Trypanocidal Activity of Flavanone Derivatives
Abstract
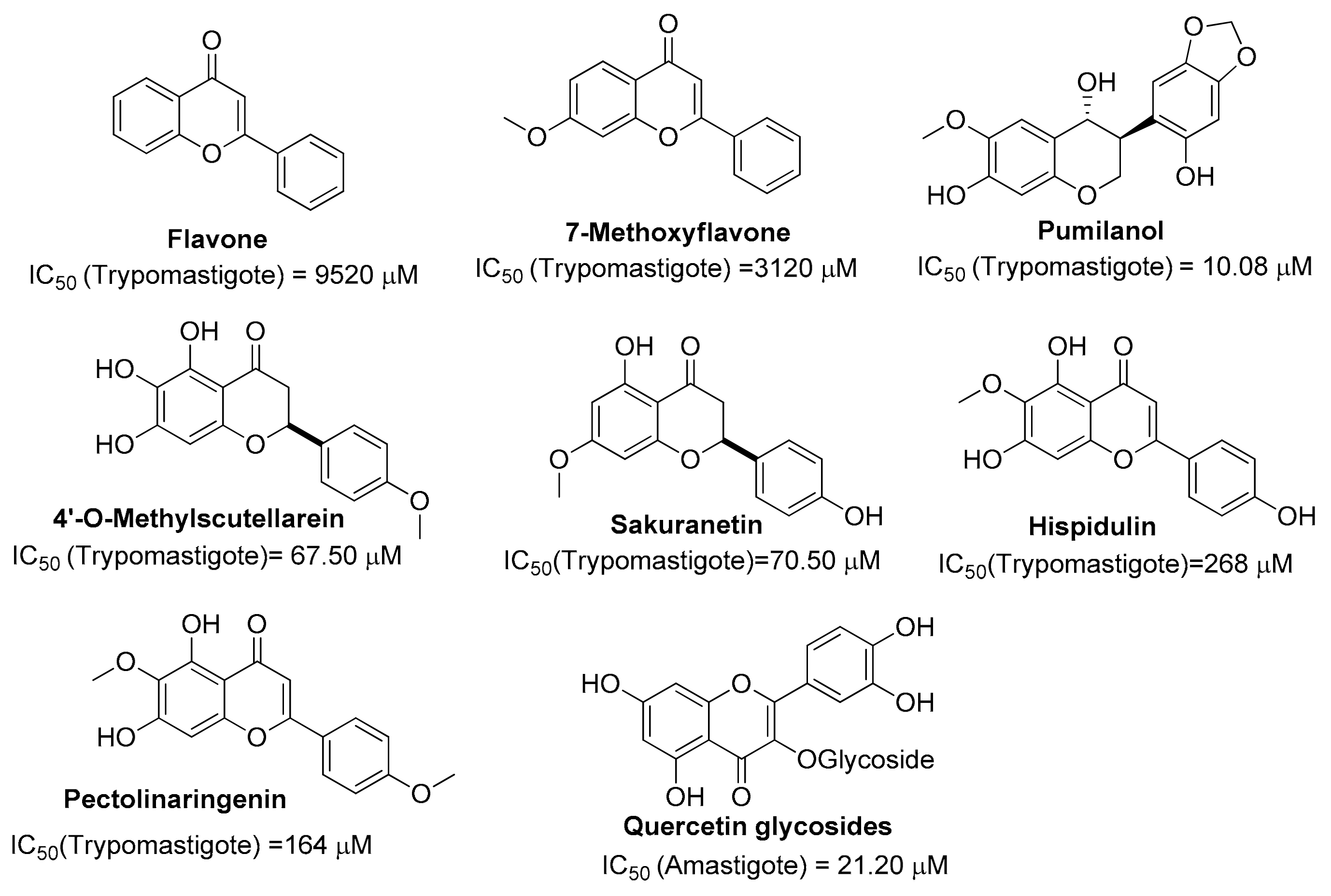
:1. Introduction
2. Results
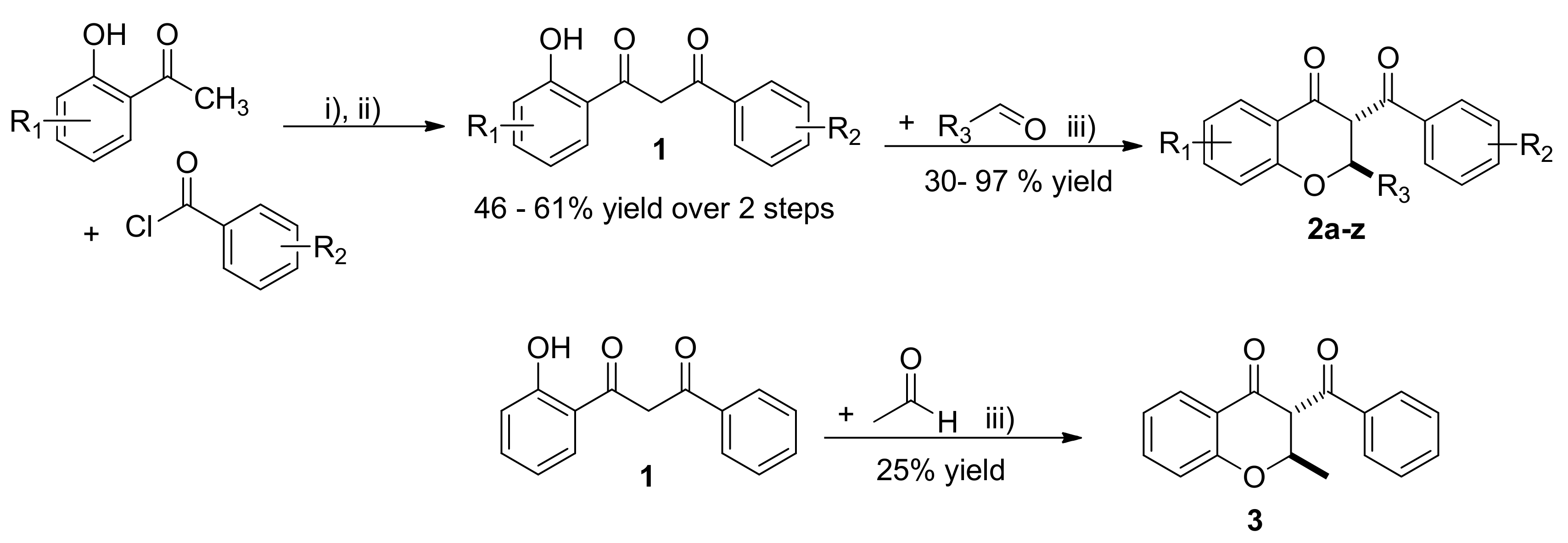
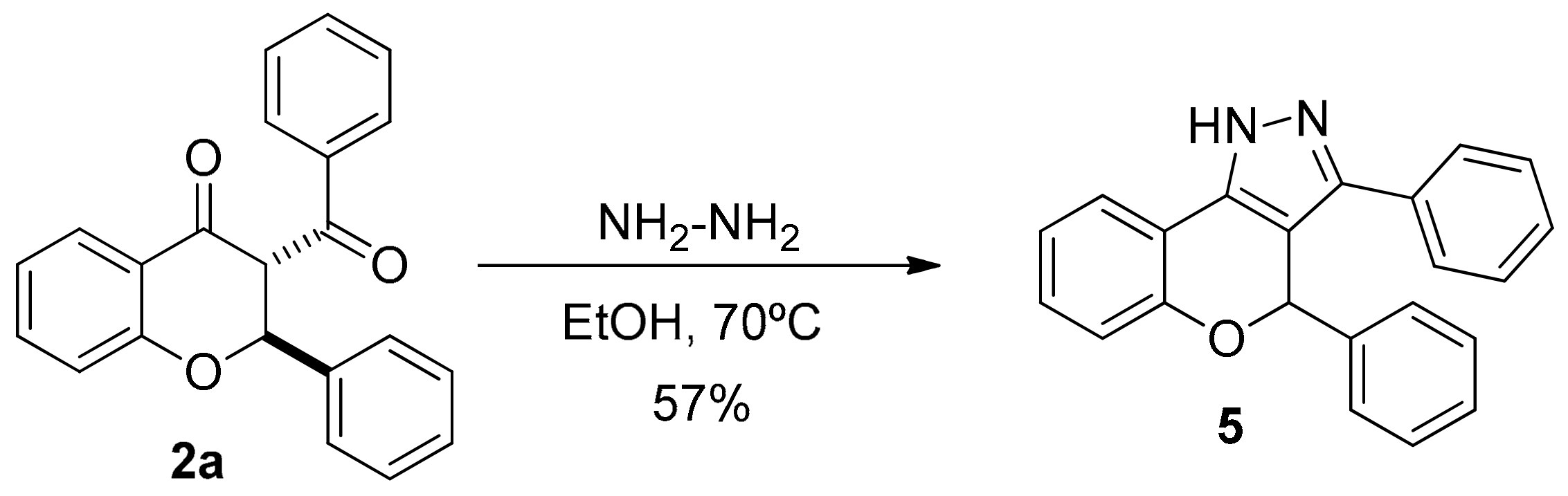
2.1. Synthesis of 3-Benzoyl Flavanones
2.2. Evaluation of In Vitro Anti-T. cruzi Activity
3. Materials and Methods
3.1. Typical Procedure for the Synthesis of Diketones 1
3.2. Typical Procedure for the Synthesis of Flavanones 2a–z and 3
3.3. Synthesis of 3-Benzoyl-3-ethyl-2-phenylchroman-4-one (4)
3.4. Synthesis of 3,4-diphenyl-1,4-dihydrochromeno[4,3-c]pyrazole (5)
3.5. Anti-Trypanosoma cruzi Activity Assay (Amastigotes and Trypomastigotes)
3.6. In Vitro Cytotoxic Test of Trypanocidal Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kropf, S.P.; Massarani, L. Carlos Chagas: A Ciência Para Combater Doenças Tropicais; Casa de Oswaldo Cruz/Fiocruz: Rio de Janeiro, Brasil, 2009; pp. 1–20. [Google Scholar]
- Nabavi, F.S.; Sureda, A.; Daglia, M.; Izadi, M.; Rastrelli, L.; Nabavi, S.M. Flavonoids and Chagas’ Disease: The Story So Far! Bentham Sci. 2017, 17, 460–466. [Google Scholar]
- Coura, J.R. Tripanosomose, Doença de Chagas. Ciênc. Cult. 2003, 55, 30–33. [Google Scholar]
- Organization World Health. Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis: Technical Report of the TDR Disease Reference Group on Chagas Disease, Human African Trypanosomiasis Leishmaniasis; Organization World Health: Geneva, Switzerland, 2012. [Google Scholar]
- Doença de Chagas: O Que é, Causas, Sintomas, Tratamento e Prevenção. Portal Ministério da Saúde. Available online: portalarquivos2.saude.gov.br/images/pdf/2018/dezembro/10/Distribui-o-dos-Casos-de-Doen--a-de-Chagas-Aguda--segundo-UF-de-resid--ncia--2008-a-2017.pdf (accessed on 21 December 2018).
- Ribeiro, A.L.; Nunes, M.P.; Teixeiras, M.M.; Rocha, M.O. Diagnosis and management of chagas disease cardiomyopathy. Nat. Rev. 2012, 10, 576–589. [Google Scholar] [CrossRef]
- Nifurtmox Drug Information Professional. Available online: www.drugs.com/mmx/nifurtimox.html (accessed on 9 March 2018).
- Ambrozim, A.R.P.; Vieira, P.C. Trypanocidal Activity of Meliacea and Rutacea Plant Extracts. Mem. Inst. Oswaldo Cruz 2004, 99, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Ganapaty, S.; Pannakal, S.T.; Srilakshmi, G.V.; Lakshmmi, P.; Waterman, P.G.; Brun, R. Pumilanol, an antiprotozoal isoflavanol from Tephrosia pumila. Phytochem. Lett. 2008, 1, 175–178. [Google Scholar] [CrossRef]
- Grecco, S.S.; Reimão, J.Q.; Tempone, A.G.; Sartorelli, P.; Romoff, P.; Fereira, M.J.; Fávero, O.A.; Lago, J.H. Isolation of an antileishemanial and antitrypanosomal flavanone from the leaves of Baccharis retusa DC. (Asteraceae). Parasitol. Res. 2010, 106, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Grecco, S.S.; Reimão, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.; Romoff, P.; Fereira, M.J.; Fávero, O.A.; Lago, J.H. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae). Exp. Parasitol. 2012, 130, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grecco, S.S.; Felix, M.J.; Pinto, E.G.; Tempone, A.G.; Romoff, P.; Ferreira, M.J.; Sartorelli, P. Anti-trypanosomal phenolic derivates from Bacchariss uncinella. Nat. Prod. Commun. 2014, 9, 171–173. [Google Scholar]
- Marin, C.; Dias, J.G.; Maiques, D.I.; Ramirez-Macias, I.; Rosales, M.J.; Guitierrez-Sanchez, R.; Cañas, R.; Sacchez-Moreno, M. Antitrypanosomatid activity of flavonoid glycosides isolated from Delphinium gracile, D. staphisagria, Consolida oliveriana and from Aconitum napellus subsp. Lusitanicum. Phytochem. Lett. 2017, 19, 196–209. [Google Scholar] [CrossRef]
- Rao, A.R.; Gaitonde, A.S.; Prakash, K.R.C.; Rao, S.P. A concise synthesis of chiral 2-methyl chroman-4-ones: Stereo selective build-up of the chromanol moiety of anti-HIV agent calanolide A. Tetrahedron Lett. 1994, 34, 6347–6350. [Google Scholar] [CrossRef]
- Golveia, A.P.; Taylor, J.G. Access to 3-Benzoylchromanones from Dibenzoylmethanes via an Iron-Catalyzed a-Methylenation Reaction. ChemistrySelect. 2018, 3, 3965–3969. [Google Scholar]
- Clarke, D.S.; Gabbutt, C.D.; Hepworth, J.D.; Heron, B.M. Synthesis of 3-alkenyl-2-arylchromones and 2,3-dialkenylchromones via acid-catalysed retro-Michael ring opening of 3-acylchroman-4-ones. Tetrahedron Lett. 2005, 46, 5515–5519. [Google Scholar] [CrossRef]
- Coelho, G.S.; Andrade, J.S.; Xavier, V.F.; Sales Júnior, P.A.; Rodrigues de Araujo, B.C.; Fonseca, K.D.S.; Caetano, M.S.; Murta, S.M.F.; Vieira, P.M.; Carneiro, C.M.; et al. Design, Synthesis, Molecular Modelling and In Vitro Evaluation of Tricyclic Coumarins Against Trypanosoma Cruzi. Chem. Biol. Drug Des. 2019, 93, 337–350. [Google Scholar] [CrossRef]
- Gayosso, L.J.; Torres-Valencia, A.M.; Rojo-Domínguez, H.; Nájera-Peña, B.; Aguirre-López, J.; Salas-Pacheco, A.; Téllez-Valencia, A. Selective inactivation of triosephosphate isomerase from Trypanosoma cruzi by brevifolin carboxylate derivatives isolated from Geranium bellum Rose. Bioorg. Med. Chem. Lett. 2009, 19, 5936–5939. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.L.; Vaz, L.B.A.; de Abreu, V.P.M.; da Silva, F.K.; Carneiro, C.M.; Taylor, J.G. Synthesis and anti-trypanosoma cruzi activity of diasyldiazepines. Molecules 2014, 20, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Moreira, D.R.; Leite, A.C.; Cardoso, M.V.; Srivastava, R.M.; Hernandes, M.Z.; Rabello, M.M.; da Cruz, L.F.; Ferreira, R.S.; de Simone, C.A.; Meira, C.S.; et al. Structural Design, Synthesis and Structure–Activity Relationships of Thiazolidinones with Enhanced Anti-Trypanosoma cruzi Activity. Chem. Med. Chem. 2014, 9, 177–188. [Google Scholar] [CrossRef]
- Elias, P.R.; Coelho, G.S.; Xavier, V.F.; Sales Junior, P.A.; Romanha, A.J.; Murta, S.M.F.; Carneiro, C.M.; Taylor, J.G. Synthesis of Xylitan Derivatives and Preliminary Evaluation of in Vitro Trypanocidal Activity. Molecules 2016, 21, 1342. [Google Scholar] [CrossRef] [Green Version]
- de Souza, A.A.; Xavier, V.F.; Coelho, G.S.; Sales Junior, P.A.; Romanha, A.J.; Murta, S.M.F.; Carneiro, C.M.; Taylor, J.G. Design, Synthesis, Synthesis of 3,5-Diarylisoxazole Derivatives and Evaluation of in vitro Trypanocidal Activity. J. Braz. Chem. Soc. 2018, 29, 269–277. [Google Scholar]
- Romanha, A.J.; Castro, S.L.; Soeiro, M.N.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Rodriguez, S.V.; Guíñez, R.F.; Matos, M.J.; Azar, C.O.; Maya, J.D. Synthesis and Trypanocidal Properties of New Coumarin-Chalcone Derivatives. Med. Chem. 2015, 5, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Sambaiah, M.; Raghavulu, K.; Shiva, K.; Yennam, S.; Behera, M. Synthesis of novel fused chromone–pyrimidine hybrids and 2,4,5-trisubstituted pyrimidine derivatives via ANRORC rearrangement. J. Chem. 2017, 41, 10020–10026. [Google Scholar] [CrossRef]
- Rout, S.K.; Guin, S.; Banerjee, A.; Khatun, N.; Gogoi, A.; Patel, B. Pd-Catalyzed Aldehyde to Ester Conversion: A Hydrogen Transfer Approach. Org. Lett. 2013, 15, 4106–4109. [Google Scholar] [CrossRef] [PubMed]
- Saberi, D.; Heydari, A. A Click Strategy for the Immobilization of MacMillan Organocatalysts onto Polymers and Magnetic Nanoparticles. Tetrahedron 2013, 54, 4178–4418. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, S. A new synthesis of flavones and pyranoflavone by intramolecular photochemical Wittig reaction in water. Tetrahedron Lett. 2011, 52, 7189–7194. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, M.V.; de Siqueira, L.R.P.; da Silva, E.B.; Costa, L.B.; Hernandes, M.Z.; Rabello, M.M.; Ferreira, R.S.; da Cruz, L.F.; Moreira, D.R.M.; Pereira, V.R.A.; et al. 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: Structural design, synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2014, 86, 48–59. [Google Scholar] [CrossRef]
- Palace-Berla, F.; Pasqualoto, K.F.M.; Jorge, S.D.; Zingales, B.; Zorzi, R.R.; Silva, M.N.; Ferreira, A.K.; de Azevedo, R.A.; Teixeira, S.F.; Tavares, L.C. Designing and exploring active N′-[(5-nitrofuran-2-yl) methylene] substituted hydrazides against three Trypanosoma cruzi strains more prevalent in Chagas disease patients. Eur. J. Med. Chem. 2015, 96, 330–339. [Google Scholar] [CrossRef]
- Farias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bugri, M.D.L.M.; Pietro, C.; Iglessias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar]
- Yu, Y.; Hu, Y.; Weiyan, S.; Huang, J.; Zuo, Y.; Huo, Y.; An, L.; Jun, D.; Xianzhang, B. Synthesis of Multi-Functionalized Chromeno[2,3-c]pyrrol-9(2H)-ones: Investigation and Application of Baker-Venkataraman Rearrangement Involved Reactions Catalyzed by 4-(Dimethylamino)pyridine. J. Org. Chem. 2011, 24, 4551–4563. [Google Scholar] [CrossRef]
- Wang, R.; Han, J.; Li, C.; Zhang, J.; Liang, Y.; Wang, T.; Zhang, Z. One-pot synthesis of 3-fluoroflavones via 1-(2-hydroxyphenyl)-3-phenylpropane-1,3-diones and selectfluor at room temperature. Org. Biomol. Chem. 2018, 16, 2479–2488. [Google Scholar] [CrossRef]
- Xu, G.D.; Huang, K.L.; Huang, Z.Z. Rh(III)-Catalyzed Aldehydic C−H Functionalization Reaction between Salicylaldehydes and Sulfoxonium Ylide. Adv. Synth. Catal. 2019, 361, 3318–3323. [Google Scholar] [CrossRef]
- Han, J.; Wang, T.; Liang, Y.; Li, Y.; Li, C.; Wang, R.; Feng, S.; Zhang, Z. Transition-Metal-Free Photoinduced Intramolecular Annulation of 2,3-Di(hetero)arylchromen-4-one. Org. Lett. 2017, 19, 3552–3555. [Google Scholar] [CrossRef] [PubMed]
- Fulla, E.; Talbot, J.; Abuhammed, A.; Westwood, I.; Davies, S.; Russell, A. Design, synthesis and structure–activity relationships of 3,5-diaryl-1H-pyrazoles as inhibitors of arylamine N-acetyltransferase. Bioorg. Med. Chem. Lett. 2013, 23, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Buceta, N.N.; Védova, C.O.D.; Romanelli, G.P.; Jios, J.C. Deuterium isotopic effect on 13C NMR chemical shifts of 1-(2-hydroxyphenyl)-3-aryl-1,3-propanediones: Hydrogen bond and substituenteffects. J. Mol. Struct. 2008, 878, 50–59. [Google Scholar] [CrossRef]
- Loewe, W.; Matzanke, N. Synthesis of heterocyclic sulfonylureas. J. Heterocycl. Chem. 1996, 33, 943–948. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Z.; Hu, S.; Wen, P.; Tao, B.; Deng, Q. Piperidinium Acetate-Catalyzed the Synthisis of 2,3-Disubstituted Chroman-4-one and 2,2-Disubstituted Benzofuran-3-one Derivatives. J. Org. Chem. 2016, 36, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Kedar, R.M.; Vidhale, N.N.; Chincholkar, M.M. Synthesis of new heterocycles and their antimicrobial study. Orient. J. Chem. 1997, 13, 143–148. [Google Scholar]
- Wu, L.L.; Tang, L.; Zhou, S.G.; Peng, Y.J.; He, X.D.; Guan, Z.; He, Y.H. Rose Bengal-photosensitized oxidation of tertiary amines for the synthesis of bis-1,3-dicarbonyl compounds. Tetrahedron 2017, 73, 6471–6478. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| In Vitro Activity | Lipinski’s Rule of Five | Risk Toxicity | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Trypanocide IC50(μM) ± 0.2 | Cytotoxicity CC50(μM) ± 2.0 | SI | HBA | HBD | MW (g.mol−1) | log P | Violations | TPSA (A2) | Volume A3 | NRB | M | T | I | RE |
| 2a | 8.8 | 80 | 9.1 | 3 | 0 | 328.37 | 4.43 | 0 | 43.38 | 296.63 | 3 | NR | NR | NR | NR |
| 2b | 10.74 | 441.90 | 40.3 | 3 | 0 | 362.81 | 5.06 | 1 | 43.38 | 310.16 | 3 | NR | NR | NR | NR |
| 2c | 21.95 | 231.14 | 10.5 | 3 | 0 | 346.36 | 4.59 | 0 | 43.38 | 301.56 | 3 | NR | NR | NR | NR |
| 2d | 5.8 | 223.40 | 38.5 | 4 | 0 | 358.39 | 4.49 | 0 | 52.61 | 322.17 | 4 | NR | NR | NR | NR |
| 2e | 183.90 | 243.10 | 1.3 | 4 | 0 | 329.36 | 3.26 | 0 | 56.27 | 292.47 | 3 | NR | NR | NR | NR |
| 2f | 7.60 | 92.13 | 12.1 | 4 | 0 | 434.39 | 6.08 | 1 | 52.61 | 393.82 | 6 | NR | NR | NR | NR |
| 2g | Inactive | - | - | 3 | 0 | 342.39 | 4.88 | 0 | 43.38 | 313.19 | 3 | NR | NR | NR | NR |
| 2h | 22.49 | 111.10 | 4.9 | 3 | 0 | 360.38 | 5.04 | 1 | 43.38 | 318.12 | 3 | NR | NR | NR | NR |
| 2i | 24.99 | 107.48 | 4.3 | 4 | 0 | 372.42 | 4.93 | 0 | 52.61 | 338.73 | 4 | NR | NR | NR | NR |
| 2j | 63.02 | 62.48 | 1 | 3 | 0 | 376.84 | 5.51 | 1 | 43.38 | 326.72 | 3 | NR | NR | NR | NR |
| 2k | 29.30 | 110.50 | 3.8 | 3 | 0 | 362.81 | 5.11 | 1 | 43.38 | 310.16 | 3 | NR | NR | NR | NR |
| 2l | 223.40 | 223.40 | 1 | 4 | 0 | 358.39 | 4.49 | 0 | 52.61 | 322.17 | 4 | NR | NR | NR | NR |
| 2m | 37.35 | 103.05 | 2.8 | 5 | 0 | 388.42 | 4.54 | 0 | 61.84 | 347.72 | 5 | NR | NR | NR | NR |
| 2n | 212.70 | - | - | 4 | 0 | 376.38 | 4.65 | 0 | 52.61 | 327.10 | 4 | NR | NR | NR | NR |
| 2o | 26.52 | 408.10 | 15.4 | 4 | 0 | 392.84 | 5.12 | 1 | 52.61 | 335.71 | 4 | NR | NR | NR | NR |
| 2p | Inactive | - | - | 4 | 0 | 358.39 | 4.46 | 0 | 52.61 | 322.17 | 4 | NR | NR | NR | NR |
| 2q | 141.18 | 206.12 | 1.4 | 5 | 0 | 388.42 | 4.52 | 0 | 61.84 | 347.72 | 5 | NR | NR | NR | NR |
| 2r | 17.55 | 106.35 | 6.1 | 4 | 0 | 376.38 | 4.63 | 0 | 52.61 | 327.10 | 4 | NR | NR | NR | NR |
| 2s | 18.36 | 102.02 | 5.5 | 4 | 0 | 392.84 | 5.09 | 1 | 52.61 | 335.71 | 4 | NR | NR | NR | NR |
| 2t | 23.20 | 220.95 | 9.5 | 3 | 0 | 362.81 | 5.08 | 1 | 43.38 | 310.16 | 3 | NR | NR | NR | NR |
| 2u | 59.17 | 204.95 | 3.5 | 4 | 0 | 392.84 | 5.14 | 1 | 52.61 | 335.71 | 4 | NR | NR | NR | NR |
| 2v | Inactive | - | - | 4 | 0 | 318.33 | 3.69 | 0 | 56.52 | 278.19 | 3 | NR | NR | NR | NR |
| 2w | Inactive | - | - | 5 | 0 | 348.35 | 3.74 | 0 | 65.75 | 303.74 | 4 | NR | NR | NR | NR |
| 2x | Inactive | - | - | 4 | 0 | 336.32 | 3.85 | 0 | 56.52 | 283.12 | 3 | NR | NR | NR | NR |
| 2y | 113.80 | 503.0 | 4.4 | 4 | 1 | 318.33 | 4.72 | 0 | 59.67 | 277.82 | 3 | NR | NR | NR | NR |
| 2z | 2.6 | 27.54 | 10.6 | 7 | 1 | 363.32 | 4.80 | 0 | 105.50 | 301.15 | 6 | LR | NR | NR | NR |
| 3 | Inactive | - | - | 3 | 0 | 266.30 | 3.21 | 0 | 43.38 | 241.78 | 2 | NR | NR | NR | NR |
| 4 | 13.2 | 449.26 | 34.03 | 3 | 0 | 256.43 | 5.01 | 1 | 43.38 | 329.67 | 4 | NR | NR | NR | NR |
| 5 | 8.3 | 38.56 | 4.6 | 3 | 1 | 324.38 | 5.01 | 1 | 37.92 | 294.07 | 2 | NR | NR | NR | NR |
| Bnz | 3.8 | 2381 | 625 | - | - | 260.25 | 0.78 | 0 | 92.75 | 224.99 | 5 | NR | NR | NR | HR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciel Diogo, G.; Andrade, J.S.; Sales Junior, P.A.; Maria Fonseca Murta, S.; Dos Santos, V.M.R.; Taylor, J.G. Trypanocidal Activity of Flavanone Derivatives. Molecules 2020, 25, 397. https://doi.org/10.3390/molecules25020397
Maciel Diogo G, Andrade JS, Sales Junior PA, Maria Fonseca Murta S, Dos Santos VMR, Taylor JG. Trypanocidal Activity of Flavanone Derivatives. Molecules. 2020; 25(2):397. https://doi.org/10.3390/molecules25020397
Chicago/Turabian StyleMaciel Diogo, Gabriela, Josimara Souza Andrade, Policarpo Ademar Sales Junior, Silvane Maria Fonseca Murta, Viviane Martins Rebello Dos Santos, and Jason Guy Taylor. 2020. "Trypanocidal Activity of Flavanone Derivatives" Molecules 25, no. 2: 397. https://doi.org/10.3390/molecules25020397





