Molecular Engineering Enhances the Charge Carriers Transport in Wide Band-Gap Polymer Donors Based Polymer Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
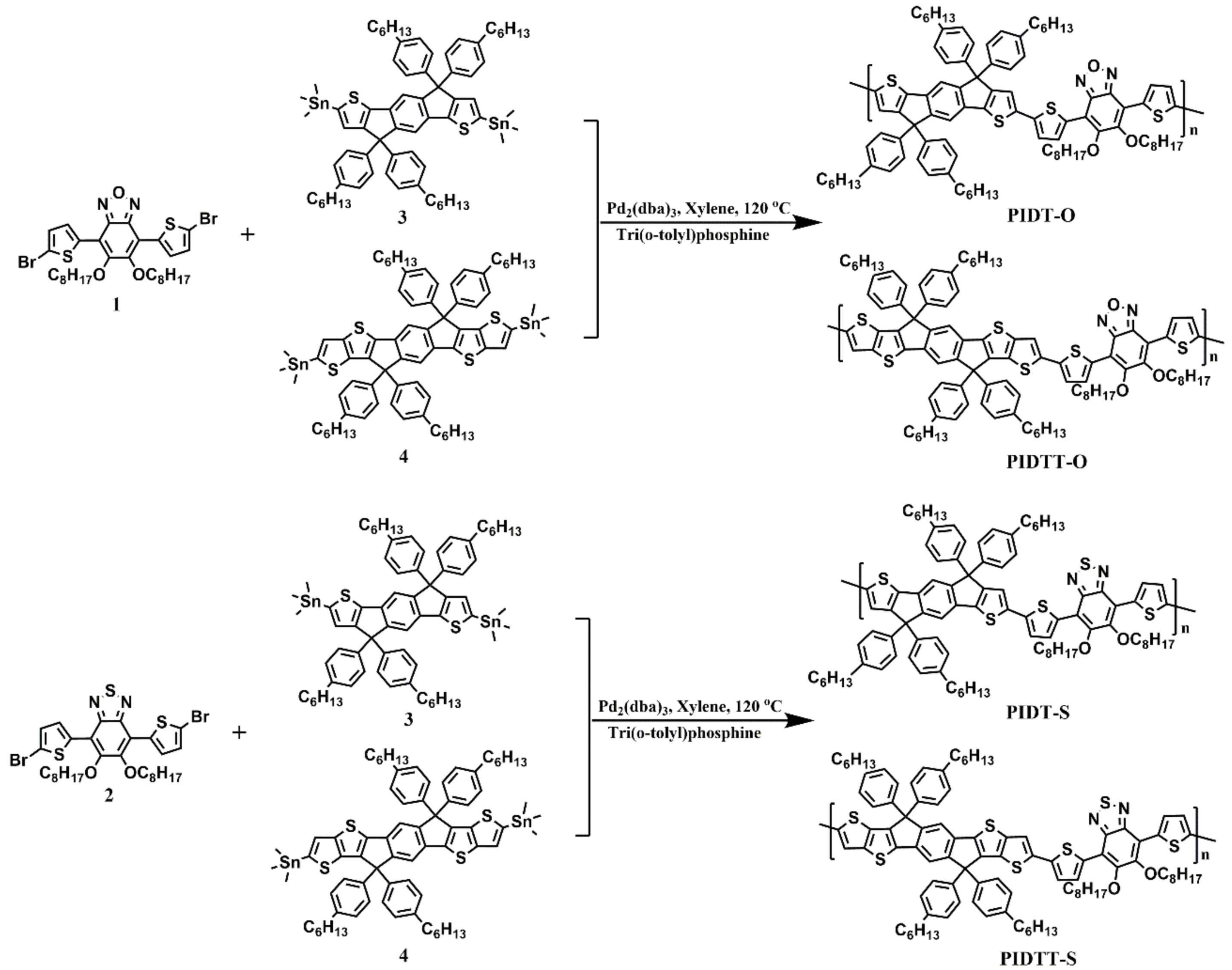
2.1. Synthesis
2.2. Thermal Properties
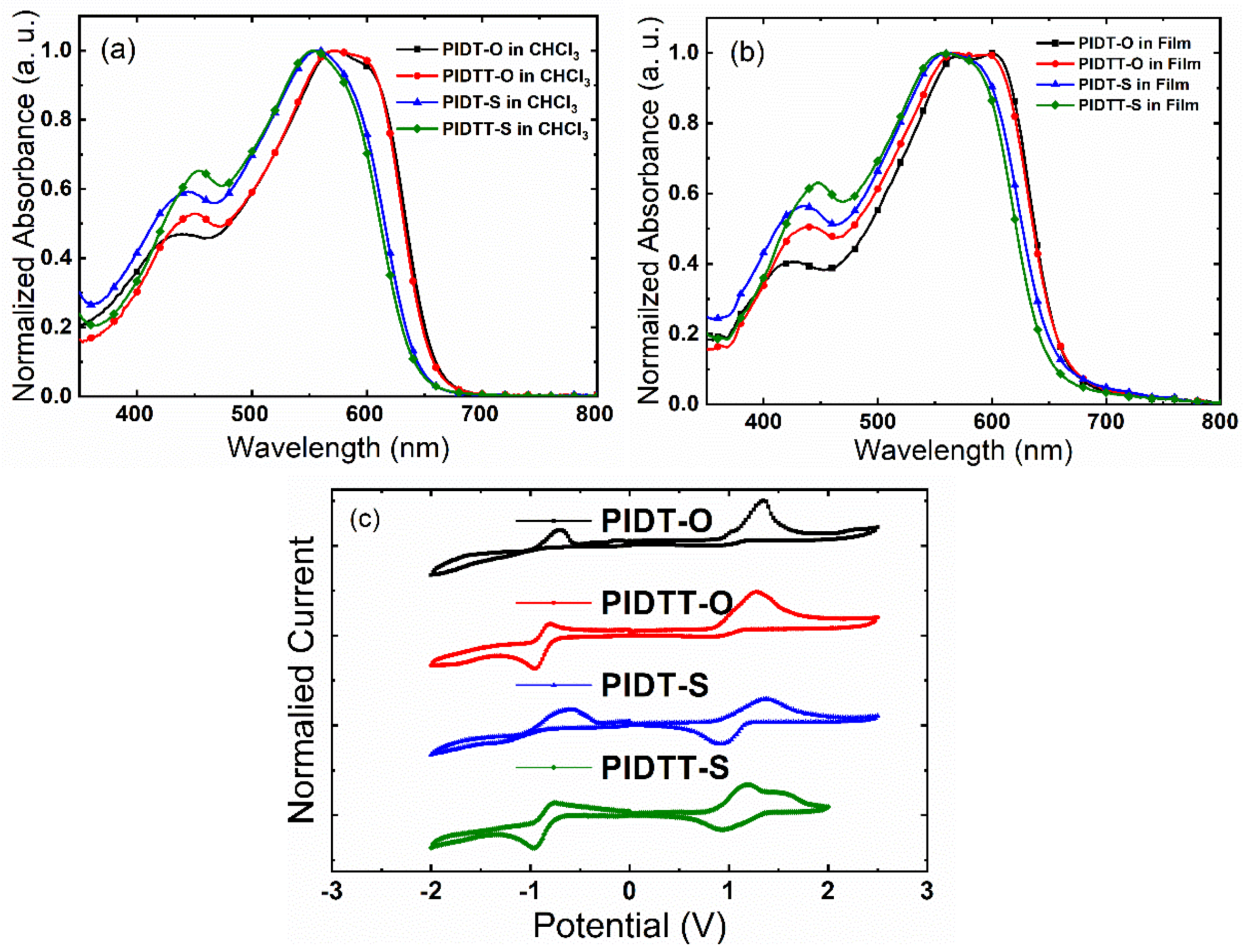
2.3. UV-vis Absorption and Electrochemical Properties
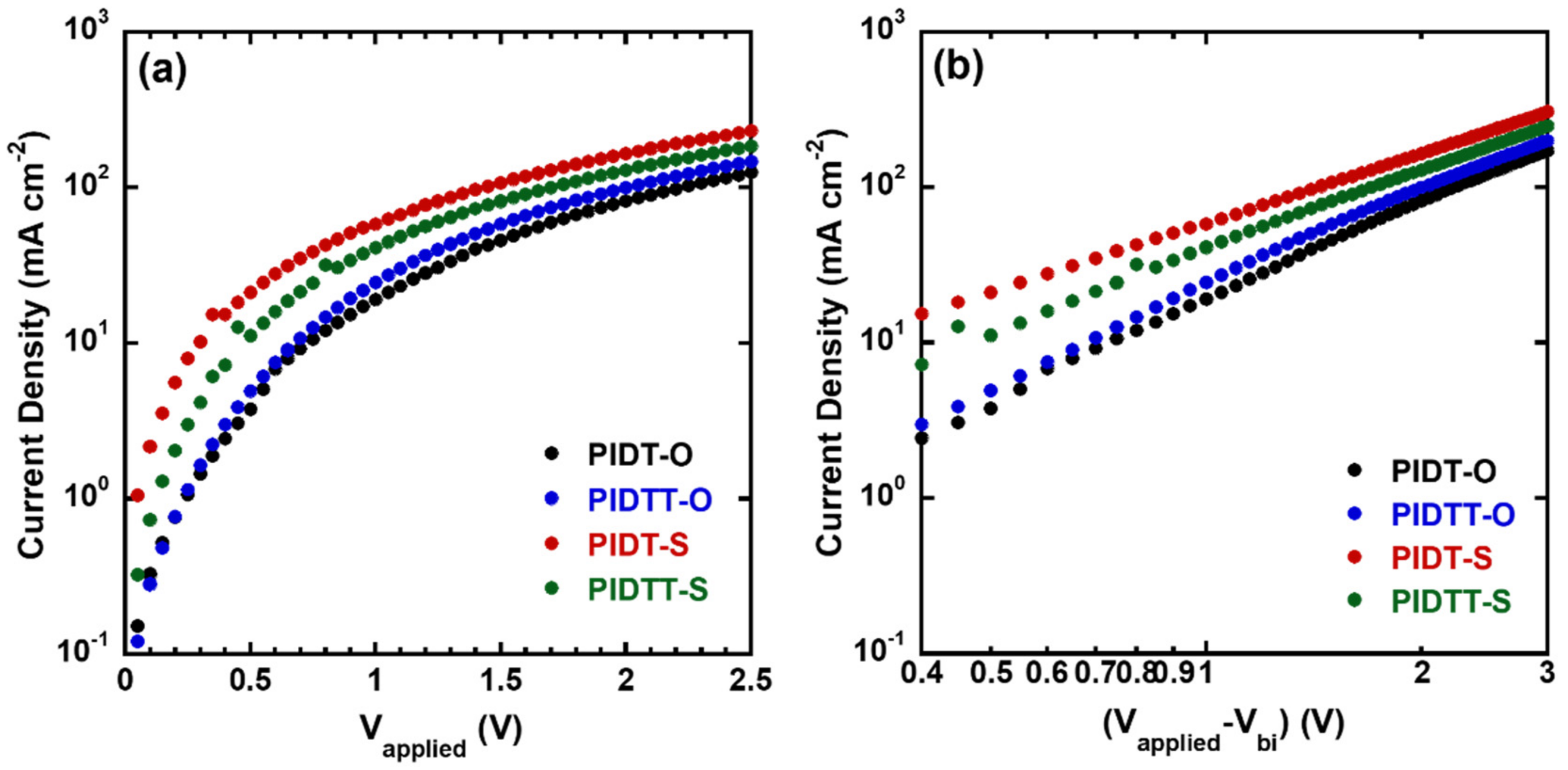
2.4. Hole Mobilities
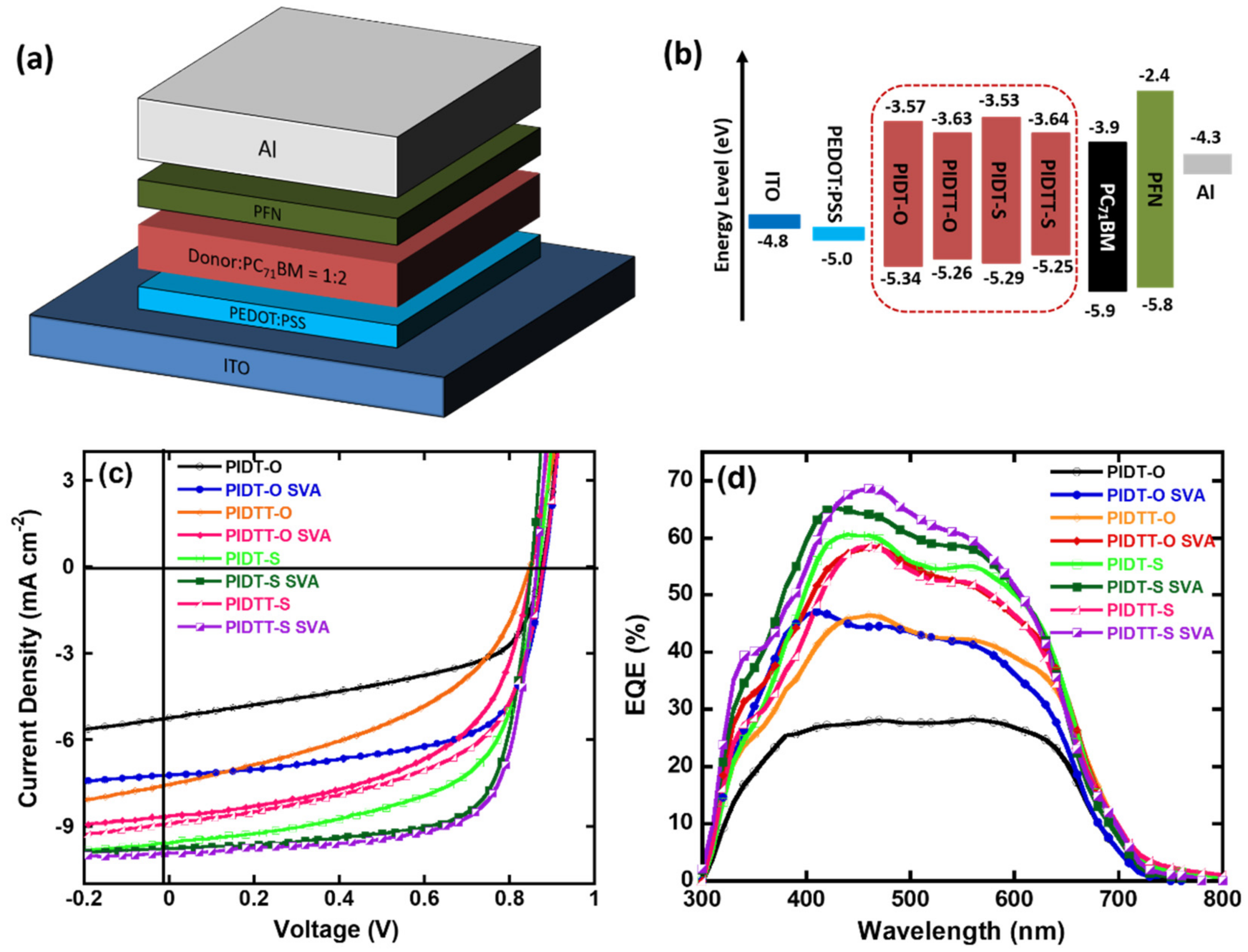
2.5. Photovoltaic Properties
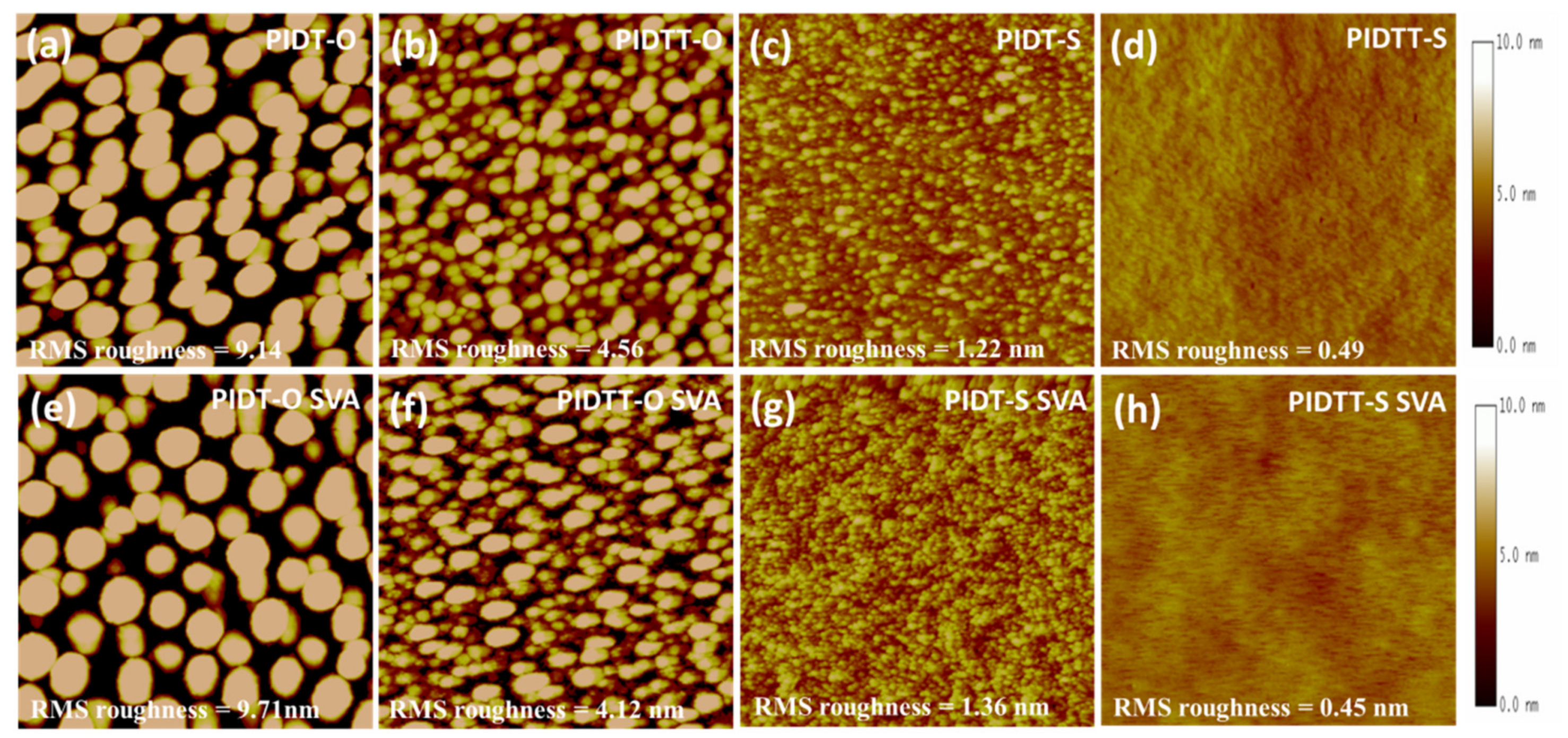
2.6. Atomic Force Microscopy Topographies
3. Experimental Section
3.1. Characterization and Instrumentation
3.2. Device Fabrication
3.3. Synthesis of Polymers
3.3.1. General Procedure for Preparing Polymers
3.3.2. Poly(indacenodithiophene-alt-4,7-di(thiophen-2-yl)-5,c-bis(octyloxy) benzoxadiazole) (PIDT-O)
3.3.3. Poly(indacenodithieno[3,2-b]thiophene-alt-4,7-di(thiophen-2-yl)-5,6-bis(octyloxy) benzoxadiazole) (PIDTT-O)
3.3.4. Poly(indacenodithiophene-alt-4,7-di(thiophen-2-yl)-5,6-bis(octyloxy) benzothiadizole) (PIDT-S)
3.3.5. Poly(indacenodithieno[3,2-b]thiophene-alt-4,7-di(thiophen-2-yl)-5,6-bis(octyloxy) benzothiadizole) (PIDTT-S)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brabec, C.J. Organic photovoltaics: Technology and market. Sol. Energy Mater. Sol. Cells. 2004, 83, 273–279. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, S.; Adhikari, N.; Chen, J.; Ngo, E.C.; Dubey, A.; Galipeau, D.W.; Qiao, Q. Interplay of nanoscale domain purity and size on charge transport and recombination dynamics in polymer solar cells. Nanoscale 2014, 6, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.; Abdelsamie, M.; Toney, M.F.; Bazan, G.C. Solvent additives: Key morphology-directing agents for solution-processed organic solar cells. Adv. Mater. 2018, 30, 1707114. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, C.; Zhan, X. Morphology control in organic solar cells. Adv. Energy Mater. 2018, 8, 1703147. [Google Scholar] [CrossRef]
- Sieval, A.B.; Hummelen, J.C. Device physics and manufacturing technologies. In Organic Photovoltaics: Materials; Brabec, C., Scherf, U., Dyakonov, V., Eds.; Wiley-Vchweinheim: Weinheim, Germany, 2014; Volume 8, pp. 209–238. [Google Scholar]
- Clarke, T.M.; Durrant, J.R. Charge photogeneration in organic solar cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef]
- Gregg, B.A. Entropy of charge separation in organic photovoltaic cells: The benefit of higher dimensionality. J. Phys. Chem. Lett. 2011, 2, 3013–3015. [Google Scholar] [CrossRef]
- Baran, D.; Kirchartz, T.; Wheeler, S.; Dimitrov, S.; Abdelsamie, M.; Gorman, J.; Ashraf, R.S.; Holliday, S.; Wadsworth, A.; Gasparini, N. Restricting the liquid-liquid phase separation of PTB7-Th:PF12TBT:PC71BM by enhanced PTB7-Th solution aggregation to optimize the interpenetrating network. Adv. Mater. 2017, 139, 17913–17922. [Google Scholar]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Huang, B.; Chen, L.; Jin, X.; Chen, D.; An, Y.; Xie, Q.; Tan, Y.; Lei, H.; Chen, Y. Alkylsilyl functionalized copolymer donor for annealing-free high performance solar cells with over 11% efficiency: Crystallinity induced small driving force. Adv. Funct. Mater. 2018, 28, 1800606. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Y.; Tsao, C.; Saeki, A.; Li, J.; Chen, C.; Wang, H.; Chen, H.; Meng, D.; Wu, K.; et al. Enhancing photovoltaic performance by tuning the domain sizes of a small-molecule acceptor by side-chain-engineered polymer donors. J. Mater. Chem. A 2019, 7, 3072–3082. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Zhou, R.; Shi, Y.; Yang, C.; Zhang, Y.; Zhang, J.; Zou, W.; Deng, D.; Lu, K.; et al. Liquid-crystalline small molecules for nonfullerene solar cells with high fill factors and power conversion efficiencies. Adv. Energy Mater. 2019, 9, 1803175. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.; Speller, E.M.; Kim, J. Twist and degrade—Impact of molecular structure on the photostability of nonfullerene acceptors and their photovoltaic blends. Adv. Energy Mater. 2019, 9, 1803755. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Feng, Y.; Larson, W.; Su, M.; Li, Y.; Yuan, J. Understanding the interplay of transport-morphology-performance in PBDB-T-based polymer solar cells. Sol. RRL 2020, 4, 1900524. [Google Scholar]
- Zhang, J.; Jiang, K.; Yang, G.; Ma, T.; Liu, J.; Li, Z.; Lai, J.Y.L.; Ma, W.; Yan, H. Tuning energy levels without negatively affecting morphology: A promising approach to achieving optimal energetic match and efficient nonfullerene polymer solar cells. Adv. Energy Mater. 2017, 7, 1602119. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H. Indacenodithiophene-based D-A conjugated polymers for application in polymer solar cells. Org. Electron. 2017, 50, 443–457. [Google Scholar] [CrossRef]
- Chueh, C.; Yao, K.; Yip, H.; Chang, C.; Xu, Y.; Chen, K.; Li, C.; Liu, P.; Huang, F.; Chen, Y.; et al. Non-halogenated solvents for environmentally friendly processing of high-performance bulk-heterojunction polymer solar cells. Energy Environ. Sci. 2013, 6, 3241–3248. [Google Scholar] [CrossRef]
- Xu, Y.; Chueh, C.; Yip, H.; Chang, C.; Liang, P.; Intemann, J.J.; Chenb, W.; Alex, K.Y. Indacenodithieno[3,2-b]thiophene-based broad bandgap polymers for high efficiency polymer solar cells. Polym. Chem. 2013, 4, 5220–5223. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Xue, X.; Wei, D.; Huo, L.; Sun, Y. High-performance wide-bandgap copolymers based on indacenodithiophene and indacenodithieno[3,2-b]thiophene units. J. Mater. Chem. C 2017, 5, 7777–7783. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Wang, M.; Xiao, H.; Gong, X.; Yang, W.; Cao, Y. Highly efficient polymer solar cells based on poly (carbazole-alt-thiophene-benzofurazan). New J. Chem. 2012, 36, 2042–2047. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, L.; Fan, L.; Wang, N.; Hu, L.; Yang, W. Indolo[3,2-b]carbazole and benzofurazan based narrow band-gap polymers for photovoltaic cells. New J. Chem. 2014, 38, 4587–4593. [Google Scholar] [CrossRef]
- Meng, D.; Fu, H.; Xiao, C.; Meng, X.; Winands, T.; Ma, W.; Wei, W.; Fan, B.; Huo, L.; Doltsinis, N.L.; et al. Three-bladed rylene propellers with three- dimensional network assembly for organic electronics. J. Am. Chem. Soc. 2016, 138, 10184–10190. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, L.; Facchetti, A.; Marks, T.J. Organic and polymeric semiconductors enhanced by noncovalent conformational locks. Chem. Rev. 2017, 117, 10291–10318. [Google Scholar] [CrossRef]
- Brabec, C.J.; Winder, C.; Sariciftci, N.S.; Hummelen, J.C.; Dhanabalan, A.; van Hal, P.A.; Janssen, R.A.J. A low-bandgap semiconducting polymer for photovoltaic devices and infrared emitting diodes. Adv. Funct. Mater. 2002, 12, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Inganas, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Wang, K.; Azouz, M.; Babics, M.; Cruciani, F.; Marszalek, T.; Saleem, Q.; Pisula, W.; Beaujuge, P.M. Solvent annealing effects in dithieno[3,2-b:2′,3′-d]pyrrole-5,6-Difluorobenzo[c][1,2,5]thiadiazole small molecule donors for bulkheterojunction solar cells. Chem. Mater. 2016, 28, 5415–5425. [Google Scholar] [CrossRef] [Green Version]
- Babics, M.; Liang, R.; Wang, K.; Cruciani, F.; Kan, Z.; Wohlfahrt, M.; Tang, M.; Laquai, F.; Beaujuge, P.M. Solvent vapor annealing-mediated crystallization directs charge generation, recombination and extraction in BHJ solar cells. Chem. Mater. 2018, 30, 789–798. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Abdelsamie, M.; Shi, Q.; Zhang, Y.; Parker, T.C.; Jucov, E.V.; Timofeeva, T.V.; Amassian, A.; Bazan, G.C.; et al. Intermediate-sized conjugated donor molecules for organic solar cells: Comparison of benzodithiophene and benzobisthiazole-based cores. Chem. Mater. 2017, 29, 7880–7887. [Google Scholar] [CrossRef]
- Yi, S.; Deng, W.; Sun, S.; Lan, L.; He, Z.; Yang, W.; Zhang, B. Trifluoromethyl-substituted large band-gap polytriphenylamines for polymer solar cells with high open-circuit voltages. Polymers 2018, 10, 52. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the PIDT-O, PIDTT-O, PIDT-S and PIDTT-S are available from the authors. |

| Polymers | Mn | Mw | PDI | Td (°C) |
|---|---|---|---|---|
| PIDT-O | 26,300 | 45,000 | 1.71 | 337 |
| PIDTT-O | 43,700 | 91,200 | 2.09 | 343 |
| PIDT-S | 26,800 | 53,400 | 1.99 | 335 |
| PIDTT-S | 49,400 | 120,100 | 2.34 | 362 |
| Polymers | λabs, Solution (nm) | λabs, Film (nm) | λabs, Onset (nm) | Eg, Opt (eV) | Eox (eV) | Ered (eV) | EHOMO (eV) | ELUMO (eV) |
|---|---|---|---|---|---|---|---|---|
| PIDT-O | 439,573 | 426,570,600 | 660 | 1.88 | 0.92 | −0.85 | −5.34 | −3.57 |
| PIDTT-O | 453,574 | 438,564,598 | 661 | 1.87 | 0.84 | −0.79 | −5.26 | −3.63 |
| PIDT-S | 447,556 | 436,558 | 655 | 1.89 | 0.87 | −0.89 | −5.29 | −3.53 |
| PIDTT-S | 453,553 | 448,556 | 650 | 1.91 | 0.85 | −0.78 | −5.25 | −3.64 |
| Polymers | Thickness (nm) | Hole Mobility (cm2 V−1 s−1) |
|---|---|---|
| PIDT-O | 100 | 6.01 × 10−4 |
| PIDTT-O | 100 | 7.72 × 10−4 |
| PIDT-S | 100 | 1.83 × 10−3 |
| PIDTT-S | 100 | 1.29 × 10−3 |
| Polymers | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| PIDT-O | 0.88 | 5.22 | 52.39 | 2.41 |
| PIDT-O SVA a | 0.88 | 7.22 | 64.83 | 4.12 |
| PIDTT-O | 0.85 | 7.56 | 45.02 | 2.89 |
| PIDTT-O SVA | 0.85 | 8.64 | 55.22 | 4.06 |
| PIDT-S | 0.88 | 9.58 | 60.42 | 5.09 |
| PIDT-S SVA | 0.86 | 9.76 | 72.04 | 6.05 |
| PIDTT-S | 0.88 | 8.89 | 56.66 | 4.43 |
| PIDTT-S SVA | 0.86 | 9.92 | 71.79 | 6.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yi, S.; Qing, P.; Li, W.; Gu, B.; He, Z.; Zhang, B. Molecular Engineering Enhances the Charge Carriers Transport in Wide Band-Gap Polymer Donors Based Polymer Solar Cells. Molecules 2020, 25, 4101. https://doi.org/10.3390/molecules25184101
Liu S, Yi S, Qing P, Li W, Gu B, He Z, Zhang B. Molecular Engineering Enhances the Charge Carriers Transport in Wide Band-Gap Polymer Donors Based Polymer Solar Cells. Molecules. 2020; 25(18):4101. https://doi.org/10.3390/molecules25184101
Chicago/Turabian StyleLiu, Siyang, Shuwang Yi, Peiling Qing, Weijun Li, Bin Gu, Zhicai He, and Bin Zhang. 2020. "Molecular Engineering Enhances the Charge Carriers Transport in Wide Band-Gap Polymer Donors Based Polymer Solar Cells" Molecules 25, no. 18: 4101. https://doi.org/10.3390/molecules25184101