Gliadin Nanoparticles Pickering Emulgels for β-Carotene Delivery: Effect of Particle Concentration on the Stability and Bioaccessibility
Abstract
:1. Introduction
2. Results and Discussion
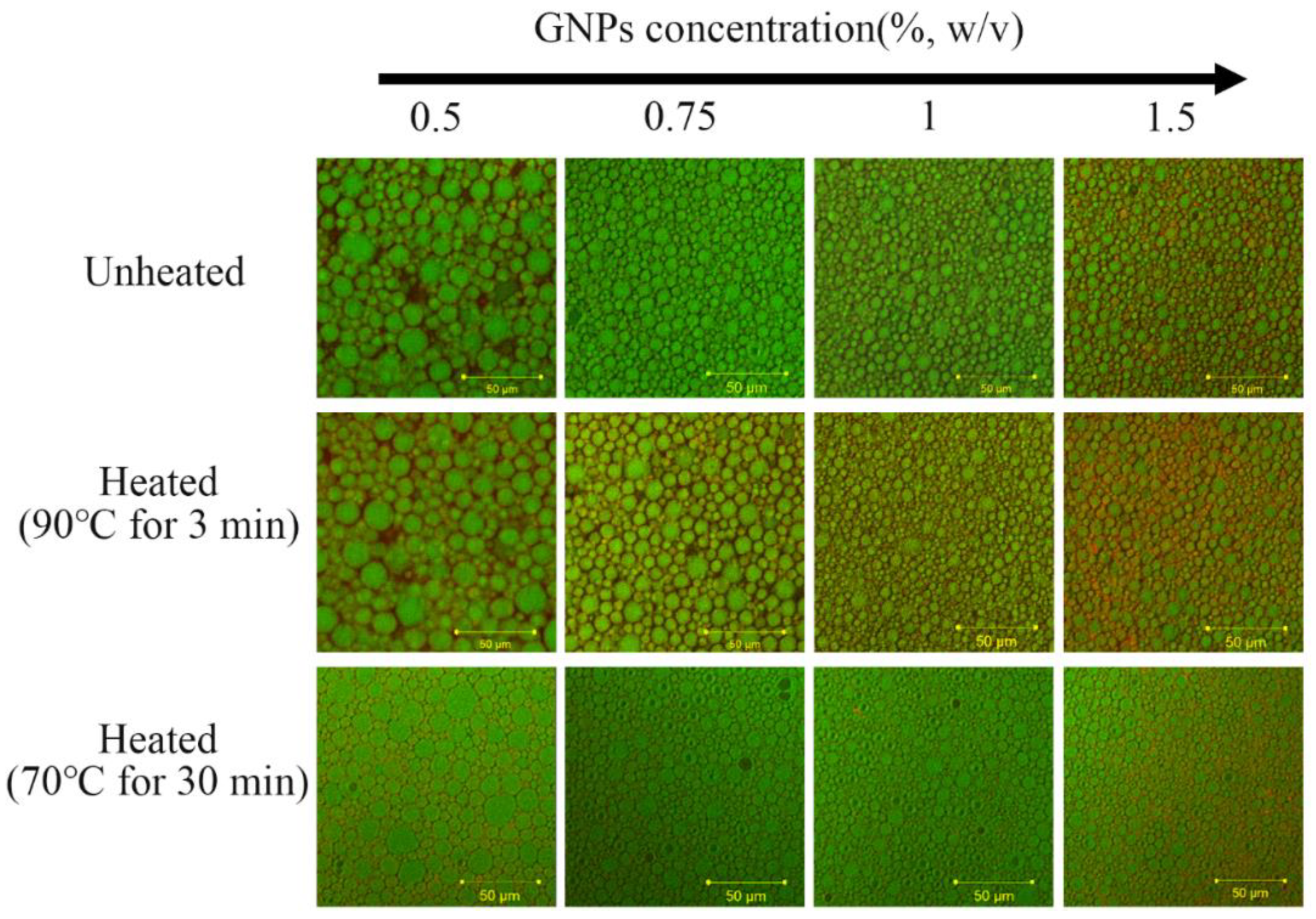
2.1. Formation and Microstructure of Pickering Emulgels
2.2. Rheological Property of β-Carotene Pickering Emulgels
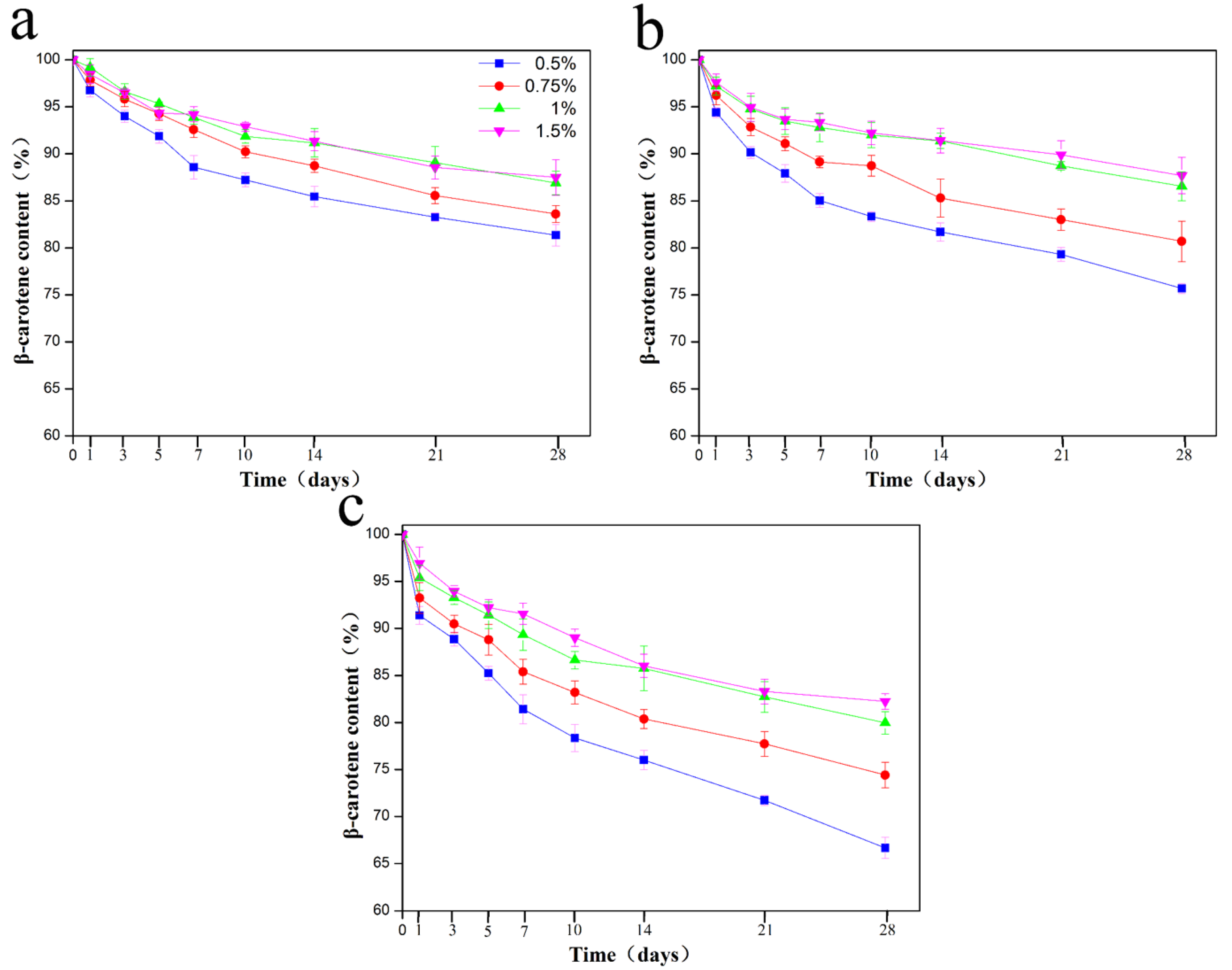
2.3. Thermal and Storage Stability of β-Carotene Pickering Emulgels
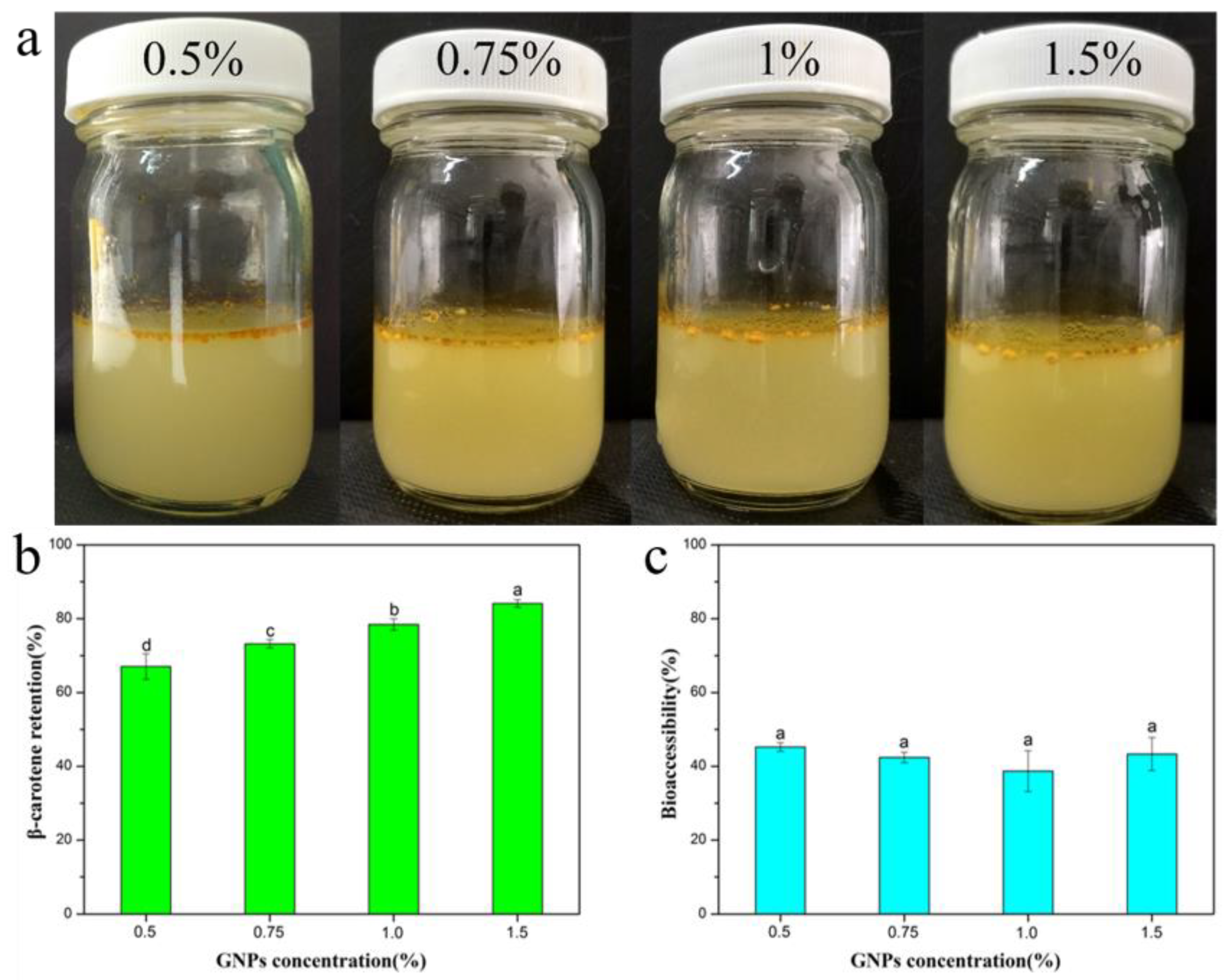
2.4. Retention and Bioaccessibility of β-Carotene during In Vitro Digestion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Gliadin Nanoparticles
3.3. Preparation of β-Carotene Pickering Emulgels
3.4. Droplet Size Analysis
3.5. Microstructure Observation and Rheology Measurement
3.5.1. Microstructure Observation
3.5.2. Rheology Measurement
3.6. Measurement of Thermal and Storage Stability of β-Carotene Pickering Emulgels
3.7. In Vitro Digestion
3.7.1. Mouth Phase
3.7.2. Gastric Phase
3.7.3. Small Intestine Phase
3.7.4. Determination of β-Carotene Bioaccessibility and Stability
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.F.; Marisca, O.; Oprea, I.; Rugina, D.; Suciu, M.; Nistor, M.; Tofana, M.; Leopold, N.; Coman, C. Cellular Internalization of Beta-Carotene Loaded Polyelectrolyte Multilayer Capsules by Raman Mapping. Molecules 2020, 25, 1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basar, A.O.; Prieto, C.; Durand, E.; Villeneuve, P.; Sasmazel, H.T.; Lagaron, J. Encapsulation of beta-Carotene by Emulsion Electrospraying Using Deep Eutectic Solvents. Molecules 2020, 25, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.L.; Wu, H.Y.; Liang, Y.; Song, J.; Gan, W.Q.; Hou, H.M. Influence of Oxygen-Containing Sulfur Flavor Molecules on the Stability of beta-Carotene under UVA Irradiation. Molecules 2019, 24, 318. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, D.; Sun, C.; Gao, Y. Influence of polysaccharides on the physicochemical properties of lactoferrin-polyphenol conjugates coated β-carotene emulsions. Food Hydrocoll. 2016, 52, 661–669. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Zheng, H.; Chen, H.; Gao, Y. Characterization of β-carotene loaded emulsion gels containing denatured and native whey protein. Food Hydrocoll. 2020, 102, 105600. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Chen, X.; Liang, R.; Zhong, F.; Yokoyama, W.H. Effect of beta-carotene status in microcapsules on its in vivo bioefficacy and in vitro bioaccessibility. Food Hydrocoll. 2020, 106, 105848. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Chen, X.; McClements, D.J.; Zou, L.; Liu, W. Pickering-stabilized emulsion gels fabricated from wheat protein nanoparticles: Effect of pH, NaCl and oil content. J. Dispers. Sci. Technol. 2017, 39, 826–835. [Google Scholar] [CrossRef]
- Chen, H.; Mao, L.; Hou, Z.; Yuan, F.; Gao, Y. Roles of additional emulsifiers in the structures of emulsion gels and stability of vitamin E. Food Hydrocoll. 2020, 99, 105372. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Tang, C. Novel pickering high internal phase emulsion gels stabilized solely by soy β-conglycinin. Food Hydrocoll. 2019, 88, 21–30. [Google Scholar] [CrossRef]
- de Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Gao, L.; Zhong, G.; Fan, Y. Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for beta-carotene stabilization and delivery. Food Funct. 2020, 11, 768–778. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C. Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH 3.0. Lwt-Food Sci. Technol. 2014, 58, 463–469. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Yin, S.W.; Zhu, J.H.; Qi, J.R.; Guo, J.; Wu, L.Y.; Tang, C.H.; Yang, X.Q. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocoll. 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy glycinin as food-grade Pickering stabilizers: Part. III. Fabrication of gel-like emulsions and their potential as sustained-release delivery systems for β-carotene. Food Hydrocoll. 2016, 56, 434–444. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Edible Pickering emulsions stabilized by ovotransferrin-gum arabic particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.H. Gel-like pea protein Pickering emulsions at pH 3.0 as a potential intestine-targeted and sustained-release delivery system for β-carotene. Food Res. Int. 2016, 79, 64–72. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, B.; Wang, Y.; Teng, Y. Effects of colloidal complexes formation between resveratrol and deamidated gliadin on the bioaccessibility and lipid oxidative stability. Food Hydrocoll. 2017, 69, 466–472. [Google Scholar] [CrossRef]
- Yang, S.; Dai, L.; Mao, L.; Liu, J.; Yuan, F.; Li, Z.; Gao, Y. Effect of sodium tripolyphosphate incorporation on physical, structural, morphological and stability characteristics of zein and gliadin nanoparticles. Int. J. Biol. Macromol. 2019, 136, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ezpeleta, I.; Irache, J.M.; Stainmesse, S.; Chabenat, C.; Gueguen, J.; Popineau, Y.; Orecchioni, A.M. Gliadin nanoparticles for the controlled release of all-trans-retinoic acid. Int. J. Pharm. 1996, 131, 191–200. [Google Scholar] [CrossRef]
- Arangoa, M.A.; Campanero, M.A.; Renedo, M.J.; Ponchel, G.; Irache, J.M. Gliadin nanoparticles as carriers for the oral administration of lipophilic drugs. Relationships between bioadhesion and pharmacokinetics. Pharm. Res. 2001, 18, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; McClements, D.J. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef]
- Du, Z.; Wang, P. Gelatin Hydrolysate Hybrid Nanoparticles as Soft Edible Pickering Stabilizers for Oil-in-Water Emulsions. Molecules 2020, 25, 393. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Guo, J.; Wan, Z.L.; Liu, Y.-Y.; Ruan, Q.J.; Yang, X.Q. Wheat gluten-stabilized high internal phase emulsions as mayonnaise replacers. Food Hydrocoll. 2018, 77, 168–175. [Google Scholar] [CrossRef]
- Kondaraju, S.; Farhat, H.; Lee, J.S. Study of aggregational characteristics of emulsions on their rheological properties using the lattice Boltzmann approach. Soft Matter 2012, 8, 1374–1384. [Google Scholar] [CrossRef]
- Zhu, Y.; McClements, D.J.; Zhou, W.; Peng, S.; Zhou, L.; Zou, L.; Liu, W. Influence of ionic strength and thermal pretreatment on the freeze-thaw stability of Pickering emulsion gels. Food Chem. 2020, 303, 125401. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Iwami, K.; Kawabata, M.; Ibuki, F. Antioxidant effects of wheat gliadin and hen’s egg white in powder model systems: Protection against oxidative deterioration of safflower oil and sardine oil. Agric. Biol. Chem 2016, 52, 539–545. [Google Scholar]
- Villiere, A.; Viau, M.; Bronnec, I.; Moreau, N.; Genot, C. Oxidative Stability of Bovine Serum Albumin- and Sodium Caseinate-Stabilized Emulsions Depends on Metal Availability. J. Agric. Food Chem. 2005, 53, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Chaudhari, A.; Pan, Y.; Nitin, N. Beverage emulsions: Comparison among nanoparticle stabilized emulsion with starch and surfactant stabilized emulsions. Food Res. Int. 2015, 69, 156–163. [Google Scholar] [CrossRef]
- Mao, Y.; McClements, D.J. Influence of electrostatic heteroaggregation of lipid droplets on their stability and digestibility under simulated gastrointestinal conditions. Food Funct. 2012, 3, 1025–1034. [Google Scholar] [CrossRef]
- Gal, J.Y.; Fovet, Y.; Adib-Yadzi, M. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, H. Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva. Food Hydrocoll. 2009, 23, 1270–1278. [Google Scholar] [CrossRef]
- Liu, W.; Gao, H.; McClements, D.J.; Zhou, L.; Wu, J.; Zou, L. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocoll. 2019, 88, 210–217. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| GNPs Concentration (%) | η | k | n | R2 |
|---|---|---|---|---|
| 0.5 | 0.365 ± 0.012 d | 11.989 ± 0.085 d | 0.214 ± 0.001 a | 0.998 |
| 0.75 | 0.614 ± 0.015 c | 23.478 ± 0.492 c | 0.181 ± 0.012 b | 0.998 |
| 1 | 0.818 ± 0.024 b | 34.860 ± 1.245 b | 0.156 ± 0.026 b | 0.993 |
| 1.5 | 1.090 ± 0.041 a | 50.018 ± 0.746 a | 0.088 ± 0.008 c | 0.982 |
| GNPs Concentration (%) | d4,3 (μm) | ||
|---|---|---|---|
| Unheated | 90 °C 3 min | 70 °C 30 min | |
| 0.5 | 11.34 ± 0.46 b | 13.78 ± 0.45 a | 13.69 ± 0.43 a |
| 0.75 | 9.71 ± 0.52 c | 11.37 ± 0.33 b | 10.99 ± 0.46 b |
| 1 | 7.53 ± 0.48 d | 7.67 ± 0.36 d | 7.81 ± 0.28 d |
| 1.5 | 4.94 ± 0.28 e | 4.91 ± 0.29 e | 5.09 ± 0.36 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Gao, Y.; Wu, Z.; Miao, J.; Gao, H.; Ma, L.; Zou, L.; Peng, S.; Liu, C.; Liu, W. Gliadin Nanoparticles Pickering Emulgels for β-Carotene Delivery: Effect of Particle Concentration on the Stability and Bioaccessibility. Molecules 2020, 25, 4188. https://doi.org/10.3390/molecules25184188
Cheng C, Gao Y, Wu Z, Miao J, Gao H, Ma L, Zou L, Peng S, Liu C, Liu W. Gliadin Nanoparticles Pickering Emulgels for β-Carotene Delivery: Effect of Particle Concentration on the Stability and Bioaccessibility. Molecules. 2020; 25(18):4188. https://doi.org/10.3390/molecules25184188
Chicago/Turabian StyleCheng, Ce, Yi Gao, Zhihua Wu, Jinyu Miao, Hongxia Gao, Li Ma, Liqiang Zou, Shengfeng Peng, Chengmei Liu, and Wei Liu. 2020. "Gliadin Nanoparticles Pickering Emulgels for β-Carotene Delivery: Effect of Particle Concentration on the Stability and Bioaccessibility" Molecules 25, no. 18: 4188. https://doi.org/10.3390/molecules25184188






