The Effect of Moisture on the Adhesion Energy and Nanostructure of Asphalt-Aggregate Interface System Using Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Results and Discussion
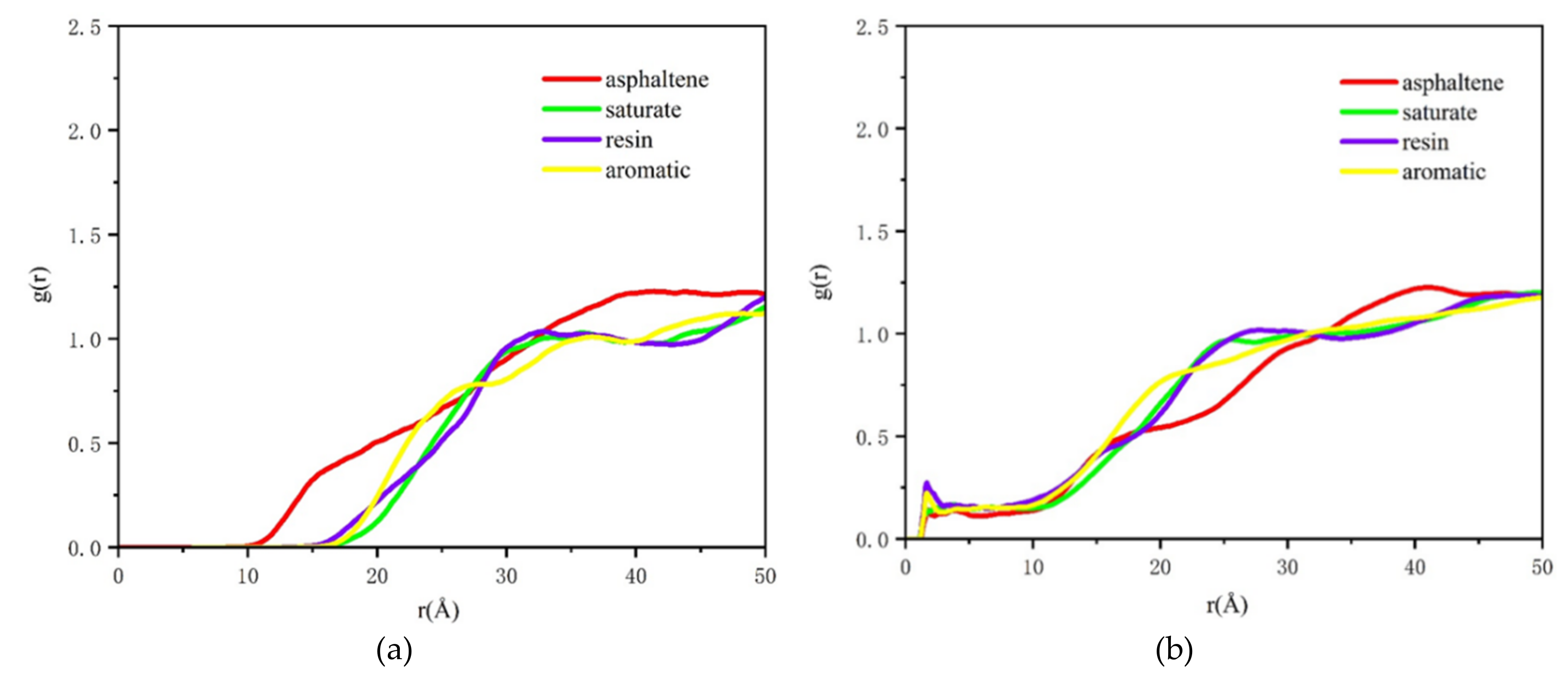
2.1. The Distribution of Asphalt Compositions on Aggregates
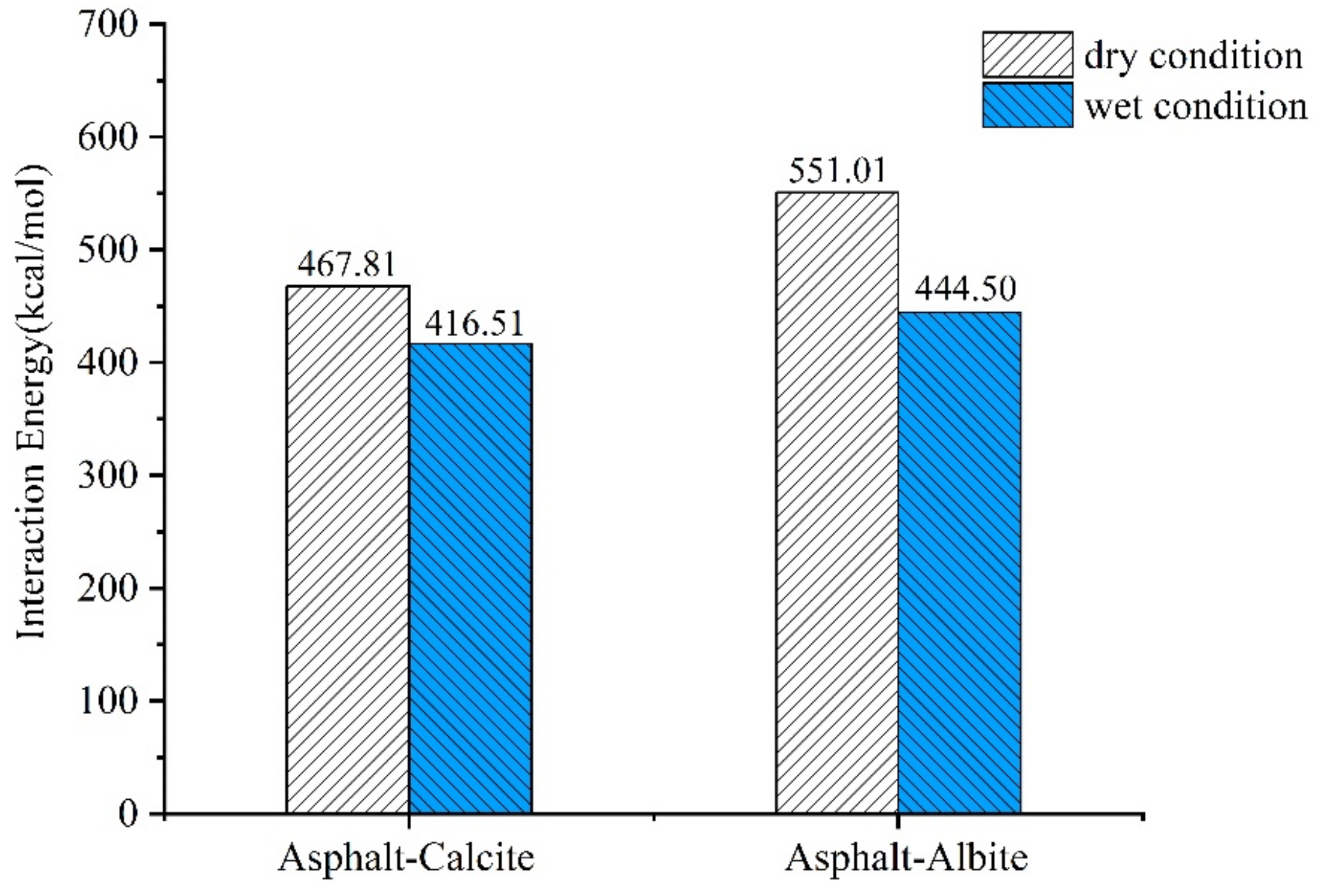
2.2. Adhesion Energy of the Asphalt-Aggregate System
3. Models and Simulation Methods
3.1. Asphalt Binder Model
3.2. Aggregate Mineral Model
3.3. Asphalt-Aggregate Mineral System Model
3.4. Radial Distribution Function
4. Conclusions
- (1)
- According to hyperspectral technology and image processing results, calcite, dolomite, and magnesite were the main mineral components of limestone, where calcite accounted for 73.2%. Also, albite, quartz, and biotite were the main mineral components of granite, where albite accounted for 68.8%. Therefore, calcite and albite were used as aggregates in MD simulations.
- (2)
- Asphaltene and resin tended to accumulate near the surface of weakly alkaline aggregates due to charge interactions. Molecular interactions between bitumen and acid minerals were weak and the distribution of SARA components near the surface of acid aggregates was more uniform.
- (3)
- Moisture intrusion into the asphalt-aggregate interface could affect asphalt nanostructure and the distribution of SARA components on aggregate surface.
- (4)
- The existence of interfacial moisture declined asphalt-aggregate adhesion work. The adhesion works of limestone and granite were decreased by 11.0 and 19.3%, respectively. That is to say, the water damage resistance of asphalt mixture relied on adhesion work and debonding energy between asphalt and aggregate with the intrusion of water into the asphalt-aggregate interface system.
Author Contributions
Funding
Conflicts of Interest
References
- Ji, X.; Sun, E.; Zou, H. Study on the multiscale adhesive properties between asphalt and aggregate. Constr. Build. Mater. 2020, 249, 118693. [Google Scholar] [CrossRef]
- Guo, F.; Pei, J.; Zhang, J. Study on the adhesion property between asphalt binder and aggregate: A state-of-the-art review. Constr. Build. Mater. 2020, 256, 119474. [Google Scholar] [CrossRef]
- Xiong, S.; Yin, S.; Wang, Y. Organic/inorganic electrochromic nanocomposites with various interfacial interactions: A review. Mater. Sci. Eng. B. 2017, 221, 41–53. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Wang, L. Evaluation of physicochemical interaction between asphalt binder and mineral filler through interfacial adsorbed film thickness. Constr. Build. Mater. 2020, 252, 119135. [Google Scholar] [CrossRef]
- Wang, L.; Ren, M.; Xing, Y.; Chen, G. Study on affecting factors of interface crack for asphalt mixture based on microstructure. Constr. Build. Mater. 2017, 156, 1053–1062. [Google Scholar] [CrossRef]
- Sun, Y.; Du, C. Analysis of load-induced top-down cracking initiation in asphalt pavements using a two-dimensional microstructure-based multiscale finite element method. Eng. Fract. Mech. 2019, 216, 106497. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L. A review and perspective for research on moisture damage in asphalt pavement induced by dynamic pore water pressure. Constr. Build. Mater. 2019, 204, 631–642. [Google Scholar] [CrossRef]
- Kakar, M.R.; Hamzah, M.O.; Valentin, J. A review on moisture damages of hot and warm mix asphalt and related investigations. J. Clean. Prod. 2015, 99, 39–58. [Google Scholar] [CrossRef]
- Nobakht, M.; Zhang, D. Characterization of the adhesive and cohesive moisture damage for asphalt concrete. Constr. Build. Mater. 2020, 247, 118616. [Google Scholar] [CrossRef]
- Wang, W.; Luo, R. Evaluation on moisture sensitivity induced by dynamic pore water pressure for asphalt mixture and its components using the bending beam rheometer method. Constr. Build. Mater. 2020, 251, 118942. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Q. Temperature and pressure dependent behaviors of moisture diffusion in dense asphalt mixture. Constr. Build. Mater. 2020, 246, 118500. [Google Scholar] [CrossRef]
- Luo, R.; Huang, T. Water vapor diffusion in asphalt mixtures under different relative humidity differentials. Constr. Build. Mater. 2017, 136, 126–138. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H. Moisture effect on nanostructure and adhesion energy of asphalt on aggregate surface: A molecular dynamics study. Appl. Surf. Sci. 2020, 510, 145435. [Google Scholar] [CrossRef]
- Cui, B.; Gu, X.; Hu, D. A multiphysics evaluation of the rejuvenator effects on aged asphalt using molecular dynamics simulations. J. Clean. Prod. 2020, 259, 120629. [Google Scholar] [CrossRef]
- Long, Z.W.; You, L.Y. Analysis of interfacial adhesion properties of nano-silica modified asphalt mixtures using molecular dynamics simulation. Constr. Build. Mater. 2020, 255, 119354. [Google Scholar] [CrossRef]
- Liu, J.Z.; Yu, B.; Hong, Q.Z. Molecular dynamics simulation of distribution and adhesion of asphalt components on steel slag. Constr. Build. Mater. 2020, 255, 119332. [Google Scholar] [CrossRef]
- Xu, G.J.; Wang, H. Molecular dynamics study of oxidative aging effect on asphalt binder properties. Fuel 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- Ziegel, K.D.; Romanov, A. Modulus reinforcement in elastomer composites. I. inorganic fillers. J. Appl. Polym. Sci. 1973, 17, 1119–1131. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, Y.S. Study of melting properties of basalt based on their mineral components. Compos. Part. B 2017, 116, 53–60. [Google Scholar] [CrossRef]
- Rinnen, S.; Stroth, C. Characterization and identification of minerals in rocks by ToF-SIMS and principal component analysis. Appl. Surf. Sci. 2015, 349, 622–628. [Google Scholar] [CrossRef]
- Xu, G.J.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comput. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- Bhasin, A.; Masad, E.; Little, D.; Lytton, R. Limits on adhesive bond energy for improved resistance of hot-mix asphalt to moisture damage. Transport. Res. Rec. 2006, 1970, 2–13. [Google Scholar] [CrossRef]
- Cala, A.; Caro, S. Impact of the chemical composition of aggregates on the adhesion quality and durability of asphalt-aggregate systems. Constr. Build. Mater. 2019, 216, 661–672. [Google Scholar] [CrossRef]
- Zhang, J.; Apeagyei, A.K.; Airey, G.D.; Grenfell, J.R. Influence of aggregate mineralogical composition on water resistance of aggregate-bitumen adhesion. Int. J. Adhes. Adhes. 2015, 62, 45–54. [Google Scholar] [CrossRef]
- Corbett, L.W. Composition of asphalt based on generic fractionation using solvent deasphaltening elution-adsorption chromatography and densimetric characterization. Anal. Chem. 1969, 41, 576. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Khabaz, F.; Khare, R. Glass transition and molecular mobility in styrene-butadiene rubber modified asphalt. J. Phys. Chem. B. 2015, 119, 14261–14269. [Google Scholar] [CrossRef]
- Gao, Y.M.; Zhang, Y.Q.; Wang, H. Impact of minerals and water on bitumen-mineral adhesion and debonding behaviors using molecular dynamics simulations. Constr. Build. Mater. 2018, 171, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Aslett, Z.; Taranik, J.V. Mapping rock forming minerals at Boundary Canyon, Death Valey National Park, California, using aerial SEBASS thermal infrared hyperspectral image data. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 326–339. [Google Scholar] [CrossRef]
- Abdolmaleki, M.; Tabaei, M. Selecting optimum base wavelet for extracting spectral alteration features associated with porphyry copper mineralization using hyperspectral images. Int. J. Appl. Earth Obs. Geoinf. 2017, 58, 134–144. [Google Scholar] [CrossRef]
- Laakso, K.; Middleton, M. Assessing the ability to combine hyperspectral imaging (HSI) data with Mineral Liberation Analyzer (MLA) data to characterize phosphate rocks. Int. J. Appl. Earth Obs. Geoinf. 2018, 69, 1–12. [Google Scholar] [CrossRef]
- Mahesh, K.T.; Govil, H. Evaluation of AVIRIS-NG hyperspectral images for mineral identification and mapping. Heliyon 2018, 5, e02931. [Google Scholar]
- Green, A.A.; Berman, M.; Switzer, P.; Craig, M.D. A transformation for ordering multispectral data in terms of image quality with implications for noise removal. IEEE Trans. Geosci. Remote Sens. 1988, 26, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Vandermeer, F.D.; Harald, M.A. Multi- and hyperspectral geologic remote sensing: A review. Int. J. Appl. Earth Obs. Geoinf. 2012, 14, 112–128. [Google Scholar] [CrossRef]
- Boardman, J.W.; Kruse, F.A.; Green, R.O. Mapping target signatures via partial unmixing of aviris data. Jet. Propuls. Lab. 1995, 23, 23–26. [Google Scholar]
- Gao, Y.; Zhang, Y.; Yang, Y.; Zhang, J.; Gu, F. Molecular dynamics investigation of interfacial adhesion between oxidised bitumen and mineral surfaces. Appl. Surf. Sci. 2019, 479, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Liu, Z.; Wang, P.; Gong, X. Nanostructure characterization of asphalt-aggregate interface through molecular dynamics simulation and atomic force microscopy. Fuel 2017, 189, 155–163. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B. 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Zhang, J.P.; Fan, Z.P.; Wang, H.; Sun, W.; Pei, J.Z.; Wang, D.W. Prediction of dynamic modulus of asphalt mixture using micromechanical method with radial distribution functions. Mater. Struct. 2019, 52, 1–12. [Google Scholar] [CrossRef]










| Models | ||||
|---|---|---|---|---|
| Asphalt-Calcite Asphalt-Albite | 7531.17 8219.80 | −18,4317.15 −109,904.05 | −170,136.62 −98,228.57 | −6649.36 −3455.68 |
| Models | ER (-) | ||
|---|---|---|---|
| Asphalt-Calcite Asphalt-Albite | 467.81 551.01 | −6649.36 −3455.68 | 0.07 0.16 |
| Chemical Fractions | Molecules | Formula | Number | Mass Fraction (%) |
|---|---|---|---|---|
| Asphaltene Saturate Aromatic Resin | Phenol Pyrrole Thiophene | C42H54O C66H81N C51H62S | 3 2 2 | 17.1 |
| Squalane Hopane | C30H62 C35H62 | 4 6 | 16.0 | |
| Perhydrophe-nanthrene-naphthalene (PHPN) Dioctyl-cyclohexane-naphthalene (DOCHN) | C35H44 C30H46 | 13 16 | 43.6 | |
| Quinolinohopane Thioisorenieratane Benzobisbenzothiophene Pyridinohopane Trimethylbenzeneoxane | C40H59N C40H60S C18H10S2 C36H57N C29H50O | 2 2 9 2 2 | 23.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Huang, W.; Xiao, Z.; Xie, J.; Hu, B.; Cai, X.; Wu, K. The Effect of Moisture on the Adhesion Energy and Nanostructure of Asphalt-Aggregate Interface System Using Molecular Dynamics Simulation. Molecules 2020, 25, 4165. https://doi.org/10.3390/molecules25184165
Cui W, Huang W, Xiao Z, Xie J, Hu B, Cai X, Wu K. The Effect of Moisture on the Adhesion Energy and Nanostructure of Asphalt-Aggregate Interface System Using Molecular Dynamics Simulation. Molecules. 2020; 25(18):4165. https://doi.org/10.3390/molecules25184165
Chicago/Turabian StyleCui, Wentian, Wenke Huang, Zhicheng Xiao, Jiawen Xie, Bei Hu, Xu Cai, and Kuanghuai Wu. 2020. "The Effect of Moisture on the Adhesion Energy and Nanostructure of Asphalt-Aggregate Interface System Using Molecular Dynamics Simulation" Molecules 25, no. 18: 4165. https://doi.org/10.3390/molecules25184165






