Identification of Odor Active Compounds in Physalis peruviana L.
Abstract
:1. Introduction
2. Results
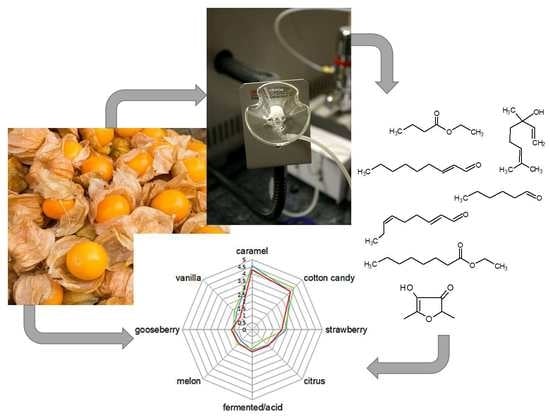
2.1. Sensory Characterization of Cape Gooseberry Fruit (Physalis peruviana L.)
2.2. Screening the Cape Gooseberry Volatiles for Aroma-Active Compounds by the Means of GC-O AEDA Analysis
3. Discussion
4. Materials and Methods
4.1. Fruit Samples
4.2. Chemical Standards
4.3. Isolation Method
4.4. Gas Chromatography-Olfactometry (GC-O)
4.5. Gas Chromatography/Mass Spectrometry
4.6. Aroma Extract Dilution Analysis (AEDA)
4.7. Quantitation of Odorants
4.8. Aroma Recombination
4.9. Sensory Evaluation
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y. (Ed.) Berry Fruit: Value-Added Products for Health Promotion; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Steven, G.; Pratt, M.D.; Matthews, K. Super Foods Rx: Fourteen Foods that Will Change Your Life; Harper Collins Publishers: New York, NY, USA, 2005. [Google Scholar]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Vuuren, S.F.; Van Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Frag. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Jamin, E. Superfruits: Are they authentic? Fruit Process. 2009, 19, 170–175. [Google Scholar]
- Crozier, S.J.; Preston, A.G.; Hurst, J.W.; Payne, M.J.; Mann, J.; Hainly, L.; Miller, D.L. Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products. Chem. Cent. J. 2011, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Tenorio, M.L.; Dekker, M.; Verkerk, R.; van Boekel, A.J.S. Health-Promoting compounds in cape gooseberry (Physalis peruviana L.): Review from a supply chain perspective. Trends Food Sci. Techn. 2016, 57, 83–92. [Google Scholar] [CrossRef]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Rodríguez, S.; Rodríguez, E. Efecto de la ingesta de Physalisperuviana (aguaymanto) sobre la glicemia postprandial enadultosjóvenes. Rev. Médica Val. 2007, 4, 43–52. [Google Scholar]
- Salazar, M.; Jones, J.; Chaves, B.; Cooman, A. A model for the potential production and dry matter distribution of cape gooseberry (Physalis peruviana L.). Sci. Hortic. 2008, 115, 142–148. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Moersel, J.T. Impact of enzymatic treatment on chemical composition, physicochemical properties and radical scavenging activity of golden berry (Physalis peruviana L.) juice. J. Sci. Food Agric. 2007, 87, 452–460. [Google Scholar] [CrossRef]
- Kupska, M.; Jeleń, H.H. In-tube extraction for the determination of the main volatile compounds in Physalis peruviana L. J. Sep. Sci. 2017, 40, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kupska, M.; Wasilewski, T.; Jędrkiewicz, R.; Gromadzka, J.; Namieśnik, J. Determination of terpene profiles in potential superfruits. Int. J. Food Prop. 2016, 19, 2726–2738. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Mapping the Combinatorial Code of Food Flavors by Means of Molecular Sensory Science Approach. In Food Flavors. Chemical, Sensory and Technological Properties; Jeleń, H., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 413–438. [Google Scholar]
- Li, J.-X.; Schieberle, P.; Steinhaus, M. Characerization of the major odor-active compounds in Thai durian (Durio zibethinus L. ‘Monthong’) by aroma extract dilution analysis and headspace gas chromatography-Olfactometry. J. Agric. Food Chem. 2012, 60, 11253–11262. [Google Scholar] [CrossRef] [PubMed]
- Munafo, J.P., Jr.; Didzbalis, J.; Schnell, R.J.; Steinhaus, M. Insights into the key aroma compounds of mango (Mangifera indica L. Haden) fruits by stable isotope dilution quantitation and aroma simulation experiments. J. Agric. Food Chem. 2016, 64, 4312–4318. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Jane, L.; Strli, M.; Rusjan, D. Analysis of free and bound aroma compounds in grape berries using headspace solid-phase microextraction with GC-MS and a preliminary study of solid-phase extraction with LC-MS. Acta Chim. Slov. 2007, 54, 25–32. [Google Scholar]
- Van Gemert, L.J.; Odour, T. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Berger, R.G.; Drawert, F.; Kollmannsberger, H. The flavor of cape gooseberry (Physalis peruviana L.). Z. Lebensm Unters. Forsch. 1989, 188, 122–126. [Google Scholar] [CrossRef]
- Yilmaztekin, M. Characterization of potent aroma compounds of cape gooseberry (Physalis peruviana L.) fruits grown in Antalya through the determination of odor activity values. Int. J. Food Prop. 2014, 17, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Yilmaztekin, M. Analysis of volatile components of cape gooseberry (Physalis peruviana L.) grown in Turkey by HS-SPME and GC-MS. Sci. World J. 2014, 796097. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| No | Compound | odor | RI Supelcowax-10 | RI SPB-5 | FD |
|---|---|---|---|---|---|
| 1 | 2-Methylpropanal | fruity | 900 | <600 | 4 |
| 2 | Ethyl 2-methyl propanoate | fruity, anise-like | 950 | 750 | 4 |
| 3 | Butane-2,3-dione | buttery | 980 | <600 | 16 |
| 4 | Ethyl butanoate | fruity | 1027 | 802 | 512 |
| 5 | Hexanal | fresh grass | 1072 | 803 | 8 |
| 6 | Ethyl hexanoate | fruity | 1200 | 1002 | 16 |
| 7 | Octanal | rancid, citrus | 1287 | 998 | 16 |
| 8 | Oct-1-en-3-ol | mushroom | 1292 | 978 | 8 |
| 9 | 2-Acetyl-1-pyrroline | popcorn | 1350 | 911 | 8 |
| 10 | Ethyl octanoate | fruity | 1419 | 1195 | 128 |
| 11 | Methional | boiled potato | 1450 | 907 | 128 |
| 12 | 2,3-Diethyl-5-methyl pyrazine | earthy | 1495 | 1156 | 128 |
| 13 | (E)-Non-2-enal | fatty, green | 1530 | 1159 | 64 |
| 14 | β-Linalool | fruity | 1550 | 1101 | 256 |
| 15 | (E2, Z6)-Nona-2,6-dienal | cucumber | 1580 | 1150 | 64 |
| 16 | 2-Phenylacetaldehyde | rosy, honey-like | 1640 | 1048 | 8 |
| 17 | 2-Phenylethanol | flowery, honey-like | 1901 | 1118 | 64 |
| 18 | Furaneol | cotton candy | 2015 | 1080 | 512 |
| Compound | OT a [µg/L] | Concentration b [µg/kg] | OAV c |
|---|---|---|---|
| Ethyl butanoate | 0.76 | 383.0 | 504 |
| β-Linalol | 0.089 | 32.0 | 360 |
| (E)-Non-2-enal | 0.08 | 24.0 | 300 |
| (2E, 6Z)-Nona-2,6-dienal | 0.02 | 5.0 | 250 |
| Hexanal | 4.50 | 450.0 | 100 |
| Ethyl octanoate | 5.00 | 411.0 | 82 |
| Furaneol | 30.0 | 1350.0 | 45 |
| Ethyl hexanoate | 1.20 | 45.0 | 38 |
| Butane-2,3-dione | 15.0 | 500.0 | 33 |
| 2-Methylpropanal | 1.90 | 50.0 | 26 |
| Octanal | 0.70 | 15.0 | 21 |
| Ethyl 2-methyl propanoate | 0.089 | 1.8 | 20 |
| Methional | 0.20 | 36.0 | 18 |
| 2-Phenylacetaldehyde | 4.00 | 25.0 | 6 |
| Oct-1-en-3-ol | 1.00 | 3.0 | 3 |
| 2,3-Diethyl-5-methyl pyrazine | 1.00 | <0.1 | <1 |
| 2-Phenylethanol | 1000 | 65.0 | <1 |
| 2-Acetyl-1-pyrroline | 0.1 | <0.01 | <1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majcher, M.A.; Scheibe, M.; Jeleń, H.H. Identification of Odor Active Compounds in Physalis peruviana L. Molecules 2020, 25, 245. https://doi.org/10.3390/molecules25020245
Majcher MA, Scheibe M, Jeleń HH. Identification of Odor Active Compounds in Physalis peruviana L. Molecules. 2020; 25(2):245. https://doi.org/10.3390/molecules25020245
Chicago/Turabian StyleMajcher, Małgorzata A., Magdalena Scheibe, and Henryk H. Jeleń. 2020. "Identification of Odor Active Compounds in Physalis peruviana L." Molecules 25, no. 2: 245. https://doi.org/10.3390/molecules25020245