural resonance theory weightings and bond orders by Gram-based convex programming. J. Comput. Chem. 2019, 40, 2028–2035. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Resonance theory reboot. J. Am. Chem. Soc. 2019, 141, 4156–4166. [Google Scholar] [CrossRef]
- Nori-Shargh, D.; Weinhold, F. Natural bond orbital theory of pseudo Jahn-Teller effects. J. Phys. Chem. A 2018, 122, 4490–4498. [Google Scholar] [CrossRef]
- Bersuker, I.B. Pseudo-Jahn-Teller effect—A two-state paradigm in formation, deformation, and transformation of molecular systems and solids. Chem. Rev. 2013, 113, 1351–1390. [Google Scholar] [CrossRef]
- Ullrich, C. Time-Dependent Density-Functional Theory: Concepts and Applications; Oxford U. Press: London, UK, 2012. [Google Scholar]
- Mulliken, R.S.; Person, W.B. Molecular Complexes; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Shaik, S.S. What happens to molecules as they react? A valence bond approach to reactivity. J. Am. Chem. Soc. 1981, 103, 3692–3701. [Google Scholar] [CrossRef]
- Shaik, S.S.; Pross, A. SN2 reactivity of CH3X derivatives. A valence bond approach. J. Am. Chem. Soc. 1982, 104, 2708–2719. [Google Scholar] [CrossRef]
- Shaik, S.S. The collage of SN2 reactivity patterns. A state correlation diagram model. Prog. Phys. Org. Chem. 1985, 15, 197–337. [Google Scholar]
- Sini, G.; Shaik, S.; Hiberty, P.C. Quantitative valence-bond computations of curve crossing diagrams for a gas-phase SN2 reactions, F− + CH3F → FCH3 + F−. J. Chem. Soc. Perkin Trans. 1992, 2, 1019–1025. [Google Scholar] [CrossRef]
- Shaik, S.S.; Schlegel, H.B.; Wolfe, S. Theoretical Aspects of Physical Organic Chemistry. Application to the SN2 Transition State; Wiley Interscience: New York, NY, USA, 1992. [Google Scholar]
- Song, L.; Wu, W.; Hiberty, P.C.; Shaik, S. The identity SN2 reactions X− + CH3-X → X-CH3 + X− (X = F, Cl, Br and I) in vacuum and in aqueous solution: A valence bond study. Chem. Eur. J. 2006, 12, 7458–7466. [Google Scholar] [CrossRef]
- Marcus, R.A. On the theory of oxidation-reduction reactions involving electron transfer. J. Chem. Phys. 1956, 24, 966–978. [Google Scholar] [CrossRef] [Green Version]
- Coulson, C.A. The nature of the bonding in xenon fluorides and related molecules. J. Chem. Soc. 1964, 1964, 1442–1454. [Google Scholar] [CrossRef]
- Pimentel, G.C. The bonding of trihalide and bifluoride ions by the molecular orbital method. J. Chem. Phys. 1951, 19, 446–448. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC recommendations 2011). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Weinhold, F.; Klein, R.A. What is a hydrogen bond? Resonance covalency in the supramolecular domain. Chem. Educ. Res. Pract. 2014, 15, 276–285. [Google Scholar] [CrossRef]
- Jiao, Y.; Weinhold, F. What is the nature of supramolecular bonding? Comprehensive NBO/NRT picture of halogen and pnicogen bonding in RPH2···IF/FI complexes (R=CH3, OH, CF3, CN, NO2). Molecules 2019, 24, 2090. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. The pnicogen bond: Its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Elguero, J.; Scheiner, S. (Eds.) Noncovalent Forces. Challenges and Advances in Computational Chemistry and Physics; Springer Cham: New York, NY, USA, 2015; Volume 19. [Google Scholar]
- Legon, A.C. Prereactive complexes of dihalogens XY with Lewis bases B in the gas phase: A systematic case for the halogen analogue B∙∙∙XY of the hydrogen bond B∙∙∙HX. Angew. Chem. Int. Ed. 1999, 38, 2686–2714. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model 2007, 7, 305–311. [Google Scholar] [CrossRef]
- Bauza, A.; Frontera, A. Aerogen bonding interaction: A new supramolecular force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef]
- Miao, J.; Xiong, Z.; Gao, Y. The effects of aergogen bonding on the geometries and spectral properties of serveral small molecular clusters containing XeO3. J. Phys. Cond. Matter 2018, 30, 444001. [Google Scholar] [CrossRef]
- Arunan, E. Hydrogen bond seen, halogen bond defined and carbon bond proposed: Intermolecular bonding, a field that is maturing! Curr. Sci. 2013, 105, 892–894. [Google Scholar]
- Michalczyk, M.; Zierkiewicz, W.; Wysokinski, R.; Scheiner, S. Theoretical studies of IR and NMR spectral change induced by sigma-hole hydrogen, halogen, chalcogen, pnicogen, and tetrel bonds in a model protein environment. Molecules 2019, 24, 3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauling, L.C. The theory of resonance in chemistry. Proc. Roy. Soc. 1977, A356, 433–441. [Google Scholar]
- Frenking, G.; Krapp, A. Unicorns in the world of chemical bonding models. J. Comput. Chem. 2007, 28, 15–24. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford U. Press: London, UK, 1994. [Google Scholar]
- Weinhold, F.; Landis, C.R. Discovering Chemistry with Natural Bond Orbitals; Wiley: Hoboken, NJ, USA, 2012; Section 5.3. [Google Scholar]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 3rd ed.; Gaussian Inc.: Wallingford, CT, USA, 2015. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 7.0: New vistas in localized and delocalized chemical bonding theory. J. Comput. Chem. 2019, 40, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0; Theoretical Chemistry Institute, U. Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
- Weinhold, F. Chemical bonding as a superposition phenomenon. J. Chem. Educ. 1999, 76, 1141–1146. [Google Scholar] [CrossRef]
- Shahi, A.; Arunan, E. Hydrogen bonding, halogen bonding and lithium bonding: An atoms in molecules and natural bond orbital perspective towards conservation of total bond order, inter- and intra-molecular bonding. Phys. Chem. Chem. Phys. 2014, 16, 22935–22952. [Google Scholar] [CrossRef]
- Coulson, C.A. The electronic structure of some polyenes and aromatic molecules. VII. Bonds of fractional order in the molecular orbital method. Proc. R. Soc. London A 1939, 169, 413–428. [Google Scholar]
- Brown, R.L. Rate constants for H-atom transfer reactions by the BEBO method. J. Res. Nat. Bur. Std. 1981, 86, 605–654. [Google Scholar] [CrossRef]
- Badger, R.M. A relation between internuclear distances and bond force constants. J. Chem. Phys. 1934, 2, 138. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Casida, M.E.; Huix-Rotlant, M. Progress in time-dependent density-functional theory. Ann. Rev. Phys. Chem. 2012, 63, 287–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeshi, Y.; Tew, D.P.; Handy, N.C. A new hybrid-exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Weinhold, F.; Glendening, E.D. NBO 7.0 Manual. Available online: http://nbo7.chem.wisc.edu/nboman.pdf (accessed on 2 September 2020).
- Weinhold, F.; Phillips, D.; Glendening, E.D.; Foo, Z.Y.; Hanson, R.M. NBOPro7@Jmol; Theoretical Chemistry Institute, U. Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
- Ingold, C.K. Principles of an electronic theory of organic reactions. Chem. Rev. 1934, 15, 225–274. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Landis, C.R.; Weinhold, F. 3c/4e σ-type long-bonding: A novel NBO motif toward the metallic delocalization limit. Inorg. Chem. 2013, 52, 5154–5166. [Google Scholar] [CrossRef]
- Mazziotti, D.A. Structure of fermionic density matrices: Complete N-representability conditions. Phys. Rev. Lett. 2012, 108, 263002. [Google Scholar] [CrossRef] [Green Version]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dyes Pigm. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Kuhn, H.A. Quantum-mechanical theory of light absorption of organic dyes and similar compounds. J. Chem. Phys. 1949, 17, 1198–1212. [Google Scholar] [CrossRef] [Green Version]
- Platt, J.R. Classification of spectra of cata-condensed hydrocarbons. J. Chem. Phys. 1949, 17, 484–495. [Google Scholar] [CrossRef]
- Le Guennic, L.; Jacquemin, D. Taking up the cyanine challenge with quantum tools. Acc. Chem. Res. 2015, 17, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.; Ayres, P.W.; Boto, R.; Carbó-Dorca, R.; Ciolowski, J.; Chermette, H.; Contreras García, J.; Cooper, D.; Frenking, G.; Gatti, C.; et al. Nine questions on energy decomposition analysis. J. Comput. Chem. 2019, 40, 2248–2283. [Google Scholar] [CrossRef] [PubMed]
- Morokuma, K. Why do molecules interact? The origin of electron donor-acceptor complexes, hydrogen bonding and proton affinity. Acc. Chem. Res. 1977, 10, 294–300. [Google Scholar] [CrossRef]
- Bickelhaupt, F.M.; Baerends, E.J. Kohn-Sham density functional theory: Predicting and understanding chemistry. In Reviews in Computational Chemistry; Lipkowitz, K.B., Boyd, D.B., Eds.; Wiley-VCH: New York, NY, USA, 2000; Volume 15, pp. 1–86. [Google Scholar]
- Mo, Y.; Gao, J. Energy decomposition analysis of intermolecular interactions using a block-localized wave function approach. J. Chem. Phys. 2000, 112, 5530–5538. [Google Scholar] [CrossRef]
- Khaliullin, R.Z.; Cobar, E.A.; Lochan, R.C.; Bell, A.T.; Head-Gordon, M. Unravelling the origin of intermolecular interactions using absolutely localized molecular orbitals. J. Phys. Chem. A 2007, 111, 8753–8765. [Google Scholar] [CrossRef]
- Horn, P.R.; Mao, Y.; Head-Gordon, M. Probing non-covalent interactions with a second generation energy decomposition analysis using localized molecular orbitals. Phys. Chem. Chem. Phys. 2016, 18, 23067–23079. [Google Scholar] [CrossRef]
- Glendening, E.D.; Streitwieser, A. Natural energy decomposition analysis: An energy partitioning procedure for molecular interactions with application to weak hydrogen bonding, strong ionic, and moderate donor-acceptor interactions. J. Chem. Phys. 1994, 100, 2900–2909. [Google Scholar] [CrossRef]
- Schenter, G.K.; Glendening, E.D. Natural energy decomposition analysis: The linear response electrical self energy. J. Phys. Chem. 1996, 100, 17152–17156. [Google Scholar] [CrossRef]
- Glendening, E.D. Natural energy decomposition analysis: Extension to density functional methods and analysis of cooperative effects in water clusters. J. Phys. Chem. A 2005, 109, 11936–11940. [Google Scholar] [CrossRef]
- Weinhold, F.; Carpenter, J.E. Some remarks on nonorthogonal orbitals in quantum chemistry. J. Mol. Struct. THEOCHEM 1988, 165, 189–202. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Phipps, M.J.S.; Fox, T.; Tautermann, C.S.; Skylaris, C.-K. Energy decomposition analysis approaches and their evaluation on prototypical protein-drug interaction patterns. Chem. Soc. Rev. 2015, 44, 3177–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |
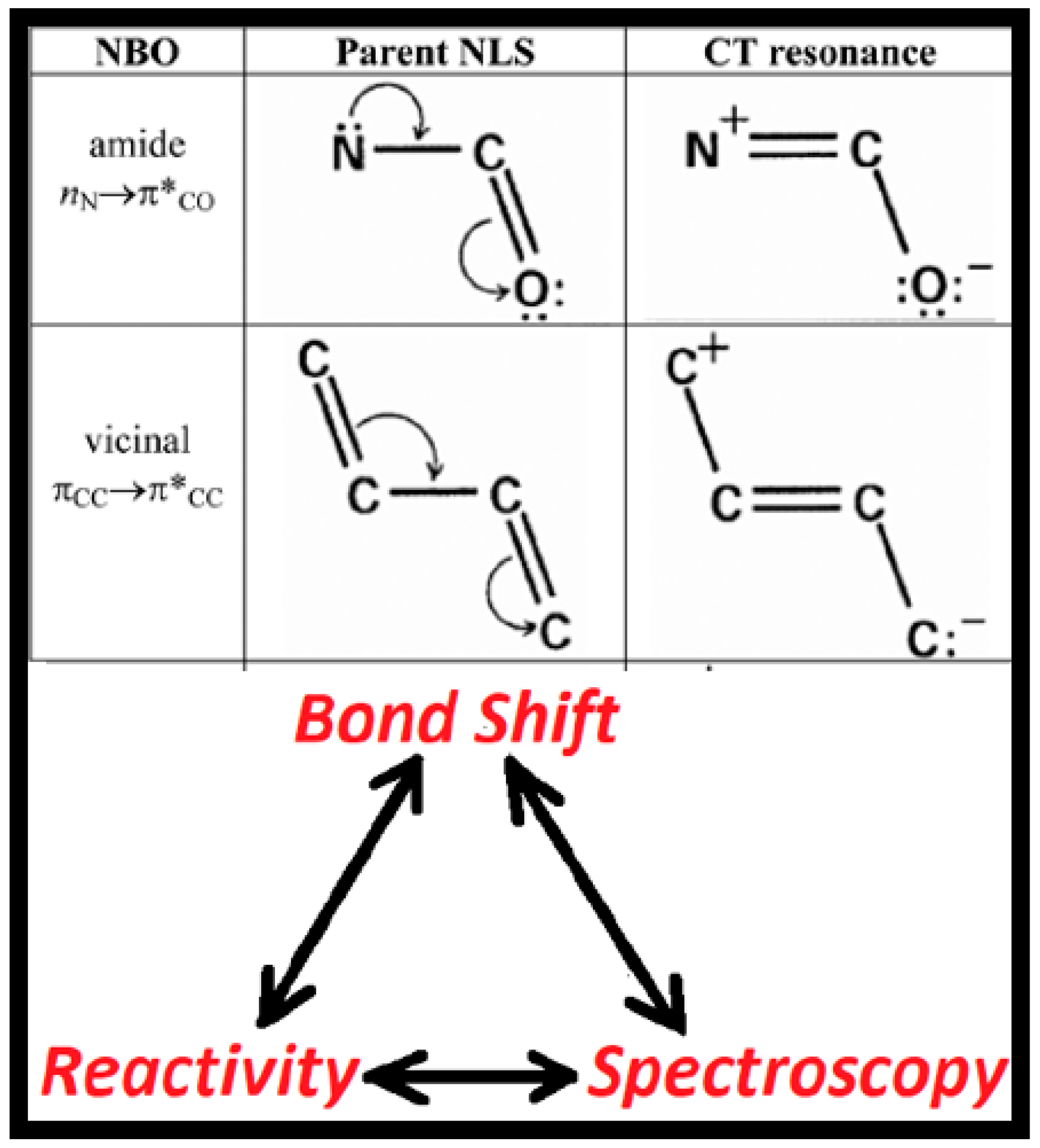






| Resonance Structure | I | II |
|---|---|---|
| (i) reactivity | reactant (R) | product (P) |
| (ii) spectroscopy | ground-state (g.s.) | excited state (x.s.) |
| (iii) single-determinant wavefunction | ΨI(SD) | ΨII(SD) |
| (iv) NBO configuration type | NLS | 2e L→NL excitation |
| Method | HF | B3LYP | CAM-B3LYP | M06 | wB97XD |
|---|---|---|---|---|---|
| ΔE(2 − st) | 160 | 171 | 162 | 169 | 158 |
| ΔE(CIS/TD) | 213 | 214 | 228 | 208 | 230 |
| (a) nN→πCC* | n = 2 | n = 3 | n = 4 | n = 5 |
| ΔEDA(2) | 60.85 | 57.74 | 55.77 | 54.34 |
| ΔEDA($DEL) | 64.71 | 61.71 | 59.88 | 58.97 |
| (b) πCC→πCN* | ||||
| ΔEDA(2) | 58.53 | 60.42 | 61.73 | 62.88 |
| ΔEDA($DEL) | 67.14 | 66.30 | 64.99 | 64.14 |
| (c) πCC→πCC* | ||||
| ΔEDA(2) | 35.04 | 34.68 30.93 | 29.06 30.48 34.90 | 27.95 27.20 29.00 35.87 |
| ΔEDA($DEL) | 41.83 | 36.56 35.96 | 33.80 31.09 33.39 | 33.22 27.54 26.53 31.80 |
| δn(av) | 57.89 | 50.13 | 44.63 | 40.37 |
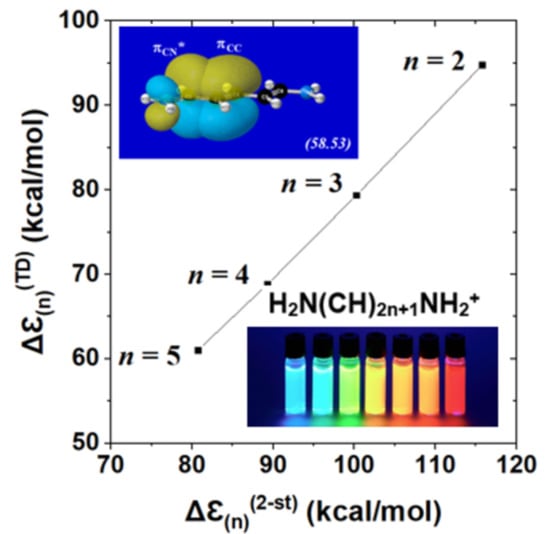
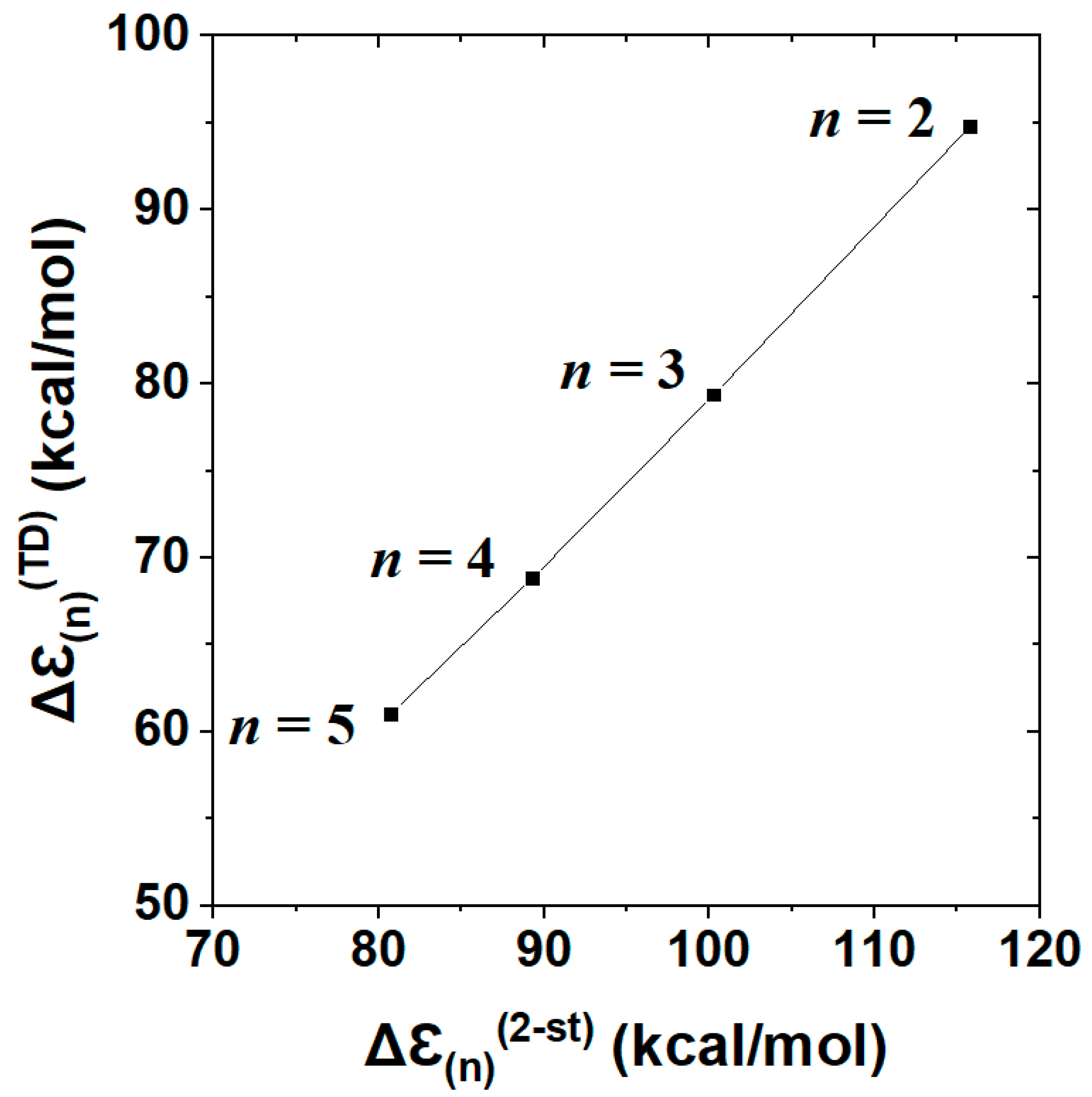
| n | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Δℇ(n)(2 − st) | 115.78 | 100.26 | 89.26 | 80.74 |
| Δℇ(n)(TD) | 94.81 | 79.38 | 68.79 | 61.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Weinhold, F. NBO/NRT Two-State Theory of Bond-Shift Spectral Excitation. Molecules 2020, 25, 4052. https://doi.org/10.3390/molecules25184052
Jiao Y, Weinhold F. NBO/NRT Two-State Theory of Bond-Shift Spectral Excitation. Molecules. 2020; 25(18):4052. https://doi.org/10.3390/molecules25184052
Chicago/Turabian StyleJiao, Yinchun, and Frank Weinhold. 2020. "NBO/NRT Two-State Theory of Bond-Shift Spectral Excitation" Molecules 25, no. 18: 4052. https://doi.org/10.3390/molecules25184052