Evaluating Quality Parameters, the Metabolic Profile, and Other Typical Features of Selected Commercial Extra Virgin Olive Oils from Brazil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quality Parameters, p-Anisidine Value, Antioxidant Capacity, Oxidative Stability Index, and Total Phenolic Content
2.2. Fatty Acid Composition in EVOO Samples
2.3. Minor Components
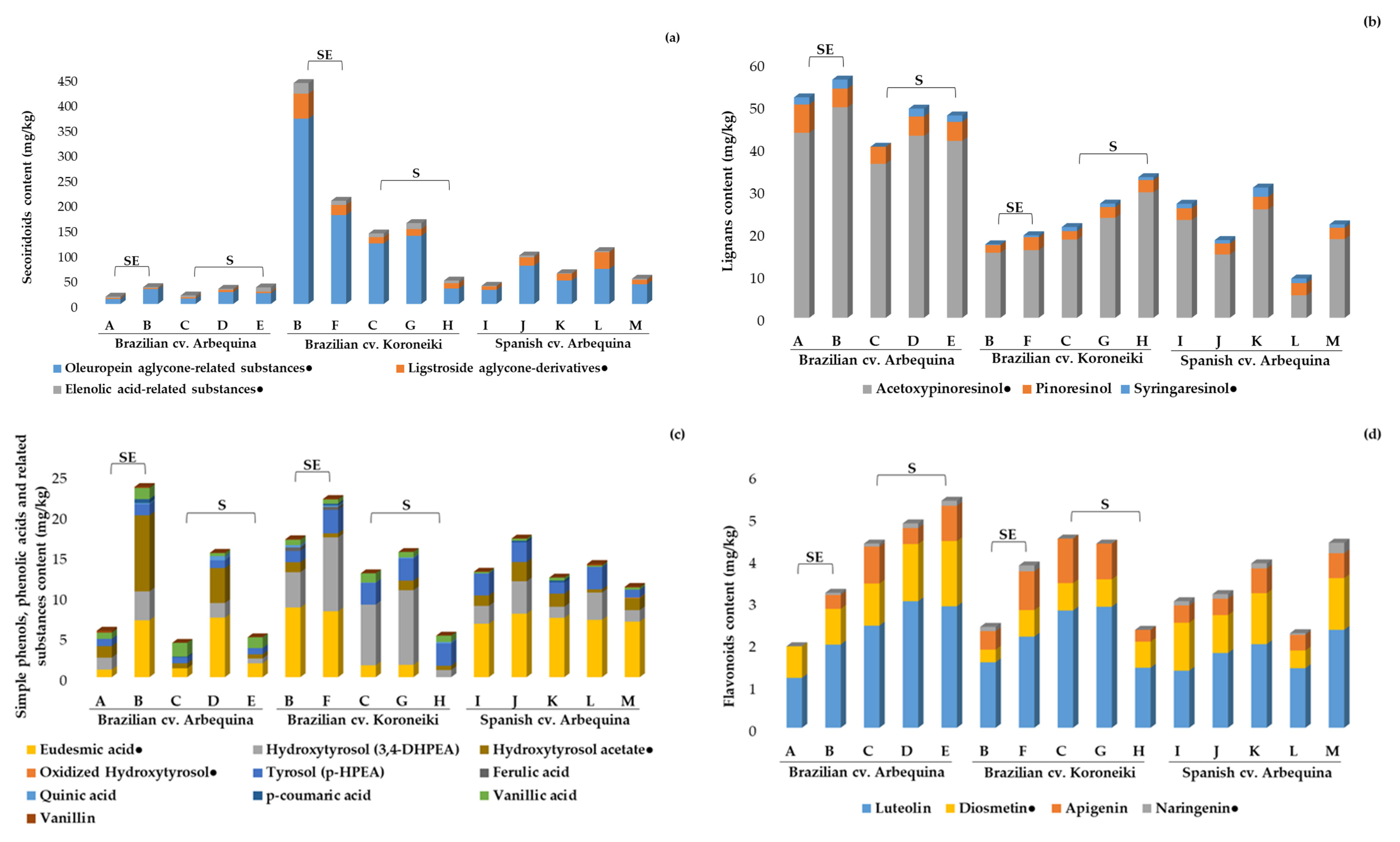
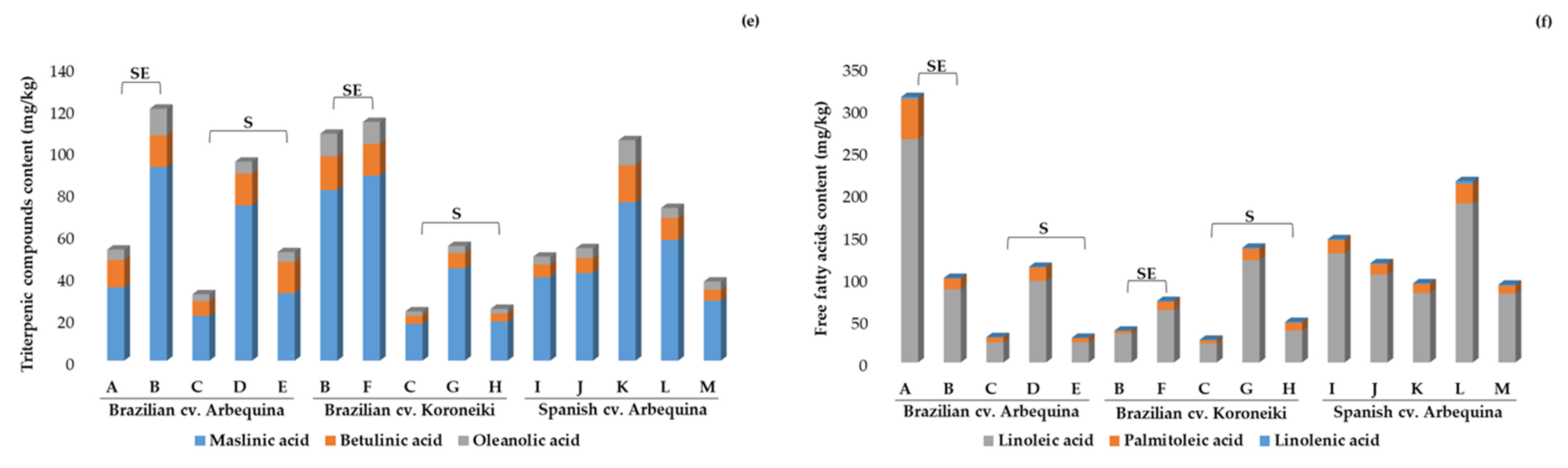
2.3.1. Phenolic Compounds, Pentacyclic Triterpenes, and Free Fatty Acids
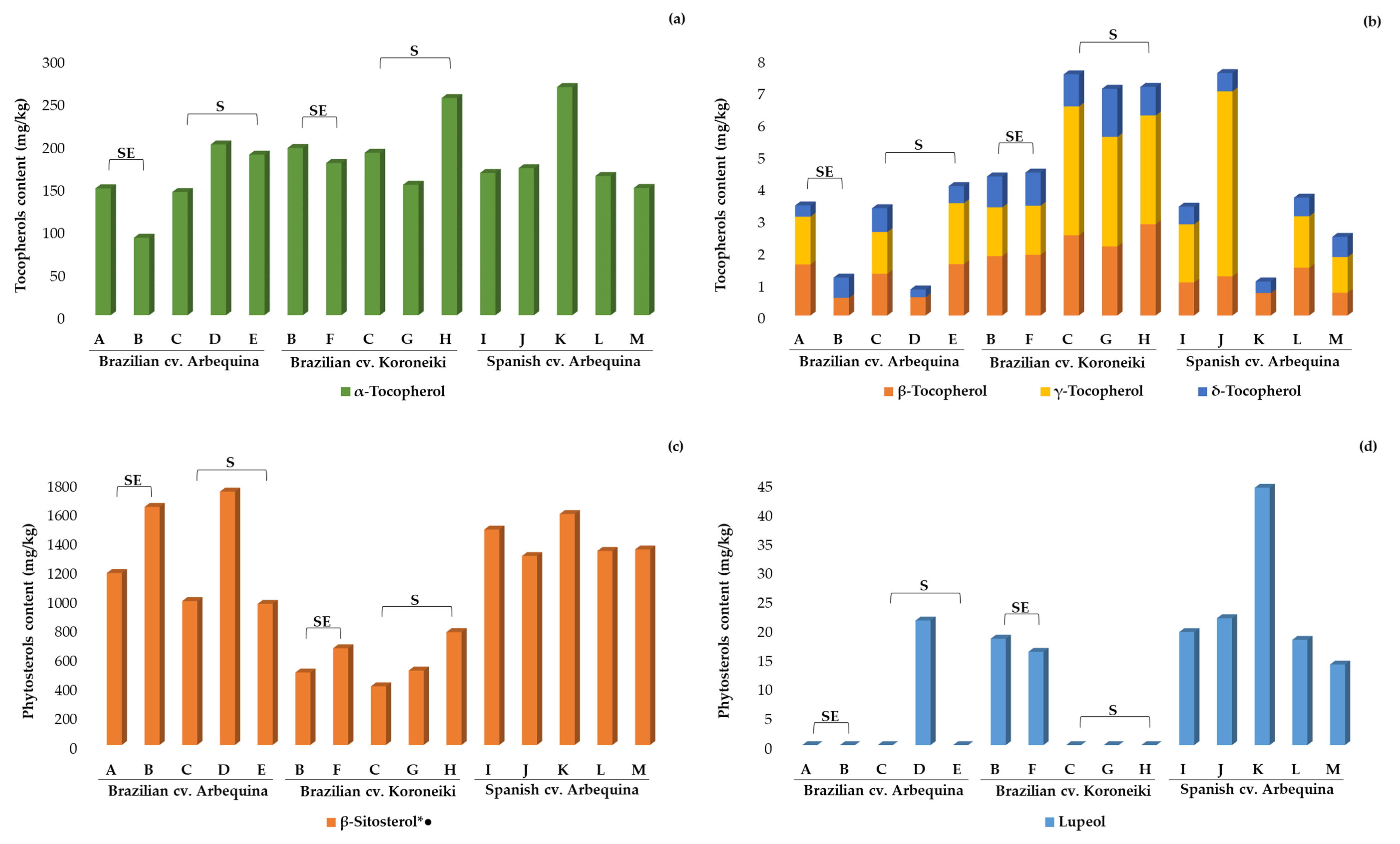
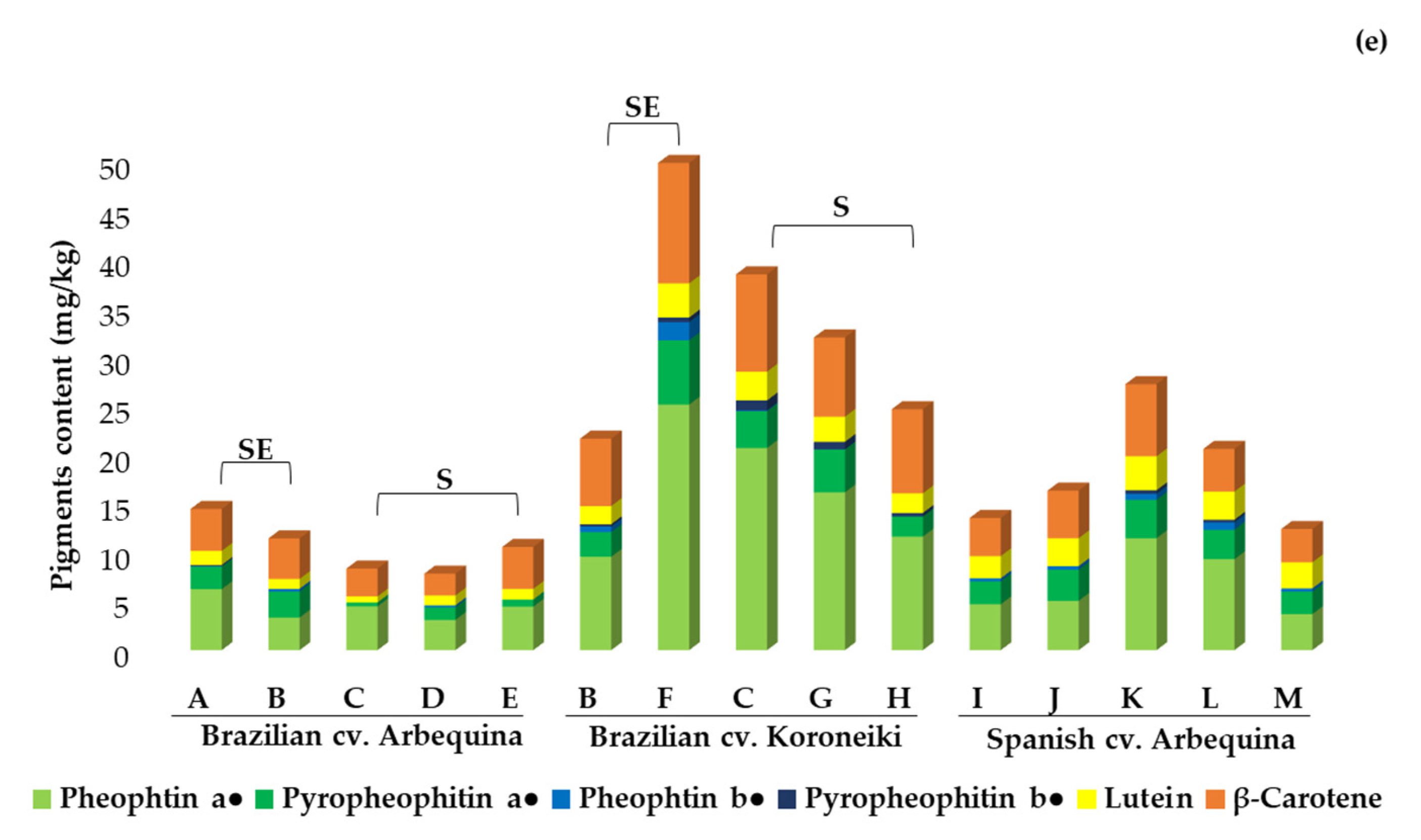
2.3.2. Tocopherols, Sterols, and Pigments
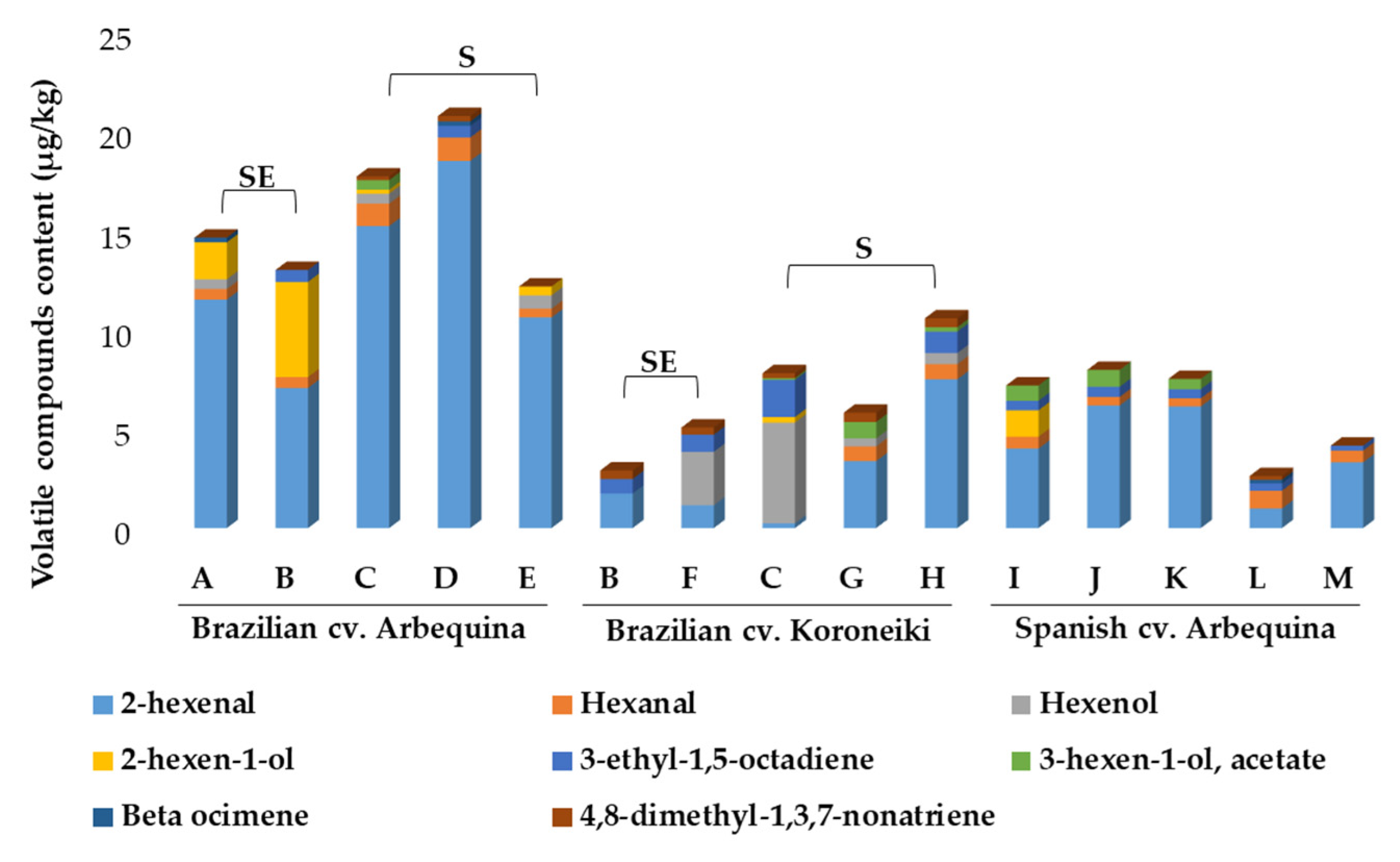
2.3.3. Volatile Composition
3. Methods and Materials
3.1. Materials
3.2. Extra Virgin Olive Oil Samples
3.3. Quality Parameters, p-Anisidine Values, Antioxidant Capacity, Oxidative Stability Index, and Total Phenolic Content
3.4. Fatty Acid Composition by GC-FID
3.5. Minor Component Profiling of EVOO Samples
3.5.1. Analysis of Minor Components in EVOO by RP-LC-MS
3.5.2. Analysis of Tocopherols, Phytosterols, Chlorophylls, and Carotenoids by NP-LC-DAD/FLD
3.5.3. Analysis of Volatile and Semi-Volatile Compounds by SPME-GC-MS
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paiva-Martins, F.; Kiritsakis, A. Olive Fruit and Olive Oil Composition and Their Functional Compounds; Wiley: Chichester, UK, 2017; pp. 81–115. [Google Scholar]
- Blekas, G.; Tsimidou, M.; Boskou, D. Olive Oil Composition; AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. [Google Scholar]
- Konuskan, D.B.; Mungan, B. Effects of Variety, Maturation and Growing Region on Chemical Properties, Fatty Acid and Sterol Compositions of Virgin Olive Oils. J. Am. Oil Chem. Soc. 2016, 93, 1499–1508. [Google Scholar] [CrossRef]
- Dabbou, S.; Issaoui, M.; Esposto, S.; Sifi, S.; Taticchi, A.; Servili, M.; Montedoro, G.F.; Hammami, M. Cultivar and growing area effects on minor compounds of olive oil from autochthonous and European introduced cultivars in Tunisia. J. Sci. Food Agric. 2009, 89, 1314–1325. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Olive Oil Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2020/07/IOC-NEWSLETTER-151-July-2020-def.-.pdf (accessed on 6 August 2020).
- Ballus, C.A.; Meinhart, A.D.; Campos, F.A.D.S.; Silva, L.F.D.O.D.; De Oliveira, A.F.; Godoy, H.T. A quantitative study on the phenolic compound, tocopherol and fatty acid contents of monovarietal virgin olive oils produced in the southeast region of Brazil. Food Res. Int. 2014, 62, 74–83. [Google Scholar] [CrossRef]
- Higgins, Charlie. Olive Oil Times. Brazilian Olive Oil Imports Continue Boom. Available online: https://www.oliveoiltimes.com/olive-oil-business/south-america/brazilian-olive-oil-imports-boom/38050 (accessed on 12 August 2020).
- Castro, M. Produção de Azeite em Minas vai Dobrar com Safra de Azeitona. Jornal do Estado de Minas. Available online: https://www.em.com.br/app/noticia/economia/2015/04/06/internas_economia,634660/producao-de-azeite-em-minas-vai-dobrar-com-safra-de-azeitona (accessed on 6 August 2020).
- ASSOOLIVE-Associação dos Olivicultores dos Contrafortes da Mantiqueira. Minas Gerais Entra no Mapa do Azeite Gourmet. 2016. Available online: http://assoolive.blogspot.com/2016/02/minas-gerais-entra-no-mapa-do-azeite.html (accessed on 12 August 2020).
- Dal Molin, G. Azeite de Oliva Brasileiro: Meta é Dobrar Produção com Novas Regras. Available online: http://www.gazetadopovo.com.br/agronegocio/agricultura/azeite-de-oliva-brasileiro-meta-e-dobrar-producao-com-novas-regras-814eeffnruldwimq4f2lo91h1 (accessed on 12 August 2020).
- Produção de Azeite de Oliva no Brasil deve Crescer Mais de 40% em 2018. Available online: http://ppv.datamark.com.br/noticias/2018/1/producao-de-azeite-de-oliva-no-brasil-deve-crescer-mais-de-40-em-2018-238052/ (accessed on 12 August 2020).
- Scarton, S. Produção de Azeite Extravirgem Deste ano Deve ser a Maior do Estado. Available online: https://estado.rs.gov.br/producao-de-azeite-extravirgem-deve-ser-a-maior-do-rs-em-2019 (accessed on 12 August 2020).
- Ibraoliva. Projeção do Mercado Oleícola para os Próximos Anos. Available online: https://www.ibraoliva.com.br/sobre (accessed on 6 August 2020).
- Ballus, C.A.; Quirantes-Piné, R.; Bakhouche, A.; Silva, L.F.D.O.D.; De Oliveira, A.F.; Coutinho, E.F.; Da Croce, D.M.; Segura-Carretero, A.; Godoy, H.T. Profile of phenolic compounds of Brazilian virgin olive oils by rapid resolution liquid chromatography coupled to electrospray ionisation time-of-flight mass spectrometry (RRLC-ESI-TOF-MS). Food Chem. 2015, 170, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Borges, T.H.; Pereira, J.A.; Cabrera-Vique, C.; Lara, L.; Oliveira, A.F.; Seiquer, I. Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 2017, 215, 454–462. [Google Scholar] [CrossRef]
- Borges, T.H.; López, L.C.; Pereira, J.A.; Cabrera–Vique, C.; Seiquer, I. Comparative analysis of minor bioactive constituents (CoQ10, tocopherols and phenolic compounds) in Arbequina extra virgin olive oils from Brazil and Spain. J. Food Compos. Anal. 2017, 63, 47–54. [Google Scholar] [CrossRef]
- Bruscatto, M.H.; Zambiazi, R.C.; Crizel-Cardoso, M.; Piatnicki, C.M.S.; Mendonça, C.R.B.; Dutra, F.L.G.; Coutinho, E.F. Chemical characterization and oxidative stability of olive oils extracted from olive trees of Southern Brazil. Pesqui. Agropecu. Bras. 2017, 52, 1231–1240. [Google Scholar] [CrossRef]
- Zago, L.; Squeo, G.; Bertoncini, E.I.; Difonzo, G.; Caponio, F. Chemical and sensory characterization of Brazilian virgin olive oils. Food Res. Int. 2019, 126, 108588. [Google Scholar] [CrossRef]
- Rodrigues, J.F.; Resende, L.M.B.; Silva, L.F.D.O.D.; Pedroso, M.P.; Pinheiro, A.C.M.; Nunes, C.A. Quality of olive oils from southeastern Brazil. Bragantia 2019, 78, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Crizel, R.L.; Hoffmann, J.F.; Zandoná, G.P.; Lobo, P.M.S.; Jorge, R.O.; Chaves, F.C. Characterization of Extra Virgin Olive Oil from Southern Brazil. Eur. J. Lipid Sci. Technol. 2020, 122, 1900347. [Google Scholar] [CrossRef]
- Gonçalves, T.R.; Rosa, L.N.; Torquato, A.S.; Da Silva, L.F.O.; Março, P.H.; Gomes, S.T.M.; Matsushita, M.; Valderrama, P. Assessment of Brazilian Monovarietal Olive Oil in Two Different Package Systems by Using Data Fusion and Chemometrics. Food Anal. Methods 2019, 13, 86–96. [Google Scholar] [CrossRef]
- Tena, N.; Wang, S.C.; Aparicio-Ruiz, R.; García-González, D.L.; Aparicio, R. In-Depth Assessment of Analytical Methods for Olive Oil Purity, Safety, and Quality Characterization. J. Agric. Food Chem. 2015, 63, 4509–4526. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Bajoub, A.; Monasterio, R.P.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Development and validation of LC-MS-based alternative methodologies to GC-MS for the simultaneous determination of triterpenic acids and dialcohols in virgin olive oil. Food Chem. 2018, 239, 631–639. [Google Scholar] [CrossRef]
- Olmo-García, L.; Kessler, N.; Neuweger, H.; Wendt, K.; Olmo-Peinado, J.M.; Fernández-Gutiérrez, A.; Baessmann, C.; Carrasco-Pancorbo, A. Unravelling the Distribution of Secondary Metabolites in Olea europaea L.: Exhaustive Characterization of Eight Olive-Tree Derived Matrices by Complementary Platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 2018, 23, 2419. [Google Scholar] [CrossRef] [Green Version]
- Olmo-García, L.; Polari, J.J.; Li, X.; Bajoub, A.; Fernández-Gutiérrez, A.; Wang, S.C.; Carrasco-Pancorbo, A. Deep insight into the minor fraction of virgin olive oil by using LC-MS and GC-MS multi-class methodologies. Food Chem. 2018, 261, 184–193. [Google Scholar] [CrossRef]
- International Olive Oil Council (IOOC). Available online: http://www.internationaloliveoil.org/news/view/698-year-2018-news/1225-ioc-s-november-december-newsletter (accessed on 14 January 2020).
- Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa N° 1, de 30 de janeiro de 2012. Available online: http://www.lex.com.br/doc_22871594_INSTRUCAO_NORMATIVA_N_1_DE_30_DE_JANEIRO_DE_2012.aspx (accessed on 14 January 2020).
- Skiera, C.; Steliopoulos, P.; Kuballa, T.; Holzgrabe, U.; Diehl, B. 1H NMR approach as an alternative to the classical p-anisidine value method. Eur. Food Res. Technol. 2012, 235, 1101–1105. [Google Scholar] [CrossRef]
- Mansouri, F.; Ben Moumen, A.; Houmy, N.; Richard, G.; Fauconnier, M.L.; Sindic, M.; Caid, H.S.; Elamrani, A. Evaluation of the oxidative stability of blends of ‘Arbequina’olive oils with other monovarietal olive oils. Olivae 2014, 120, 23–30. [Google Scholar]
- Dabbou, S.; Brahmi, F.; Taamali, A.; Issaoui, M.; Ouni, Y.; Braham, M.; Zarrouk, M.; Hammami, M. Extra Virgin Olive Oil Components and Oxidative Stability from Olives Grown in Tunisia. J. Am. Oil Chem. Soc. 2010, 87, 1199–1209. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Allalout, A.; Krichène, D.; Methenni, K.; Taamali, A.; Oueslati, I.; Daoud, D.; Zarrouk, M. Characterization of virgin olive oil from Super Intensive Spanish and Greek varieties grown in northern Tunisia. Sci. Hortic. 2009, 120, 77–83. [Google Scholar] [CrossRef]
- Ortega, A.; Varela, L.M.; Bermudez, B.; López, S.; Abia, R.; Muriana, F.J.G.; Ortega-Gomez, A. Dietary fatty acids linking postprandial metabolic response and chronic diseases. Food Funct. 2012, 3, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Michas, G.; Micha, R.; Zampelas, A. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis 2014, 234, 320–328. [Google Scholar] [CrossRef]
- Boskou, D. Olive Fruit, Table Olives, and Olive Oil Bioactive Constituents. In Olive and Olive Oil Bioactive Constituents; AOCS Press: Urbana, IL, USA, 2015; pp. 1–30. [Google Scholar]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative1H NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative Measurement of Major Secoiridoid Derivatives in Olive Oil Using qNMR. Proof of the Artificial Formation of Aldehydic Oleuropein and Ligstroside Aglycon Isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef]
- Bajoub, A.; Ajal, E.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Evaluating the potential of phenolic profiles as discriminant features among extra virgin olive oils from Moroccan controlled designations of origin. Food Res. Int. 2016, 84, 41–51. [Google Scholar] [CrossRef]
- Olmo-García, L.; Bajoub, A.; Monasterio, R.P.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Metabolic profiling approach to determine phenolic compounds of virgin olive oil by direct injection and liquid chromatography coupled to mass spectrometry. Food Chem. 2017, 231, 374–385. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Olmo-García, L.; Ajal, E.A.; Monasterio, R.P.; Hanine, H.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach. Int. J. Mol. Sci. 2016, 18, 52. [Google Scholar] [CrossRef]
- Brenes, M.; Hidalgo, F.J.; García, A.; Ríos, J.; García, P.; Zamora, R.; Garrido, A. Pinoresinol and 1-acetoxypinoresinol, two new phenolic compounds identified in olive oil. J. Am. Oil Chem. Soc. 2000, 77, 715–720. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Mariotti, M.; Peri, C. The Composition and Nutritional Properties of Extra-Virgin Olive Oil; Wiley: Chichester, UK, 2014; pp. 21–34. [Google Scholar]
- Mendonça, R.; Carvalho, N.; Martin-Moreno, J.; Pimenta, A.; Lopes, A.; Gea, A.; Martinez-Gonzalez, M.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Triterpenic Content and Chemometric Analysis of Virgin Olive Oils from Forty Olive Cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Warleta, F.; Campos, M.; Beltrán, G.; Gaforio, J.J. Bioactive Properties of the Main Triterpenes Found in Olives, Virgin Olive Oil, and Leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Schwartz, S.; von Elbe, J.; Giusti, M.M. Colorants. In Food Chemistry, 4th ed.; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 571–638. [Google Scholar]
- Gallardo-Guerrero, L.; Gandul-Rojas, B.; Roca, M.; Mínguez-Mosquera, M.I. Effect of storage on the original pigment profile of spanish virgin olive oil. J. Am. Oil Chem. Soc. 2005, 82, 33–39. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; De Torres, A.; Moya, M.; Espínola, J.M.; Espínola, F. Fresh and Aromatic Virgin Olive Oil Obtained from Arbequina, Koroneiki, and Arbosana Cultivars. Molecules 2019, 24, 3587. [Google Scholar] [CrossRef] [Green Version]
- Kosma, I.; Vavoura, M.; Kontakos, S.; Karabagias, I.; Kontominas, M.; Apostolos, K.; Badeka, A.V. Characterization and Classification of Extra Virgin Olive Oil from Five Less Well-Known Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2016, 93, 837–848. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.; Bedgood, D.; Bishop, A.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Morales, M.T.; Tsimidou, M. El papel de los compuestos volátiles y los polifenoles en la calidad sensorial del aceite de oliva. In Manual del Aceite de Olive; Aparicio, R., Hardwood, J., Eds.; AMV Ediciones/Ediciones Mundi-Prensa: Madrid, Spain, 2003; Volume 12, pp. 381–441. [Google Scholar]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Vichi, S.; Castellote, A.I.; Pizzale, L.; Conte, L.; Buxaderas, S.; López-Tamames, E.; Castellote, A.I. Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J. Chromatogr. A 2003, 983, 19–33. [Google Scholar] [CrossRef]
- Bononi, M.; Tura, D.; Bassi, D.; Tateo, F. Solid-phase microextraction in the analysis of the volatile fraction of extra-virgin olive oil from fourteen cultivars grown in the lake Garda region (Italy). Italy J. Food Sci. 2008, 1, 119–126. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction–gas chromatography, solid-phase microextraction–gas chromatography–mass spectrometry and olfactometry. J. Chromatogr. A 2002, 976, 265–275. [Google Scholar] [CrossRef]
- Angerosa, F. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Marques, S. O Guia dos Azeites do Brasil 2017; Livrobits: São Paulo, Brazil, 2017; p. 57. [Google Scholar]
- EU Trade in Olive Oil. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20191108-1 (accessed on 24 April 2020).
- American Oil Chemists’ Society (AOCS). Official Methods and Recommended Practices of the AOCS, 6th ed.; American Oil Chemists’ Society: Chicago, IL, USA, 2012. [Google Scholar]
- Castelo-Branco, V.N.; Torres, A.G. Generalized linear model describes determinants of total antioxidant capacity of refined vegetable oils. Eur. J. Lipid Sci. Technol. 2011, 114, 332–342. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Enzym. Eng. Evol. Gen. Methods 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Tuberoso, C.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar]
- Wolff, R.L.; Bayard, C.C.; Fabien, R.J. Evaluation of sequential methods for the determination of butterfat fatty acid composition with emphasis on trans -18:1 acids. Application to the study of seasonal variations in french butters. J. Am. Oil Chem. Soc. 1995, 72, 1471–1483. [Google Scholar] [CrossRef]
- Strain, H.H.; Cope, B.T.; Svec, W.A. [42] Analytical procedures for the isolation, identification, estimation, and investigation of the chlorophylls. Enzym. Eng. Evol. Gen. Methods 1971, 23, 452–476. [Google Scholar] [CrossRef]
- Moberg, L.; Karlberg, B.; Blomqvist, S.; Larsson, U. Comparison between a new application of multivariate regression and current spectroscopy methods for the determination of chlorophylls and their corresponding pheopigments. Anal. Chim. Acta 2000, 411, 137–143. [Google Scholar] [CrossRef]
- Larick, D.K.; Parker, J.D. Chromatographic Analysis of Secondary Lipid Oxidation Products. Curr. Protoc. Food Anal. Chem. 2001, D2.2.1–D2.2.9. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, L.; Pawliszyn, J. Determination of five benzodiazepines in aqueous solution and biological fluids using solid-phase microextraction with carbowaxTM/DVB fiber coating. J. Microcolumn Sep. 1998, 10, 193–201. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.; Buxaderas, S.; López-Tamames, E. Solid-Phase Microextraction in the Analysis of Virgin Olive Oil Volatile Fraction: Modifications Induced by Oxidation and Suitable Markers of Oxidative Status. J. Agric. Food Chem. 2003, 51, 6564–6571. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |
| EVOO Samples | Free Acidity (% 18:1) | Peroxide Value (mEq O2/kg) | k232 | k270 | p-Anisidine Value | Antioxidant Capacity (mmol TE/kg) | Oxidative Stability Index (h) | Total Phenolic Content (mg GAE/100 g) |
|---|---|---|---|---|---|---|---|---|
| Brazilian cv. Arbequina | ||||||||
| Southeast A | 0.18 ± 0.00 a,g,k | 8.62 ± 0.07 a | 2.31 ± 0.01 a,i | 0.22 ± 0.01 a | 4.36 ± 0.12 a | 2.52 ± 0.02 a | 12.75 ± 0.12 a | 4.42 ± 0.39 a |
| Southeast B | 0.36 ± 0.03 b | 19.10 ± 0.27 b | 2.88 ± 0.06 b | 0.18 ± 0.01 b,c | 4.08 ± 0.02 a | 2.97 ± 0.04 b | 13.08 ± 0.19 a | 6.53 ± 0.11 b,f,i |
| South C | 0.04 ± 0.00 c | 7.27 ± 0.01 c,g | 1.98 ± 0.01 c | 0.14 ± 0.01 d,f,g,h,i | 5.78 ± 0.06 b | 2.35 ± 0.04 a | 15.58 ± 0.17 b | 4.31 ± 0.22 a |
| South D | 0.20 ± 0.02 a,i,l | 9.48 ± 0.12 d | 2.29 ± 0.01 a | 0.13 ± 0.00 d,e,j,k,l | 5.28 ± 0.05 c | 3.08 ± 0.07 a | 13.21 ± 0.07 a | 5.52 ± 0.47 a,i |
| South E | 0.11 ± 0.00 d | 9.70 ± 0.08 d | 2.36 ± 0.01 d,i | 0.15 ± 0.01 e,m,n | 7.16 ± 0.07 d | 2.40 ± 0.09 a | 16.93 ± 0.38 c | 6.04 ± 0.52 b,i |
| Brazilian cv. Koroneiki | ||||||||
| Southeast B | 0.39 ± 0.01 b | 3.89 ± 0.01 e | 1.65 ± 0.05 e | 0.13 ± 0.00 f,j,o,p | 2.80 ± 0.06 e | 3.29 ± 0.09 b | 64.72 ± 0.30 d | 18.9 ± 0.78 d |
| Southeast F | 0.44 ± 0.02 e | 6.95 ± 0.04 c | 1.47 ± 0.01 f | 0.16 ± 0.00 m,q | 1.59 ± 0.02 f | 3.19 ± 0.09 b | 43.60 ± 0.13 e | 11.3 ± 0.01 e |
| South C | 0.15 ± 0.01 d,g | 4.06 ± 0.10 e | 1.50 ± 0.01 f | 0.12 ± 0.00 f,j,r | 7.13 ± 0.14 d | 2.36 ± 0.11 a | 41.09 ± 0.73 f | 7.38 ± 0.32 b,f,g |
| South G | 0.18 ± 0.00 f,g,l | 9.22 ± 0.01 a,d | 2.09 ± 0.01 g | 0.19 ± 0.00 b | 10.8 ± 0.16 g | 2.38 ± 0.05 a | 32.75 ± 0.42 g | 9.33 ± 0.24 c,h |
| South H | 0.16 ± 0.01 g | 7.63 ± 0.09 c,g | 2.07 ± 0.02 g,j | 0.15 ± 0.00 g,m,q,s | 8.07 ± 0.31 h | 2.27 ± 0.06 a | 23.67 ± 0.50 h | 6.08 ± 0.29 b,i |
| Spanish cv. Arbequina | ||||||||
| Catalonia I | 0.28 ± 0.02 h | 13.90 ± 0.47 f | 2.87 ± 0.00 b | 0.14 ± 0.00 h,k,n,o,s | 4.42 ± 0.09 a | 3.28 ± 0.14 b | 12.52 ± 0.01 a | 7.81 ± 0.35 f,g |
| Catalonia J | 0.23 ± 0.03 i | 7.68 ± 0.44 g | 2.20 ± 0.01 h | 0.17 ± 0.00 c | 7.44 ± 0.05 d | 3.08 ± 0.21 b | 26.45 ± 0.35 i | 11.8 ± 0.05 e |
| Catalonia K | 0.31 ± 0.00 h | 5.83 ± 0.48 h | 2.00 ± 0.00 c,j | 0.14 ± 0.00 h,k,n,o,s | 4.90 ± 0.06 c | 3.04 ± 0.10 b | 16.58 ± 0.33 c,b | 8.06 ± 0.10 g,h |
| Catalonia L | 0.58 ± 0.00 j | 9.52 ± 0.05 d | 2.18 ± 0.01 h | 0.15 ± 0.00 h,n,q,s | 6.68 ± 0.23 i | 2.99 ± 0.27 b | 16.81 ± 0.23 c | 9.63 ± 0.08 c |
| Catalonia M | 0.22 ± 0.01 f,i,k | 9.52 ± 0.07 d | 2.18 ± 0.02 h | 0.13 ± 0.00 i,k,l,p,r | 5.18 ± 0.16 c | 3.07 ± 0.06 b | 20.52 ± 0.39 j | 8.47 ± 0.31 c,g |
| Established limits 1 | 0.80 | 20.0 | 2.50 | 0.22 | 10.0 * | - | - | - |
| EVOO Samples | Fatty Acid Composition (g/100 g) | ||||||
|---|---|---|---|---|---|---|---|
| 16:0 | 16:1n-7 | 18:0 | 18:1n-9 | 18:2n-6 | 18:3n-3 | M: Pratio | |
| Brazilian cv. Arbequina | |||||||
| Southeast A | 14.3 ± 0.30 a,d,f | 1.59 ± 0.05 a | 1.51 ± 0.06 a,b | 73.1 ± 0.15 a | 8.91 ± 0.16 a | 0.57 ± 0.04 a,c,d | 7.89 ± 0.16 a |
| Southeast B | 15.1 ± 0.25 a,b,g | 0.11 ± 0.02 b | 1.18 ± 0.41 a | 73.2 ± 0.14 a | 9.85 ± 0.03 b | 0.61 ± 0.00 a,c,e | 7.00 ± 0.02 b |
| South C | 16.7 ± 0.39 a,h | 2.05 ± 0.09 c | 1.40 ± 0.04 a,b | 70.0 ± 0.50 b | 9.32 ± 0.04 c | 0.57 ± 0.06 a,c,d | 7.28 ± 0.05 b, j |
| South D | 16.9 ± 1.66 b,h,i | 0.10 ± 0.00 b | 1.39 ± 0.58 a,b | 66.6 ± 1.10 c | 14.3 ± 0.20 d | 0.75 ± 0.01 b | 4.44 ± 0.01 c |
| South E | 17.1 ± 0.39 b,h,i | 1.92 ± 0.04 d | 1.60 ± 0.03 a,b | 70.1 ± 0.35 b | 8.68 ± 0.09 a | 0.61 ± 0.04 a,c,e,f | 7.75 ± 0.06 a, j |
| Brazilian cv. Koroneiki | |||||||
| Southeast B | 11.0 ± 0.49 c,e | 0.04 ± 0.00 b | 1.10 ± 0.23 a,b | 83.8 ± 0.36 d | 3.52 ± 0.02 e | 0.62 ± 0.00 a,c,e,g | 20.3 ± 0.15 d |
| Southeast F | 8.54 ± 1.21 c | 0.04 ± 0.00 b | 1.23 ± 0.65 a,b | 86.0 ± 0.72 e | 3.55 ± 0.05 e | 0.64 ± 0.00 a,e,h | 20.6 ± 0.37 d |
| South C | 13.1 ± 0.26 d,e,g,j | 0.81 ± 0.06 e | 1.28 ± 0.13 a,b | 78.8 ± 0.34 f | 4.66 ± 0.12 f | 0.68 ± 0.05 b,f,g,h,i | 14.9 ± 0.50 e |
| South G | 14.1 ± 0.37 a,j,k | 0.86 ± 0.09 e,f | 2.10 ± 0.09 a | 76.7 ± 0.42 g,h,i | 5.69 ± 0.12 g | 0.58 ± 0.04 a,c,d,e | 12.3 ± 0.20 f |
| South H | 14.5 ± 0.19 a,i,j,k | 1.04 ± 0.01 f | 1.54 ± 0.16 a | 75.2 ± 0.24 g,j | 6.27 ± 0.09 h | 0.92 ± 0.06 j | 10.6 ± 0.25 g |
| Spanish cv. Arbequina | |||||||
| Catalonia I | 15.3 ± 0.21 a,i,j | 0.21 ± 0.17 b | 1.85 ± 0.49 a,b | 69.8 ± 0.48 b | 12.3 ± 0.15 i | 0.55 ± 0.01 a,c,d | 5.44 ± 0.06 h |
| Catalonia J | 11.4 ± 0.76 e | 0.19 ± 0.11 b | 2.27 ± 0.94 a,b | 77.6 ± 0.59 f,h | 8.07 ± 0.10 j | 0.53 ± 0.01 c,d | 9.04 ± 0.14 i |
| Catalonia K | 15.5 ± 1.47 a,i,j | 0.13 ± 0.00 b | 1.61 ± 0.50 a,b | 68.5 ± 0.88 b | 13.6 ± 0.08 k | 0.66 ± 0.01 b,e | 4.80 ± 0.03 c |
| Catalonia L | 12.3 ± 1.59 e,f,k | 0.12 ± 0.01 b | 2.63 ± 0.66 b | 74.0 ± 1.17 a,j | 10.4 ± 0.26 l | 0.61 ± 0.01 a,c,e,i | 6.72 ± 0.08 b |
| Catalonia M | 11.5 ± 0.76 e | 0.11 ± 0.01 b | 1.35 ± 0.65 a,b | 77.6 ± 0.84 f,i | 8.97 ± 0.10 a,c | 0.50 ± 0.00 d | 8.21 ± 0.06 a |
| Country of Origin | Cultivar | Country Region | Designation | Latitude | Longitude | Altitude (m) |
|---|---|---|---|---|---|---|
| Southeast | A | 22°41′18″ S | 45°44′11″ W | 874 | ||
| Southeast | B | 21°57′35″ S | 44°53′29″ W | 893 | ||
| Arbequina | South | C | 31°23′44″ S | 52°41′11″ W | 408 | |
| South | D | 31°28′36″ S | 53°40′45″ W | 197 | ||
| Brazil | South | E | 30°53′23″ S | 55°31′56″ W | 215 | |
| Southeast | B | 21°57′35″ S | 44°53′29″ W | 893 | ||
| Southeast | F | 21°55′46″ S | 44°36′07″ W | 1155 | ||
| Koroneiki | South | C | 31°23′44″ S | 52°41′11″ W | 408 | |
| South | G | 30°30′59″ S | 53°29′12″ W | 430 | ||
| South | H | 30°42′16″ S | 52°06′36″ W | 134 | ||
| Catalonia | I | 41°36′51″ N | 00°37′33″ W | 169 | ||
| Catalonia | J | 41°38′49″ N | 01°08′21″ W | 385 | ||
| Spain | Arbequina | Catalonia | K | 41°32′25″ N | 00°55′07″ W | 304 |
| Catalonia | L | 41°32′25″ N | 00°55′07″ W | 304 | ||
| Catalonia | M | 41°38′49″ N | 01°08′21″ W | 385 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.G.A.d.; Olmo-García, L.; Gaspar, B.R.A.; Carrasco-Pancorbo, A.; Castelo-Branco, V.N.; Torres, A.G. Evaluating Quality Parameters, the Metabolic Profile, and Other Typical Features of Selected Commercial Extra Virgin Olive Oils from Brazil. Molecules 2020, 25, 4193. https://doi.org/10.3390/molecules25184193
Carvalho AGAd, Olmo-García L, Gaspar BRA, Carrasco-Pancorbo A, Castelo-Branco VN, Torres AG. Evaluating Quality Parameters, the Metabolic Profile, and Other Typical Features of Selected Commercial Extra Virgin Olive Oils from Brazil. Molecules. 2020; 25(18):4193. https://doi.org/10.3390/molecules25184193
Chicago/Turabian StyleCarvalho, Aline Gabrielle Alves de, Lucía Olmo-García, Bruna Rachel Antunes Gaspar, Alegría Carrasco-Pancorbo, Vanessa Naciuk Castelo-Branco, and Alexandre Guedes Torres. 2020. "Evaluating Quality Parameters, the Metabolic Profile, and Other Typical Features of Selected Commercial Extra Virgin Olive Oils from Brazil" Molecules 25, no. 18: 4193. https://doi.org/10.3390/molecules25184193






