Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques
Abstract
:1. Introduction
2. Ozonation Process
2.1. Ozonation Mechanism
2.2. Ozone Absorption
3. Characterization of Ozonated Oils
3.1. Total Unsaturation
3.2. Iodine Value
3.3. Peroxide Value
3.4. Acidity Value
3.5. Formaldehyde Determination
3.6. Viscosity
4. Analytical Control of Ozonated Oils
4.1. FT-IR Spectroscopy
4.2. 1H and 13C NMR
4.3. Gas–Liquid Chromatography
5. Ozonated Oil Stability
6. Topical Applications of Ozonated Oils
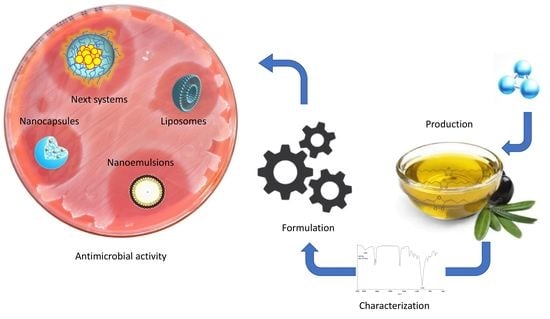
7. Ozonated Oil Delivery Systems: State of the Art and Perspectives
7.1. Ozonated Oil-Based Emulsions
7.2. Micro- and Nanoemulsions
7.3. Macro-, Micro-, and Nanoencapsulation of Oils
8. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tambe, S.M.; Sampath, L.; Modak, S.M. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J. Antimicrob. Chemother. 2001, 47, 589–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattappalil, A.; Mergenhagen, K.A. Neurotoxicity with antimicrobials in the elderly: A review. Clin. Ther. 2014, 36, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Lagacé, W.P.; Rubinstein, E. Adverse reactions to β-lactam antimicrobials. Expert Opin. Drug Saf. 2012, 11, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.S. Less-known botanical cosmeceuticals. Dermatol. Ther. 2007, 20, 330–342. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Saloom, K.S.; Al-Waili, T.N.; Al-Waili, A.N. The safety and efficacy of a mixture of honey, olive oil, and beeswax for the management of hemorrhoids and anal fissure: A pilot study. Sci. World J. 2006, 6, 1998–2005. [Google Scholar] [CrossRef]
- Martínez, S.G.; Al-Dalain, S.M.; Menéndez, S.; Re, L.; Giuliani, A.; Candelario, J.E.; Álvarez, H.; Fernández-Montequín, J.I.; León, O.S. Therapeutic efficacy of ozone in patients with diabetic foot. Eur. J. Pharm. 2005, 523, 151–161. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas. Res. 2017, 7, 212–219. [Google Scholar]
- Nagayoshi, M.; Fukuizumi, T.; Kitamura, C.; Yano, J.; Terashita, M.; Nishihara, T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol. Immunol. 2004, 19, 240–246. [Google Scholar] [CrossRef]
- Carata, E.; Tenuzzo, B.A.; Dini, L. Powerful Properties of Ozonated Extra Virgin Olive Oil. In Herbal Medicine; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Zeng, J.; Lu, J. Mechanisms of action involved in ozone-therapy in skin diseases. Int. Immunopharmacol. 2018, 56, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fenaroli, P. Gravimetric estimation of ozone; ozone numbers of oils. Gazz. Chim. 1906, 36, 292–298. [Google Scholar]
- Harada, T. Olive oil ozonide and its fungicidal quality. Bull. Chem. Soc. Jpn. 1934, 9, 192–197. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, N.R.; Beatriz, A.; de Arruda, E.J.; de Lima, D.P.; de Oliveira, L.C.S.; Micheletti, A.C. Ozonized vegetable oils: Production, chemical characterization and therapeutic potential. In Vegetable Oil: Properties, Uses and Benefits; Nova Science Publishers, Hauppauge: New York, NY, USA, 2016; pp. 129–160. [Google Scholar]
- Menéndez, S.; Falcón, L.; Maqueira, Y. Therapeutic efficacy of topical OLEOZON ® in patients suffering from onychomycosis. Mycoses 2011, 54, e272–e277. [Google Scholar] [CrossRef]
- Valacchi, G.; Zanardi, I.; Lim, Y.; Belmonte, G.; Miracco, C.; Sticozzi, C.; Bocci, V.; Travagli, V. Ozonated oils as functional dermatological matrices: Effects on the wound healing process using SKH1 mice. Int. J. Pharm. 2013, 458, 65–73. [Google Scholar] [CrossRef]
- Travagli, V.; Zanardi, I.; Valacchi, G.; Bocci, V. Ozone and ozonated oils in skin diseases: A review. Mediat. Inflamm. 2010, 2010, 610418. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.F.; Sánchez, Y.; Gómez, M.; Hernández, F.; Da, C.; Veloso, M.C.; De, P.; Pereira, P.A.; Mangrich, A.S.; De Andrade, J.B. Physicochemical characteristics of ozonated sunflower oils obtained by different procedures. Grasas Y Aceites 2012, 63, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Moureu, S.; Violleau, F.; Ali Haimoud-Lekhal, D.; Calmon, A. Ozonation of sunflower oils: Impact of experimental conditions on the composition and the antibacterial activity of ozonized oils. Chem. Phys. Lipids 2015, 186, 79–85. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chem. Int. Ed. Engl. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Kuczkowski, R.L. The structure and mechanism of formation of ozonides. Chem. Soc. Rev. 1992, 21, 79–83. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, Y.Y.; Curtis, J.M. A study of the ozonolysis of model lipids by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Church, D.F.; Mahier, T.J.; Barker, S.A.; Pryor, W.A. Separation and spectral data of the six isomeric ozonides from methyl oleate. Lipids 1992, 27, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Anachkov, M.; Batakliev, T.; Rakovsky, S. Study on the stoichiometry and reaction products of extra virgin olive oil ozonation. Bulg. Chem. Commun. 2013, 45, 203–207. [Google Scholar]
- Sega, A.; Zanardi, I.; Chiasserini, L.; Gabbrielli, A.; Bocci, V.; Travagli, V. Properties of sesame oil by detailed 1H and 13C NMR assignments before and after ozonation and their correlation with iodine value, peroxide value, and viscosity measurements. Chem. Phys. Lipids 2010, 163, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.P.; Poznyak, T.; Pérez, A.; Gómez, Y.M.; Bautista, R.M.E.; Chairez, I. Ozonation Degree of Vegetable Oils as the Factor of Their Anti-Inflammatory and Wound-Healing Effectiveness. Ozone: Sci. Eng. 2017, 39, 374–384. [Google Scholar] [CrossRef]
- Sadowska, J.; Johansson, B.; Johannessen, E.; Friman, R.; Broniarz-Press, L.; Rosenholm, J.B. Characterization of ozonated vegetable oils by spectroscopic and chromatographic methods. Chem. Phys. Lipids 2008, 151, 85–91. [Google Scholar] [CrossRef]
- Guinesi, A.S.; Andolfatto, C.; Idomeo, B.F.; Arnaldo, A.C.; Juliano, P.F.; Roberta, V.F. Ozonized Oils: A qualitative and quantitative analysis. Braz. Dent. J. 2011, 22, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Zanardi, I.; Travagli, V.; Gabbrielli, A.; Chiasserini, L.; Bocci, V. Physico-chemical characterization of sesame oil derivatives. Lipids 2008, 43, 877–886. [Google Scholar] [CrossRef]
- Guerra, B.P.; Poznyak, T.; Chairez, I.; Brito, A.M. Correlation of structural characterization and viscosity measurements with total unsaturation: An effective method for controlling ozonation in the preparation of ozonated grape seed and sunflower oils. Eur. J. Lipid Sci. Technol. 2015, 117, 988–998. [Google Scholar] [CrossRef]
- Firestone, D. Determination of the iodine value of oils and fats: Summary of collaborative study. J. AOAC Int. 1994, 77, 674–676. [Google Scholar]
- Iodine value. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2019; pp. 167–168.
- Díaz, M.F.; Hernández, R.; Martínez, G.; Vidal, G.; Gómez, M.; Fernández, H.; Garcés, R. Comparative study of ozonized olive oil and ozonized sunflower oil. J. Braz. Chem. Soc. 2006, 17, 403–407. [Google Scholar] [CrossRef]
- Günaydın, Y.; Sevim, H.; Tanyolaç, D.; Gürpınar, Ö.A. Ozonated Olive Oil with a High Peroxide Value for Topical Applications: In-Vitro Cytotoxicity Analysis with L929 Cells. Ozone Sci. Eng. 2018, 40, 37–43. [Google Scholar]
- Peroxide value. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2019; pp. 168–169.
- Peroxide Value. In AOCS Official Method Cd 8-53, 5th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1998.
- Martinez Tellez, G.; Ledea Lozano, O.; Díaz Gómez, M. Measurement of peroxidic species in ozonized sunflower oil. Ozone Sci. Eng. 2006, 28, 181–185. [Google Scholar] [CrossRef]
- Acid value. In AOCS Official Test Method Cd 3a-63, 5th ed.; American Oil Chemists’Society: Champaign, IL, USA, 1988.
- P-Anisidine Value. In AOCS Method Cd 18-90 (11), 5th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2011.
- Rodrigues De Almeida Kogawa, N.; José De Arruda, E.; Micheletti, A.C.; De Fatima Cepa Matos, M.; Silva De Oliveira, L.C.; Pires De Lima, D.; Pereira Carvalho, N.C.; Dias De Oliveira, P.; De Castro Cunha, M.; Ojeda, M.; et al. Synthesis, characterization, thermal behavior, and biological activity of ozonides from vegetable oils. RSC Adv. 2015, 5, 65427–65436. [Google Scholar] [CrossRef]
- Varol, K.; Koc, A.N.; Atalay, M.A.; Keles, I. Antifungal Activity of Olive Oil and Ozonated Olive Oil Against Candida Spp. and Saprochaete Spp. Ozone: Sci. Eng. 2017, 39, 462–470. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Kazancı, F. Synthesis and Characterization of Ozonated Oil Nanoemulsions. Jaocsjournal Am. Oil Chem. Soc. 2018, 95, 1385–1398. [Google Scholar] [CrossRef]
- Bailey, P.S. Olefinic Compounds; Academic Press: New York, NY, USA, 1978; Volume 1. [Google Scholar]
- Ledea, O.; Correa, T.; Escobar, M.; Rosado, A.; Molerio, J.; Hernández, C.; Jardines, D. Volatile components of ozonized sunflower oil “OLEOZON®”. Ozone: Sci. Eng. 2001, 23, 121–126. [Google Scholar] [CrossRef]
- Cirlini, M.; Caligiani, A.; Palla, G.; de Ascentiis, A.; Tortini, P. Stability Studies of Ozonized Sunflower Oil and Enriched Cosmetics with a Dedicated Peroxide Value Determination. Ozone: Sci. Eng. 2012, 34, 293–299. [Google Scholar] [CrossRef]
- Moureu, S.; Violleau, F.; Ali Haimoud-Lekhal, D.; Calmon, A. Influence of Storage Temperature on the Composition and the Antibacterial Activity of Ozonized Sunflower Oil. Ozone: Sci. Eng. 2016, 38, 143–149. [Google Scholar] [CrossRef]
- Boland-Nazar, N.S.; Eslamirad, Z.; Sarmadian, H.; Ghasemikhah, R. An in vitro evaluation of ozonized organic extra-virgin olive oil on Giardia Lamblia cysts. Jundishapur J. Microbiol. 2016, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Travagli, V.; Zanardi, I.; Bocci, V. Topical applications of ozone and ozonated oils as anti-infective agents: An insight into the patent claims. Recent Pat. Anti-Infect. Drug Discov. 2009, 4, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Kurtoglu, T.; Durmaz, S.; Kozaci, L.D.; Abacigil, F.; Ertugrul, B.; Erel, O. The effects of ozone on bacterial growth and thiol-disulphide homeostasis in vascular graft infection caused by MRSA in rats. Acta Cir. Bras. 2017, 32, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanardi, I.; Burgassi, S.; Paccagnini, E.; Gentile, M.; Bocci, V.; Travagli, V. What is the best strategy for enhancing the effects of topically applied ozonated oils in cutaneous infections? Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Bialoszewski, D.; Pietruczuk-Padzik, A.; Kalicinska, A.; Bocian, E.; Czajkowska, M.; Bukowska, B.; Tyski, S. Activity of ozonated water and ozone against staphylococcus aureus and pseudomonas aeruginosa bioflms. Med Sci. Monit. 2011, 17, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Ouf, S.A.; Moussa, T.A.; Abd-Elmegeed, A.M.; Eltahlawy, S.R. Anti-fungal potential of ozone against some dermatophytes. Braz. J. Microbiol. 2016, 47, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Jenerowicz, D.; Silny, W.; Dańczak-Pazdrowska, A.; Polańska, A.; Osmola-Mańkowska, A.; Olek-Hrab, K. Environmental factors and allergic diseases. Ann. Agric. Environ. Med. 2012, 19, 475–481. [Google Scholar]
- Gershwin, L.J. Comparative immunology of allergic responses. Annu. Rev. Anim. Biosci. 2015, 3, 327–346. [Google Scholar] [CrossRef]
- Di Paolo, N.; Gaggiotti, E.; Galli, F. Extracorporeal blood oxygenation and ozonation: Clinical and biological implications of ozone therapy. Redox Rep. 2005, 10, 121–130. [Google Scholar] [CrossRef]
- Bocci, V.; Zanardi, I.; Valacchi, G.; Borrelli, E.; Travagli, V. Validity of oxygen-ozone therapy as integrated medication form in chronic inflammatory diseases. Cardiovasc. Hematol. Disord. Drug Targets 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Borges, G.Á.; Elias, S.T.; da Silva, S.M.M.; Magalhães, P.O.; Macedo, S.B.; Ribeiro, A.P.D.; Guerra, E.N.S. In vitro evaluation of wound healing and antimicrobial potential of ozone therapy. J. Cranio-Maxillofac. Surg. 2017, 45, 364–370. [Google Scholar] [CrossRef]
- Song, M.; Zeng, Q.; Xiang, Y.; Gao, L.; Huang, J.; Huang, J.; Wu, K.; Lu, J. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol. Med. Rep. 2018, 17, 2449–2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Serio, F.; Pizzolante, G.; Cozzolino, G.; D’Alba, M.; Bagordo, F.; De Giorgi, M.; Grassi, T.; Idolo, A.; Guido, M.; De Donno, A. A New Formulation Based on Ozonated Sunflower Seed Oil: In Vitro Antibacterial and Safety Evaluation. Ozone: Sci. Eng. 2017, 39, 139–147. [Google Scholar] [CrossRef]
- Hernández, F.; Hernández, D.; Zamora, Z.; Díaz, M.; Ancheta, O.; Rodriguez, S.; Torres, D. Giardia duodenalis: Effects of an ozonized sunflower oil product (Oleozon®) on in vitro trophozoites. Exp. Parasitol. 2009, 121, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, M.; Aghaei, S.; Sokhanvari, F.; Ansari, N.; Hosseini, S.M.; Mohaghegh, M.-A.; Hejazi, S.H. The therapeutic effect of ozonated olive oil plus glucantime on human cutaneous leishmaniasis. Iran. J. Basic Med. Sci. 2019, 22, 25–30. [Google Scholar] [PubMed]
- Fitzpatrick, E.; Holland, O.J.; Vanderlelie, J.J. Ozone therapy for the treatment of chronic wounds: A systematic review. Int. Wound J. 2018, 15, 633–644. [Google Scholar] [CrossRef]
- Aerts, O.; Leysen, J.; Horst, N.; Lambert, J.; Goossens, A. Contact dermatitis caused by pharmaceutical ointments containing ‘ozonated’ olive oil. Contact Dermat. 2016, 75, 123–126. [Google Scholar] [CrossRef]
- Rajabi, O.; Sazgarnia, A.; Abbasi, F.; Layegh, P. The activity of ozonated olive oil against Leishmania major promastigotes. Iran. J. Basic Med Sci. 2015, 18, 915–919. [Google Scholar]
- Tiwari, S.; Avinash, A.; Katiyar, S.; Aarthi Iyer, A.; Jain, S. Dental applications of ozone therapy: A review of literature. Saudi J. Dent. Res. 2017, 8, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Greene, A.K.; Few, B.K.; Serafini, J.C. A Comparison of Ozonation and Chlorination for the Disinfection of Stainless Steel Surfaces. J. Dairy Sci. 1993, 76, 3617–3620. [Google Scholar] [CrossRef]
- Ghobashy, S.A.; El-Tokhey, H.M. In Vivo Study of the Effectiveness of Ozonized Olive Oil Gel on Inhibiting Enamel Demineralization during Orthodontic Treatment. J. Am. Sci. 2012, 8, 657–666. [Google Scholar]
- Pietrocola, G.; Ceci, M.; Preda, F.; Poggio, C.; Colombo, M. Evaluation of the antibacterial activity of a new ozonized olive oil against oral and periodontal pathogens. J. Clin. Exp. Dent. 2018, 10, e1103–e1108. [Google Scholar] [CrossRef] [PubMed]
- El Hadary, A.A.; Yassin, H.H.; Mekhemer, S.T.; Holmes, J.C.; Grootveld, M. Evaluation of the effect of ozonated plant oils on the quality of osseointegration of dental implants under the influence of cyclosporin a: An in vivo study. J. Oral Implant. 2011, 37, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Arora, N.; Puri, G.; Aravinda, K.; Dixit, A.; Jatti, D. Efficacy of ozonized olive oil in the management of oral lesions and conditions: A clinical trial. Contemp. Clin. Dent. 2016, 7, 51–54. [Google Scholar] [CrossRef]
- Tara, F.; Zand-Kargar, Z.; Rajabi, O.; Berenji, F.; Akhlaghi, F.; Shakeri, M.T.; Azizi, H. The Effects of Ozonated Olive Oil and Clotrimazole Cream for Treatment of Vulvovaginal Candidiasis. Altern.Ther. Health Med. 2016, 22, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Battinelli, L.; Daniele, C.; Cristiani, M.; Bisignano, G.; Saija, A.; Mazzanti, G. In vitro antifungal and anti-elastase activity of some aliphatic aldehydes from Olea europaea L. fruit. Phytomedicine 2006, 13, 558–563. [Google Scholar] [CrossRef]
- Rodriguez, B.R.V.L.; Menéndez, S.; Gómez, M. Application of ozonized oil as a treatment of vulvovaginitis in patients intolerant to carbohydrates. In Proceedings of the 1st Iberolatinoamerican Congress on Ozone Applications, Havana, Cuba, 31 October–3 November 1990. [Google Scholar]
- Spadea, L.; Tonti, E.; Spaterna, A.; Marchegiani, A. Use of Ozone-Based Eye Drops: A Series of Cases in Veterinary and Human Spontaneous Ocular Pathologies. Case Rep. Ophthalmol. 2018, 9, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Baeyens, V.; Felt-Baeyens, O.; Rougier, S.; Pheulpin, S.; Boisramé, B.; Gurny, R. Clinical evaluation of bioadhesive ophthalmic drug inserts (BODI®) for the treatment of external ocular infections in dogs. J. Control. Release 2002, 85, 163–168. [Google Scholar] [CrossRef]
- Fernández Torres, I.; Curtiellas Piñol, V.; Sánchez Urrutia, E.; Gómez Regueiferos, M. In vitro antimicrobial activity of ozonized theobroma oil against Candida albicans. Ozone: Sci. Eng. 2006, 28, 187–190. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- O’Malley, P. Micro-Encapsulation of Ozonated Oils. U.S. Patent 9,554,973, 31 January 2017. [Google Scholar]
- Serio, F.; Alba, M.; Cozzolino, G.; Idolo, A.; Grassi, T. Efficacy of a Dermatological Gel Based on Ozonized Sunflower Seed Oil (Oz. Or. Oil 30) on Bedsores: A Pilot Study. Clin. Derm. Res. J. 2017, 2, 2. [Google Scholar]
- Adilson, B.; De Lima, D.P.; De Arruda, E.J.; De Oliveira, L.C.S.; De Almeida, N.R. Process for obtaining gel from ozonization of vegetable oils. BR102017000897A2.
- Zhou, S.; Miao, Y.; Yuan, C.; Liu, X. An Ozonized Oil Bicontinuous Phase Emulsion and Its Preparation Method and Application in Preventing and Treating animal Bedsore. CN107088185A, 25 August 2017. [Google Scholar]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharm. 2018, 108, 1477–1494. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Cao, Z.; Spilker, T.; Fan, Y.; Kalikin, L.M.; Ciotti, S.; Lipuma, J.J.; Makidon, P.E.; Wilkinson, J.E.; Baker, J.R.; Wang, S.H. Nanoemulsion is an effective antimicrobial for methicillin-resistant Staphylococcus aureus in infected wounds. Nanomedicine 2017, 12, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.Y.; Ramalingam, K.; Bienek, D.R.; Lee, V.; You, T.; Alvarez, R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 3568–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, P.C.; Leite, G.M.; Domingues, R.J.; Silva, J.; Gibbs, P.A.; Ferreira, J.P. Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. Int. J. Food Microbiol. 2007, 118, 15–19. [Google Scholar] [CrossRef]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef] [Green Version]
- Carocci, G. Composition Comprising Microstructures, an Oil-in-Water Microemulsion, for the Controlled Release of Ozonized Oil. EP3311800A1, 25 April 2018. [Google Scholar]
- Antonaccio, S. Cosmetic complex compound comprising phosphatidylcholine, carnitine and oils. EP1938792A1, 2 July 2008. [Google Scholar]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Yip, J.; Luk, M.Y.A. Microencapsulation technologies for antimicrobial textiles. In Antimicrobial Textiles; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 19–46. [Google Scholar]
- Özyildiz, F.; Karagönlü, S.; Basal, G.; Uzel, A.; Bayraktar, O. Micro-encapsulation of ozonated red pepper seed oil with antimicrobial activity and application to nonwoven fabric. Lett. Appl. Microbiol. 2013, 56, 168–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beşen, B.S.; Balcı, O.; Güneşoğlu, C.; Orhan, M.; İnci Somuncuoğlu, E.; İrem Tatlı, I. Obtaining medical textiles including microcapsules of the ozonated vegetable oils. Fibers Polym. 2017, 18, 1079–1090. [Google Scholar] [CrossRef]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic Effect of Newly Introduced Root Canal Medicaments; Ozonated Olive Oil and Chitosan Nanoparticles, Against Persistent Endodontic Pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Cutarelli, A.; Carlini, G.; Sarno, F.; Nebbioso, A.; Garofalo, F.; Altucci, L.; Corrado, F. The Role of Ozone Carried by Liposomes in the Therapy of Infectious and Skin-Regenerating Ocular Surface. J. Biomed. Sci. Eng. 2019, 12, 347. [Google Scholar] [CrossRef] [Green Version]
- Duchêne, D.; Bochot, A.; Yu, S.C.; Pépin, C.; Seiller, M. Cyclodextrins and emulsions. Int. J. Pharm. 2003, 266, 85–90. [Google Scholar] [CrossRef]
- Beşen, B.S.; Guneşoğlu, C.; Balci, O.; Kahramanmaraş, S. Antibacterial finishing of 100% cotton fabric with β-cyclodextrin-ozonated olive oil inclusion complex. AATCC J. Res. 2016, 3, 12–18. [Google Scholar] [CrossRef]
- Jia, B.; Zhang, Z.; Chen, M.H.; Zhang, W.P. Effect of liquid oils on the properties of multiple emulsions containing liquid crystals. J. Dispers. Sci. Technol. 2017, 38, 876–882. [Google Scholar] [CrossRef]
- Silva, S.A.M.; Lacerda, R.; Bernegossi, J.; Chorilli, M.; Leonardi, G.R. Development of nanotechnology-based drug delivery systems with olive vegetable oil for cutaneous application. Braz. J. Pharm. Sci. 2016, 52, 211–219. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, P.R.; Calixto, G.M.F.; da Silva, I.C.; de Paula Zago, L.H.; Oshiro Junior, J.A.; Pavan, F.R.; Ribeiro, A.O.; Fontana, C.R.; Chorilli, M. Mucoadhesive In Situ Gelling Liquid Crystalline Precursor System to Improve the Vaginal Administration of Drugs. AAPS PharSciTech 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Landge, A.; Pawar, A.; Shaikha, K. Investigation of cochleates as carriers for topical drug delivery. Int. J. Pharm. Pharm. Sci. 2013, 5, 314–320. [Google Scholar]
- Shende, P.; Khair, R.; Gaud, R.S. Nanostructured cochleates: A multi-layered platform for cellular transportation of therapeutics. Drug Dev. Ind. Pharm. 2019, 45, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Vairo, C.; Collantes, M.; Quincoces, G.; Villullas, S.; Peñuelas, I.; Pastor, M.; Gil, A.G.; Gainza, E.; Hernandez, R.M.; Igartua, M.; et al. Preclinical safety of topically administered nanostructure lipid carriers (NLC) for wound healing application: Biodistribution and toxicity studies. Int. J. Pharm. 2019, 569, 118484. [Google Scholar] [CrossRef] [PubMed]
- Mahant, S.; Kumar, S.; Nanda, S.; Rao, R. Microsponge for dermatological applications: Perspectives and Challenges. Asian J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Shah, C.N.; Shah, D.P. Microsponges: A revolutionary path breaking modified drug delivery of topical drugs. Int. J. Pharm. Res. 2014, 6, 1–13. [Google Scholar]
- Rajeshree, M.; Harsha, P.; Vishnu, P. Microsponge for topical drug delivery system. Int. J. Pharm. Technol. 2014, 5, 2839–2851. [Google Scholar]
- Wadhwa, G.; Kumar, S.; Mittal, V.; Rao, R. Encapsulation of babchi essential oil into microsponges: Physicochemical properties, cytotoxic evaluation and anti-microbial activity. J. Food Drug Anal. 2019, 27, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Erosh, Y.; Rekha, R.; Sunil, K.; Sheefali, M.; Prakriti, V. Microsponge Based Gel of Tea Tree Oil for Dermatological Microbial Infections. Nat. Prod. J. 2018, 8, 1–12. [Google Scholar]
- Patel, N.; Padia, N.; Vadgama, N.; Raval, M.; Sheth, N. Formulation and evaluation of microsponge gel for topical delivery of fluconazole for fungal therapy. J. Pharm. Investig. 2016, 46, 221–238. [Google Scholar] [CrossRef]
- Pandit, A.P.; Patel, S.A.; Bhanushali, V.P.; Kulkarni, V.S.; Kakad, V.D. Nebivolol-Loaded Microsponge Gel for Healing of Diabetic Wound. AAPS PharSciTech 2017, 18, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Amrutiya, N.; Bajaj, A.; Madan, M. Development of microsponges for topical delivery of mupirocin. Aaps Pharmscitech 2009, 10, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obiedallah, M.M.; Abdel-Mageed, A.M.; Elfaham, T.H. Ocular administration of acetazolamide microsponges in situ gel formulations. Saudi Pharm. J. 2018, 26, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Awad, G.E.A.; Makhlouf, A.I.A. Improved vaginal retention and enhanced antifungal activity of miconazole microsponges gel: Formulation development and in vivo therapeutic efficacy in rats. Eur. J. Pharm. Sci. 2018, 114, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Reisch, M.S. Ushering cosmetics to the right spots. Chem. Eng. News 2007, 85, 15–21. [Google Scholar] [CrossRef]
- Ciriminna, R.; Sciortino, M.; Alonzo, G.; De Schrijver, A.; Pagliaro, M. From molecules to systems: Sol-gel microencapsulation in silica-based materials. Chem. Rev. 2011, 111, 765–789. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, P.; Tian, J.; Li, L.; Li, J.; Tian, J.H.; Yang, K. Ozone therapy for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]




| Vegetal Oil | Analyzed Physico-Chemical Parameters | Reference |
|---|---|---|
| Olive oil | Peroxide value Acidity value Iodine value p-anisidine value Viscosity pH | [42] |
| Olive oil Sunflower oil | Peroxide value Acidity value Iodine value | [34] |
| Flaxseed oil Sunflower oil Baru oil Oleogel (from 24 h of ozonolysis in the presence of water) | Peroxide value Acidity value Iodine value | [41] |
| Sunflower oil ozonated using medical oxygen Sunflower oil ozonated using air | Peroxide value Acidity value Iodine value Moles of double bonds Viscosity Density | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. https://doi.org/10.3390/molecules25020334
Ugazio E, Tullio V, Binello A, Tagliapietra S, Dosio F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules. 2020; 25(2):334. https://doi.org/10.3390/molecules25020334
Chicago/Turabian StyleUgazio, Elena, Vivian Tullio, Arianna Binello, Silvia Tagliapietra, and Franco Dosio. 2020. "Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques" Molecules 25, no. 2: 334. https://doi.org/10.3390/molecules25020334