Synthesis, Anti-proliferative Activity, and Molecular Docking Study of New Series of 1,3-5-Triazine Schiff Base Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
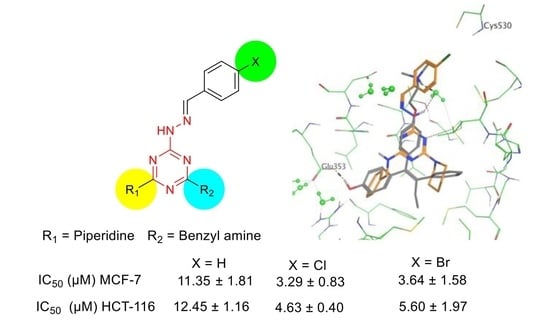
2.2.1. Anti-Proliferative Activity and Induced Apoptosis
2.2.2. Molecular Docking Study
3. Materials and Methods
3.1. General Method for the Synthesis of 1,3,5-triazine Schiff Base Derivatives
3.2. Biology
3.2.1. In Vitro Anti-Proliferative Assay
3.2.2. Annexin V/PI Apoptotic Assay:
3.2.3. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Min, J.; Liu, Z.; Young, A.; Deshazer, H.; Gao, T.; Chang, Y.T.; Kallenbach, N.R. Synthesis and biological evaluation of novel 1,3,5-triazinederivatives as antimicrobial agents. Bioorg. Med. Chem. Lett. 2008, 18, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Solankee, A.; Kapadia, K.; Ciric, A.; Sokovic, M.; Doytchinova, I.; Geronikaki, A. Synthesis of some new s-triazine based chalcones and their derivatives as potent antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Kumari, P.; Chikhalia, K.H. Design, synthesis and antimicrobial screening of s-triazinyl piperazine and piperidine derivatives. Int. J. Adv. Pharm. Sci. 2010, 1, 395–403. [Google Scholar]
- Kumar, G.V.P.; Srinivasa, R.D.; Pooja, B.; Harika, G.; Kumar, Y.A.; Sadasiva, R.G. An extensive review on 1,2,3 and 1,2,4-triazines scaffold-valuable lead molecules with potent and diverse pharmacological activities. Der Chem. Sin. 2016, 7, 101–130. [Google Scholar]
- Ma, X.; Tan, S.-T.; Khoo, C.-L.; Sim, H.-M.; Chan, L.-W.; Chui, W.K. Synthesis and antimicrobial activity of N1-benzyl or N1-benzyloxy-1, 6-dihydro-1, 3, 5 triazine-2, 4-diamines. Bioorg. Med. Chem. Lett. 2011, 21, 5428–5431. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.; Utreja, D. Recent Advances in Synthesis and antifungal activity of 1,3,5-triazines. Curr. Org. Synth. 2016, 13, 484–503. [Google Scholar] [CrossRef] [Green Version]
- Manohar, S.; Khan, S.I.; Rawat, D.S. 4-Aminoquinoline-triazine-based hybrids with improved in vitro antimalarial activity against CQ-sensitive and CQ-resistant strains of plasmodium falciparum. Chem. Biol. Drug Des. 2013, 81, 625–630. [Google Scholar] [CrossRef]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.Y.; Montero, J.L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg. Med. Chem. Lett. 2004, 14, 5427–5433. [Google Scholar] [CrossRef]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.Y.; Montero, J.L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II, and IX. Bioorg. Med. Chem. Lett. 2005, 15, 3102–3108. [Google Scholar] [CrossRef]
- Ceruso, M.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Inhibition of human carbonic anhydrase isoforms I-XIV with sulfonamides incorporating fluorine and 1,3,5-triazine moieties. Bioorg. Med. Chem. 2013, 21, 6929–6936. [Google Scholar] [CrossRef]
- Carta, F.; Garaj, V.; Maresca, A.; Wagner, J.; Avvaru, B.S.; Robbins, A.H.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII, and XIV over cytosolic isoforms I and II: Solution and X-ray crystallographic studies. Bioorg. Med. Chem. 2011, 19, 3105–3119. [Google Scholar] [CrossRef] [PubMed]
- Lolaka, N.; Akocaka, S.; Buab, S.; Supuranb, C.T. Design, synthesis and biological evaluation of novel ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as potent carbonic anhydrase IX inhibitors. Bioorg. Chem. 2019, 82, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xu, C.; Ma, J.; Sun, Y.; Du, F.; Liu, H.; Lin, L.; Li, C.; Ding, J.; Chen, K.; et al. Synthesis and antitumor evaluation of a novel series of triaminotriazine derivatives. Bioorg. Med. Chem. 2007, 15, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Keum, Y.S.; Park, S.W. Medicinal chemistry discoveries among 1,3,5- triazines: Recent advances (2000–2013) as antimicrobial, anti-TB, anti-HIV and antimalarials. Mini Rev. Med. Chem. 2014, 14, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, T.; Zhou, Z.; Du, L. A systematic review on antitumor agents with 1,3,5-triazines. Med. Chem. 2015, 5, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Cascioferro, S.; Parrino, B.; Spano, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. 1,3,5-Triazines: A promising scaffold, for anticancer drugs development. Eur. J. Med. Chem. 2017, 142, 523–549. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Desmukh, A.P.; Soni, P.K.; Kankoriya, A.; Halve, A.K.; Dixit, R. 4-Aminoantipyrine: A significant tool for the synthesis of biologically active Schiff bases and metal complexes. Int. J. Pharm. Sci. Rev. Res. 2015, 34, 162–170. [Google Scholar]
- Goel, P.; Kumar, D.; Chandra, S. Schiff’s Base Ligands and their transition metal complexes as Antimicrobial agents. J. Chem. Biol. Phy. Sci. 2014, 2014, 1946–1964. [Google Scholar]
- Ebrahimipour, S.Y.; Khosravan, M.; White, J.; Fekri, S. Preparation, crystal structure, spectroscopic studies, DFT calculations, antibacterial activities and molecular docking of a tridentate Schiff base ligand and its cis-MoO2 complex. Appl. Organomet. Chem. 2018, 32, e4233. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013, 2013, 14. [Google Scholar] [CrossRef] [Green Version]
- Uddin, N.; Rashid, F.; Ali, S.; Tirmizi, S.A.; Ahmad, I.; Zaib, S.; Zubir, M.; Diaconescu, P.L.; Tahir, M.N.; Iqbal, J.; et al. Synthesis, characterization, and anticancer activity of Schiff bases. J. Biomol. Str. Dynam. 2020, 38, 3246–3259. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Abd-Elzaher, M.M.; Labib, A.A.; Mousa, H.A.; Moustafa, S.A.; Ali, M.M.; El-Rashedy, A.A. Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety. Beni Suef Univ. J. Basic. Appl. Sci. 2016, 5, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Menear, K.A.; Gomez, S.; Malagu, K.; Bailey, C.; Blackburn, K.; Cockcroft, X.L.; Sebastian, L. Identification and optimisation of novel and selective small molecular weight kinase inhibitors of mTOR. Bioorg Med. Chem. Lett. 2009, 19, 5898–5901. [Google Scholar] [CrossRef]
- Bai, F.; Liu, H.; Tong, L.; Zhou, W.; Liu, L.; Zhao, Z.; Liu, X.; Jiang, H.; Wang, X.; Xie, H. Discovery of novel selective inhibitors for EGFR-T790M/L858R. Bioorg. Med. Chem. Lett. 2012, 22, 1365–1370. [Google Scholar] [CrossRef]
- Herke Herke, B.R.; Consler, T.G.; Go, N.; Hale, R.L.; Hohman, D.R.; Jones, S.A.; Lu, A.T.; Moore, L.B.; Moore, J.T.; Orband-Miller, L.A.; et al. A new series of estrogen receptor modulators that display selectivity for estrogen receptor. β J. Med. Chem. 2002, 45, 5492–5505. [Google Scholar]
- Hale, R.L.; Henke, B.R.; Lambert, M.H.; Lu, A.T.; Spearing, P.K.; Turnbull, P.S. Piperazinyltriazine as Estrogen Modulators. U.S. Patent 6,943,162 B2, 13 September 2005. [Google Scholar]
- Lu, X.; Huang, A.; Xiao, M.; Sun, L.; Mao, J.; Luo, G.; Xiang, H. A new class of 1,3,5-triazine-based selective estrogen receptor degraders (SERDs): Lead optimization, molecular docking and dynamic simulation. Bioorg. Chem. 2020, 97, 103666. [Google Scholar] [CrossRef]
- El-Faham, A.; Soliman, S.M.; Ghabbour, H.A.; Elnakady, Y.A.; Mohaya, T.A.; Siddiqui, M.R.; Albericio, F. Ultrasonic promoted synthesis of novel s-triazine-Schiff base derivatives; molecular structure, spectroscopic studies and their preliminary anti-proliferative activities. J. Mol. Str. 2016, 1125, 121–135. [Google Scholar] [CrossRef]
- Barakat, A.; El-Senduny, F.F.; Almarhoon, Z.; Al-Rasheed, H.H.; Badria, F.A.; Al-Majid, A.M.; El-Faham, A. Synthesis, X-ray crystal structures, and preliminary antiproliferative activities of new s-triazine-hydroxybenzylidene hydrazone derivatives. J. Chem. 2019, 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Al Rasheed, H.H.; Malebari, A.M.; Dahlous Kh., A.; El-Faham, A. Synthesis and characterization of new series of 1,3-5-triazine hydrazone derivatives with promising anti-proliferative activity. Molecules 2020, 25, 2708. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ghabbour, H.; Khan, S.T.; Beatriz, G.; Albericio, F.; El-Faham, A. Novel pyrazolyl-s-triazine derivatives, molecular structure and antimicrobial activity. J. Mol. Str. 2017, 1145, 244–253. [Google Scholar] [CrossRef]
- El-Faham, A.A.; Elnakady, Y. Synthesis, characterization of novel morpholino-1, 3, 5-triazinyl amino acid Ester derivatives and their anti-proliferation activities. Lett. Org. Chem. 2015, 12, 753–758. [Google Scholar] [CrossRef]
- Hassan, F.; Mohammed, G.; El-Hiti, G.A.; Alshanon, A.; Yousif, E. Cytotoxic effects of tamoxifen in breast cancer cells. J. Unexplored Med. Data 2018, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Al Rasheed, H.H.; Dahlous Kh., A.; Sharma, A.; Sholkamy, E.; El-Faham, A.; De La Torre, B.G.; Albericio, F. Barbiturate- and Thiobarbituarte-based s-triazine hydrazone derivatives with promising antiproliferative activities. ACS Omega 2020, 5, 15805–15811. [Google Scholar] [CrossRef]
- Alaghaz, A.; Bayoumi, H.A. Synthesis, Spectral Properties and Potentiometric Studies on Some Metal Schiff Base Complexes Derived from 4–chlorophenyl–2–aminothiazole. Int. J. Electrochem. Sci. 2013, 8, 11860–11876. [Google Scholar]
- Mojena, M.; Povo-Retana, A.; González-Ramos, S.; Fernández-García, V.; Regadera, J.; Zazpe, A.; Artaiz, I.; Martín-Sanz, P.; Ledo, F.; Boscá, L. Benzylamine and Thenylamine derived drugs induce apoptosis and reduce proliferation, migration and metastasis formation in melanoma cells. Front. Oncol. 2018, 8, 328. [Google Scholar] [CrossRef]
- Carr, M.; Knox, A.J.; Lloyd, D.G.; Zisterer, D.M.; Meegan, M.J. Development of the β-lactam type molecular scaffold for selective estrogen receptor α modulator action: Synthesis and cytotoxic effects in MCF-7 breast cancer cells. J. Enzy. Inhib. Med. Chem. 2016, 31, 130–117. [Google Scholar] [CrossRef]
- Ma, D.; Tremblay, P.; Mahngar, K.; Akbari-Asl, P.; Collins, J.; Hudlicky, T.; McNulty, J.; Pandey, S. A novel synthetic C-1 analogue of 7-deoxypancratistatin induces apoptosis in p53 positive and negative human colorectal cancer cells by targeting the mitochondria: Enhancement of activity by tamoxifen. Investig. New Drugs 2012, 30, 1012–1027. [Google Scholar] [CrossRef]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef]
- Ogawa, S.; Inoue, S.; Watanabe, T.; Hiroi, H.; Orimo, A.; Hosoi, T.; Ouchi, Y.; Muramatsu, M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem. Biophys. Res. Commun. 1998, 243, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Chemical Computing Group. Molecular Operating Environment (MOE); ULC: Montreal, QC, Canada, 2020. [Google Scholar]
- BIOVIA, Dassault Systèmes. Available online: http://accelrys.com/products/collaborative-science/biovia-pipeline-pilot/ (accessed on 20 July 2020).
Sample Availability: Samples of the compounds are available from the authors. |






| Compound no. | IC50 (μM) a MCF-7 | IC50 (μM)a HCT-116 |
|---|---|---|
| 4a | 11.35 ± 1.81 | 12.45 ± 1.16 |
| 4b | 3.29 ± 0.83 | 3.64 ± 1.58 |
| 4c | 4.63 ± 0.40 | 5.60 ± 1.97 |
| 4d | 28.62 ± 2.80 | >50 |
| 4e | 33.90 ± 0.94 | >50 |
| 4f | 18.49 ± 1.03 | >50 |
| 4g | 24.46 ± 1.83 | 14.01 ± 1.61 |
| 4h | 16.44 ± 1.13 | 8.19 ± 0.22 |
| 4i | 22.20 ± 2.59 | 12.22 ± 0.34 |
| 4j | 18.59 ± 1.62 | 24.10 ± 3.94 |
| 4k | 14.08 ± 0.06 | 42.34 ± 4.74 |
| 4l | 26.91 ± 1.20 | 37.29 ± 0.34 |
| 4m | 7.93 ± 0.77 | 5.10 ± 1.01 |
| 4n | 6.10 ± 0.42 | 4.54 ± 0.41 |
| 4o | 6.58 ± 0.65 | 10.71± 2.91 |
| 4p | 3.98 ± 0.22 | 8.45 ± 0.24 |
| 4q | 9.37 ± 1.74 | 6.26 ± 0.49 |
| 4r | 10.44 ± 0.43 | 7.57 ± 1.34 |
| Tamoxifen b | 5.12 ± 0.36 | 26.41 ± 4.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Rasheed, H.H.; Malebari, A.M.; Dahlous, K.A.; Fayne, D.; El-Faham, A. Synthesis, Anti-proliferative Activity, and Molecular Docking Study of New Series of 1,3-5-Triazine Schiff Base Derivatives. Molecules 2020, 25, 4065. https://doi.org/10.3390/molecules25184065
Al Rasheed HH, Malebari AM, Dahlous KA, Fayne D, El-Faham A. Synthesis, Anti-proliferative Activity, and Molecular Docking Study of New Series of 1,3-5-Triazine Schiff Base Derivatives. Molecules. 2020; 25(18):4065. https://doi.org/10.3390/molecules25184065
Chicago/Turabian StyleAl Rasheed, Hessa H., Azizah M. Malebari, Kholood A. Dahlous, Darren Fayne, and Ayman El-Faham. 2020. "Synthesis, Anti-proliferative Activity, and Molecular Docking Study of New Series of 1,3-5-Triazine Schiff Base Derivatives" Molecules 25, no. 18: 4065. https://doi.org/10.3390/molecules25184065