A FRET-ICT Dual-Modulated Ratiometric Fluorescence Sensor for Monitoring and Bio-Imaging of Cellular Selenocysteine
Abstract
:1. Introduction
2. Results and Discussion
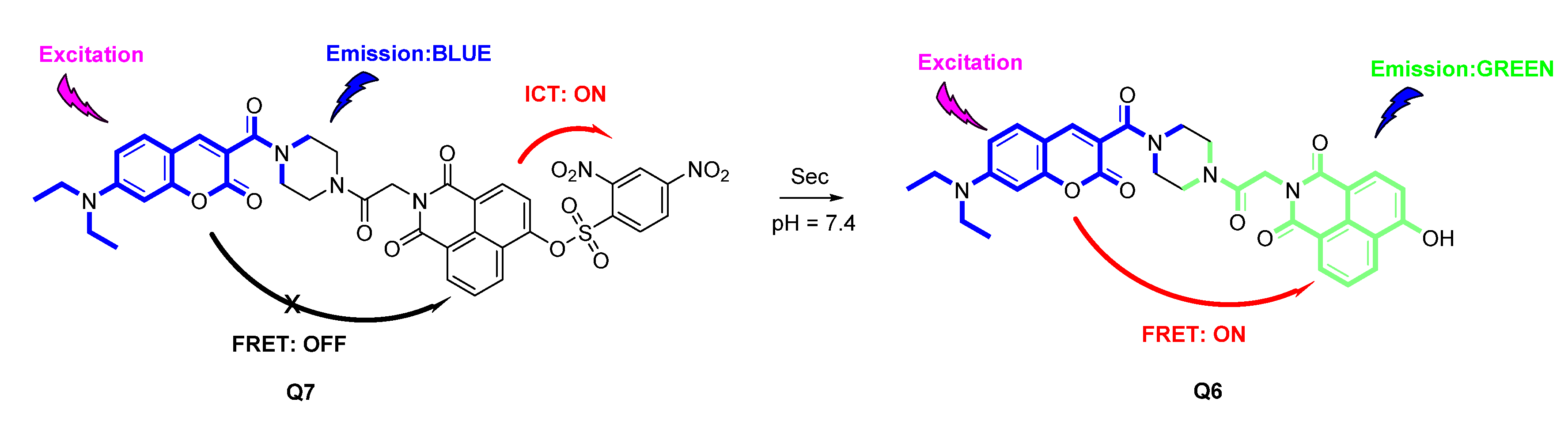
2.1. Design of Ratiometric Sensor
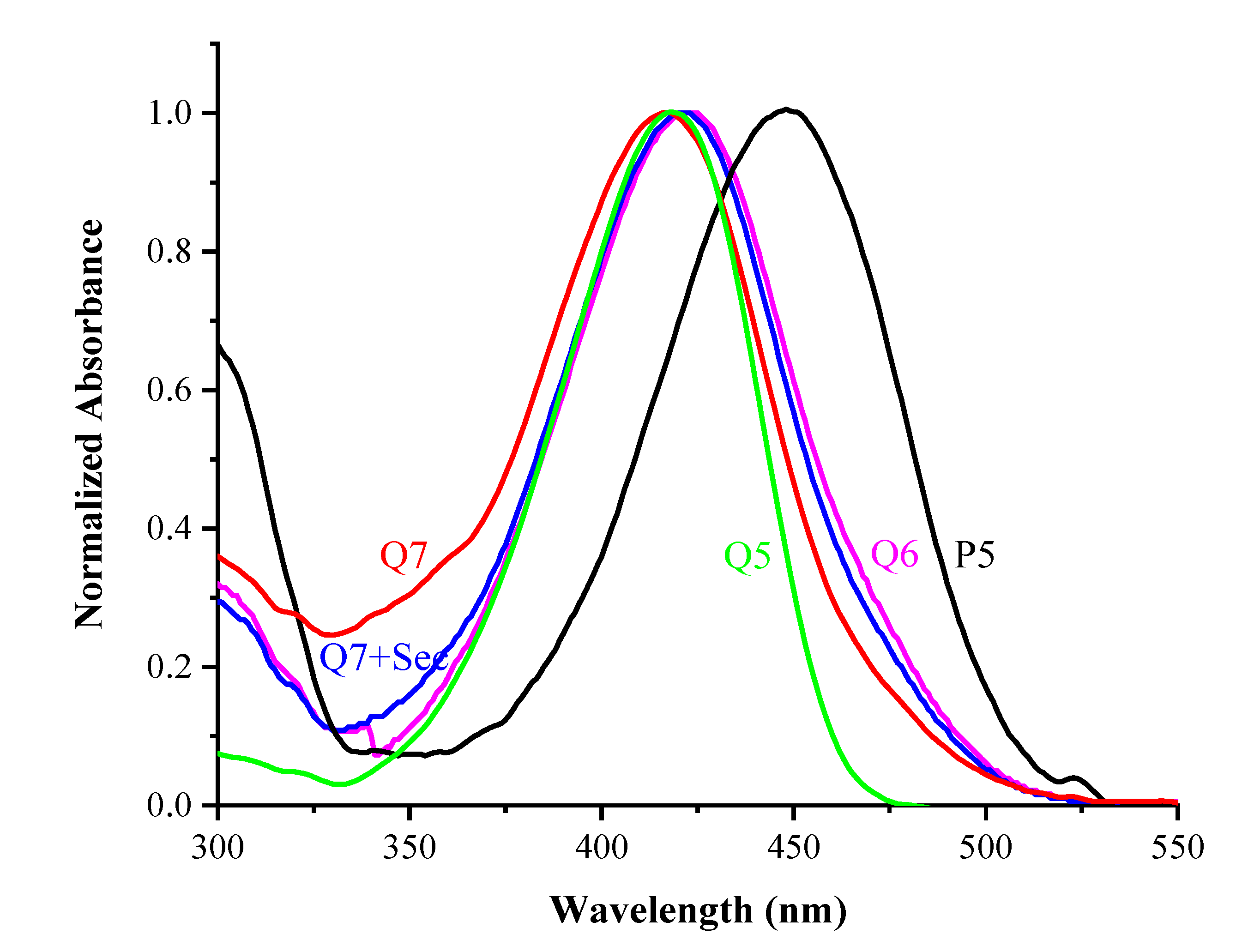
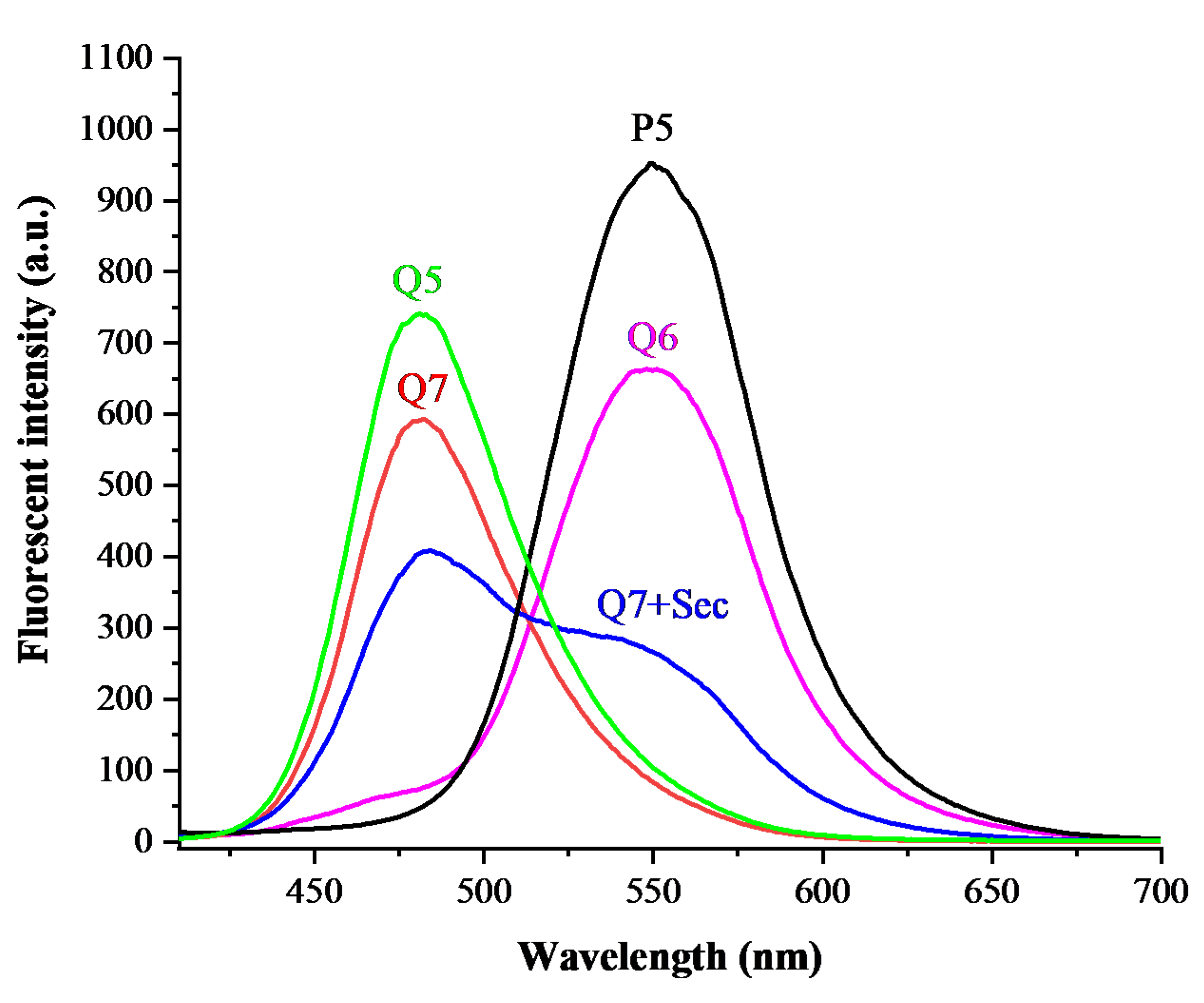
2.2. Spectral Properties of Q7
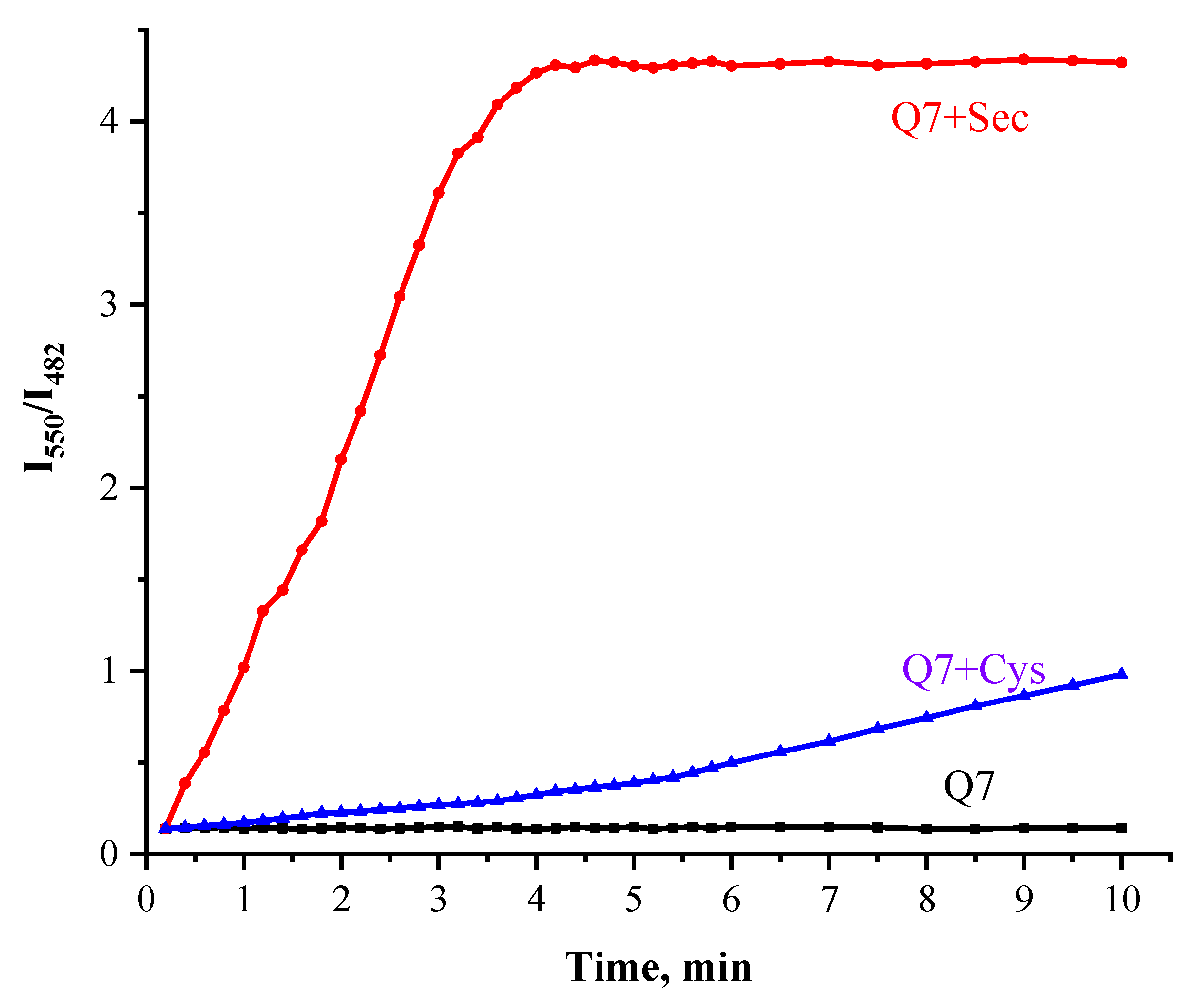
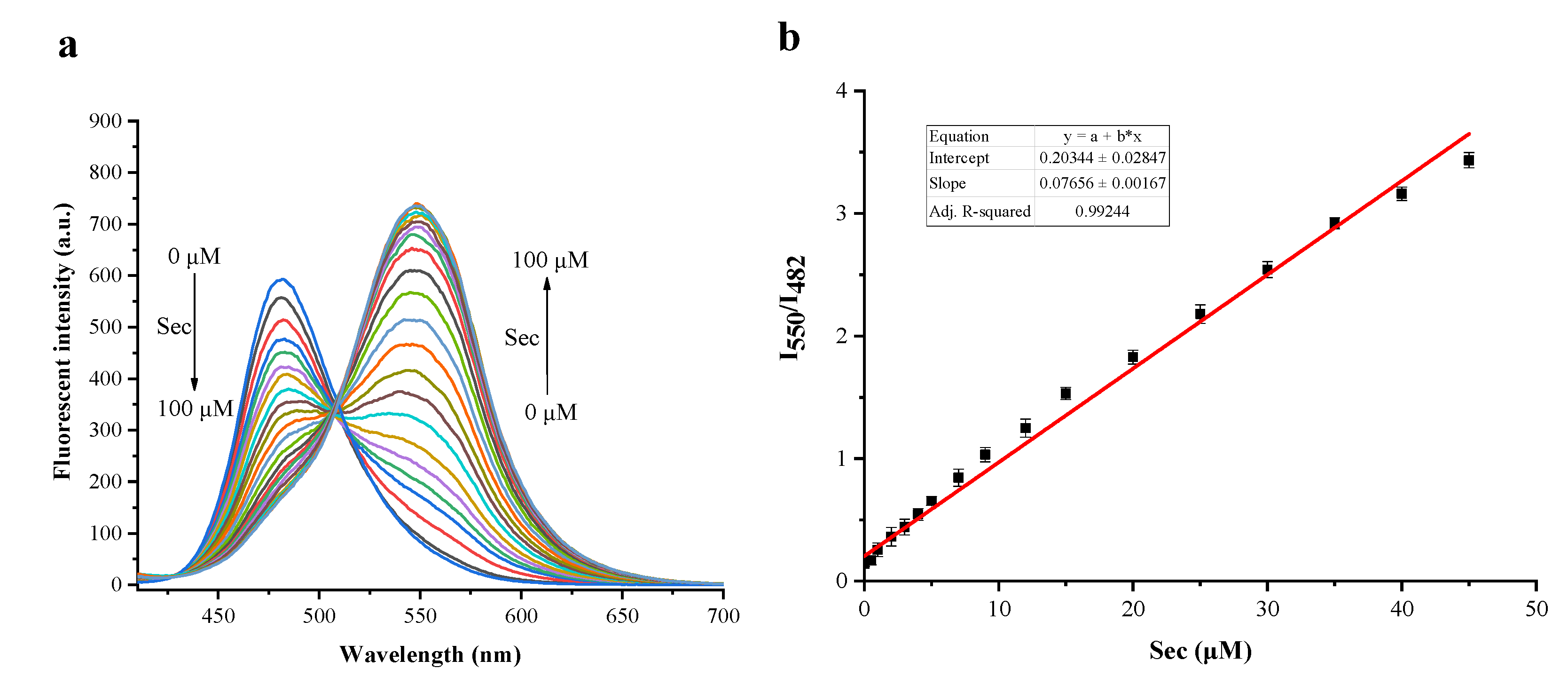
2.3. The Sensitivity Studies
2.4. The Selectivity and Anti-Interference Properties
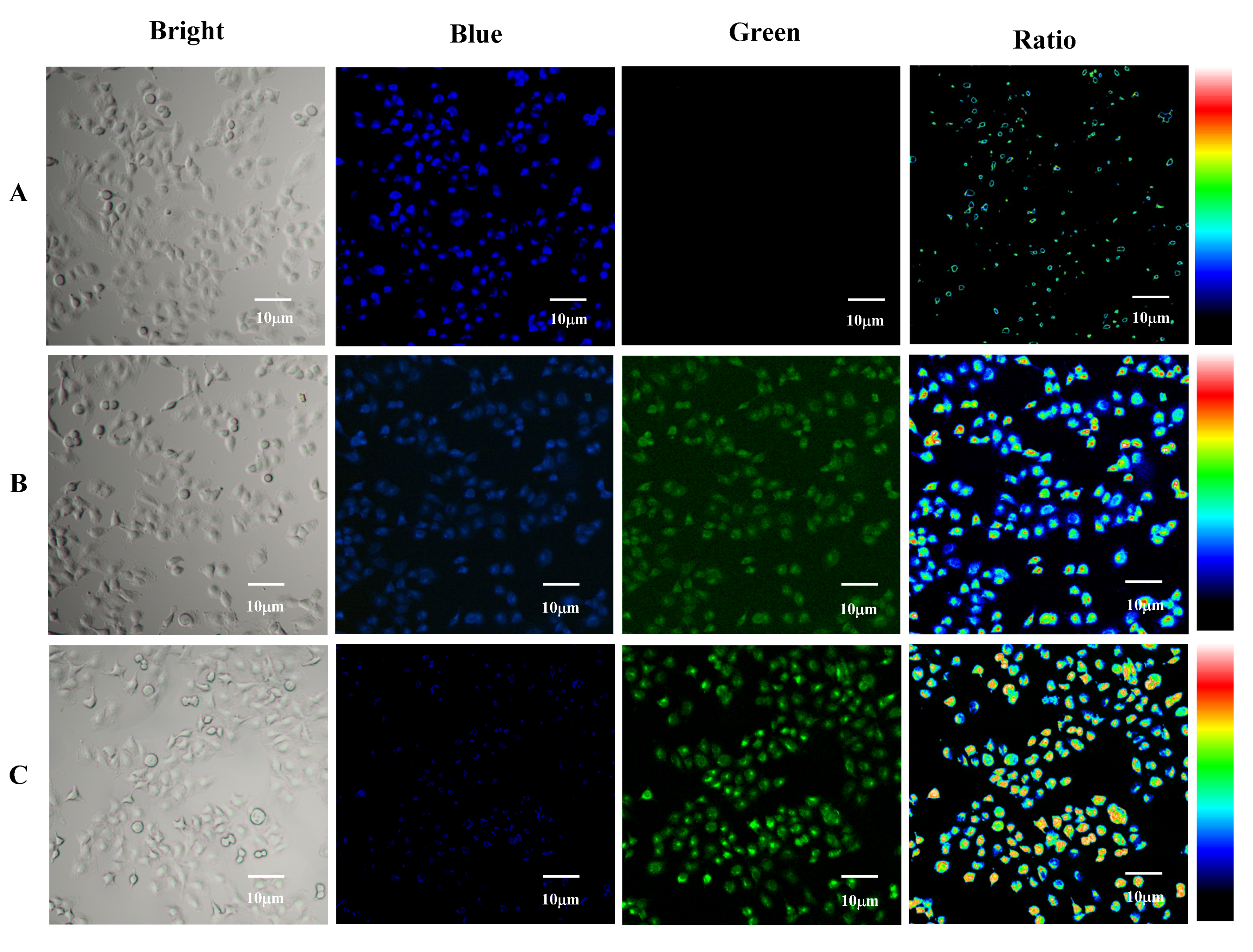
2.5. Cell Experiments
3. Materials and Methods
3.1. Materials and Equipment
3.2. Synthesis of the Fluorescent Sensor
3.3. Spectrum Analysis
3.4. Anti-Interference Assays
3.5. Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zadeh, M.H.; Farsani, G.M.; Zamaninour, N. Selenium status after Roux-en-Y gastric bypass: Interventions and recommendations. Obes. Surg. 2019, 29, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Frączek, A.; Pasternak, K. Selenium in medicine and treatment. J. Elementol. 2013, 18, 145–163. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.Y.; Kwon, N.; Yoon, J. Fluorescent probes containing selenium as a guest or host. Chem 2016, 1, 674–698. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, M.; Buntinx, F.; Muls, E.; Zeegers, M.P. Use of selenium in chemoprevention of bladder cancer. Lancet Oncol. 2006, 7, 766–774. [Google Scholar] [CrossRef]
- Kuganesan, M.; Samra, K.; Evans, E.; Singer, M.; Dyson, A. Selenium and hydrogen selenide: Essential micronutrient and the fourth gasotransmitter? Intens. Care Med. Exp. 2019, 7, 71. [Google Scholar] [CrossRef]
- Shang, N.N.; Wang, X.F.; Shu, Q.M.; Wang, H.; Zhao, L.N. The functions of selenium and selenoproteins relating to the liver diseases. J. Nanosci. Nanotechno. 2019, 19, 1875–1888. [Google Scholar] [CrossRef]
- Reddy, P.S.; Metanis, N. Small molecule diselenide additives for in vitro oxidative protein folding. Chem. Commun. 2016, 52, 3336–3339. [Google Scholar] [CrossRef] [Green Version]
- Peeler, J.C.; Falco, J.A.; Kelemen, R.E.; Abo, M.; Chartier, B.V.; Edinger, L.C.; Chen, J.J.; Chatterjee, A.; Weerapana, E. Generation of recombinant mammalian selenoproteins through genetic code expansion with photocaged selenocysteine. ACS Chem. Biol. 2020, 15, 1535–1540. [Google Scholar] [CrossRef]
- Pessione, E.; Pessione, A.; Mangiapane, E. Selenium and selenoproteins: An overview on different biological systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar]
- Zhang, X.; Zhang, L.; Zhu, J.H.; Cheng, W.H. Nuclear selenoproteins and genome maintenance. IUBMB Life 2016, 68, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef]
- Loscalzo, J. Keshan disease, selenium deficiency, and the selenoproteome. New Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef]
- Zhang, P.P.; Ding, Y.; Liu, W.M.; Niu, G.L.; Zhang, H.Y.; Ge, J.C.; Wu, J.S.; Li, Y.Q.; Wang, P.F. Red emissive fluorescent probe for the rapid detection of selenocysteine. Sens. Actuators B 2018, 264, 234–239. [Google Scholar] [CrossRef]
- Bednarik, A.; Kuta, J.; Vu, D.L.; Ranglova, K.; Hrouzek, P.; Kanicky, V.; Preisler, J. Thin-layer chromatography combined with diode laser thermal vaporization inductively coupled plasma mass spectrometry for the determination of selenomethionine and selenocysteine in algae and yeast. J. Chromatogr. A 2018, 1533, 199–207. [Google Scholar] [CrossRef]
- Thosaikham, W.; Jitmanee, K.; Sittipout, R.; Maneetong, S.; Chantiratikul, A.; Chantiratikul, P. Evaluation of selenium species in selenium-enriched pakchoi (Brassica chinensis Jusl var parachinensis (Bailey) Tsen & Lee) using mixed ion-pair reversed phase HPLC-ICP-MS. Food Chem. 2014, 145, 736–742. [Google Scholar]
- Zhang, L.; Kai, X.N.; Zhang, Y.R.; Zheng, Y.G.; Xue, Y.S.; Yin, X.X.; Zhao, J. A reaction-based near-infrared fluorescent probe that can visualize endogenous selenocysteine in vivo in tumor-bearing mice. Analyst 2018, 143, 4860–4869. [Google Scholar] [CrossRef]
- Kong, F.P.; Hu, B.; Gao, Y.; Xu, K.H.; Pan, X.H.; Huang, F.; Zheng, Q.L.; Chen, H.; Tang, B. Fluorescence imaging of selenol in HepG2 cell apoptosis induced by Na2SeO3. Chem. Commun. 2015, 51, 3102–3105. [Google Scholar] [CrossRef]
- Guo, Y.D.; Luo, Y.; Wang, N.; Tang, M.G.; Xiao, J.C.; Chen, S.W.; Wang, J.Y. Au nanoparticle-based probe for selenol in living cells and selenium-rich tea and rice. Talanta 2020, 212, 120583. [Google Scholar] [CrossRef]
- Feng, W.Y.; Li, M.X.; Sun, Y.; Feng, G.Q. Near-infrared fluorescent turn-on probe with a remarkable large stokes shift for imaging selenocysteine in living cells and animals. Anal. Chem. 2017, 89, 6107–6113. [Google Scholar] [CrossRef]
- Maeda, H.; Katayama, K.; Matsuno, H.; Uno, T. 3′-(2,4-Dinitirobenzenesulfonyl)-2′,7′-dimethyl-fluorescein as a fluorescent probe for selenols. Angew. Chem. Int. Edit. 2006, 45, 1810–1813. [Google Scholar] [CrossRef]
- Zhang, S.R.; Wang, Q.; Liu, X.W.; Zhang, J.J.; Yang, X.F.; Li, Z.; Li, H. Sensitive and selective fluorescent probe for selenol in living cells designed via a pK(a) shift strategy. Anal. Chem. 2018, 90, 4119–4125. [Google Scholar] [CrossRef]
- Dai, C.G.; Wang, J.L.; Song, Q.H. Red fluorescent probes based on a BODIPY analogue for selective and sensitive detection of selenols in solutions and in living systems. J. Mater. Chem. B 2016, 4, 6726–6733. [Google Scholar] [CrossRef]
- Liu, Y.N.; Feng, X.H.; Yu, Y.N.; Zhao, Q.Y.; Tang, C.H.; Zhang, J.M. A review of bioselenol-specific fluorescent probes: Synthesis, properties, and imaging applications. Anal. Chim. Acta 2020, 1110, 141–150. [Google Scholar] [CrossRef]
- Zhang, B.X.; Ge, C.P.; Yao, J.; Liu, Y.P.; Xie, C.H.; Fang, J.G. Selective selenol fluorescent probes: Design, synthesis, structural determinants, and biological applications. J. Am. Chem. Soc. 2015, 137, 757–769. [Google Scholar] [CrossRef]
- Li, M.X.; Feng, W.Y.; Zhai, Q.S.; Feng, G.Q. Selenocysteine detection and bioimaging in living cells by a colorimetric and near-infrared fluorescent turn-on probe with a large stokes shift. Biosens. Bioelectron. 2017, 87, 894–900. [Google Scholar] [CrossRef]
- Zhao, X.J.; Wang, C.; Yuan, G.Q.; Ding, H.Y.; Zhou, L.Y.; Liu, X.G.; Lin, Q.L. A dual-site modulated FRET-based two-photon ratiometric fluorescent probe for tracking lysosomal pH changes in living cells, tissues and zebrafish. Sens. Actuators B 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, F.Y.; Gao, C.C.; Jing, J.; Zhang, X.L. Ratiometric fluorescent imaging for endogenous selenocysteine in cancer cell matrix. J. Mater. Chem. B 2017, 5, 6890–6896. [Google Scholar] [CrossRef]
- Luo, X.Z.; Wang, R.; Lv, C.Z.; Chen, G.; You, J.M.; Yu, F.B. Detection of selenocysteine with a ratiometric near-infrared fluorescent probe in cells and in mice thyroid diseases model. Anal. Chem. 2020, 92, 1589–1597. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yuan, G.Q.; Ding, H.Y.; Zhou, L.Y.; Lin, Q.L. A TP-FRET-based fluorescent sensor for ratiometric visualization of selenocysteine derivatives in living cells, tissues and zebrafish. J. Hazard. Mater. 2020, 381, 120918. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Y.F.; Sheng, Z.J.; Zhang, Y.R.; Kai, X.N.; Li, M.Y.; Yin, X.X. Bioluminescence imaging of selenocysteine in vivo with a highly sensitive probe. ACS Sens. 2019, 4, 3147–3155. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.Y.; Xie, Y.N.; Chen, B.; Song, J.Z. Fluorescent detection of hypochlorous acid from turn-on to FRET-based ratiometry by a HOCl-mediated cyclization reaction. Chem. Eur. J. 2012, 18, 2700–2706. [Google Scholar] [CrossRef]
- Shu, W.; Yan, L.G.; Liu, J.; Wang, Z.K.; Zhang, S.; Tang, C.C.; Liu, C.Y.; Zhu, B.C.; Du, B. Highly selective fluorescent probe for the sensitive detection of inorganic and organic mercury species assisted by H2O2. Ind. Eng. Chem. Res. 2015, 54, 8056–8062. [Google Scholar] [CrossRef]
- Liang, S.C.; Yu, H.; Xiang, J.; Yang, W.; Chen, X.H.; Liu, Y.B.; Gao, C.; Yan, G.P. New naphthalimide modified polyethylenimine nanoparticles as fluorescent probe for DNA detection. Spectrochim. Acta Part A 2012, 97, 359–365. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zhu, C.C.; Cen, J.J.; Li, J.; He, W.J.; Jiao, Y.; Guo, Z.J. A reversible ratiometric sensor for intracellular Cu2+ imaging: Metal coordination-altered FRET in a dual fluorophore hybrid. Chem. Commun. 2013, 49, 7632–7634. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, M.X.; Feng, W.Y.; Feng, G.Q. Rapid and selective detection of selenocysteine with a known readily available colorimetric and fluorescent turn-on probe. Dyes Pigments 2018, 149, 475–480. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zheng, H.H.; Zhang, C.L.; Tang, D.F.; Wu, Q.Y.; Dessie, W.; Jiang, Y.R. A red emissive fluorescent turn-on sensor for the rapid detection of selenocysteine and its application in living cells imaging. Sensors 2020, 20, 4768. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, S.H.; Wu, L.; Dong, Q.J.; Yang, W.C.; Yang, G.F. Detection of intracellular selenol-containing molecules using a fluorescent probe with near-zero background signal. Anal. Chem. 2016, 88, 6084–6091. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Q5, Q6, Q7 and P5 are available from the authors. |


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hao, C.; Luo, X.; Wu, Q.; Zhang, C.; Dessie, W.; Jiang, Y. A FRET-ICT Dual-Modulated Ratiometric Fluorescence Sensor for Monitoring and Bio-Imaging of Cellular Selenocysteine. Molecules 2020, 25, 4999. https://doi.org/10.3390/molecules25214999
Wang Z, Hao C, Luo X, Wu Q, Zhang C, Dessie W, Jiang Y. A FRET-ICT Dual-Modulated Ratiometric Fluorescence Sensor for Monitoring and Bio-Imaging of Cellular Selenocysteine. Molecules. 2020; 25(21):4999. https://doi.org/10.3390/molecules25214999
Chicago/Turabian StyleWang, Zongcheng, Chenhong Hao, Xiaofang Luo, Qiyao Wu, Chengliang Zhang, Wubliker Dessie, and Yuren Jiang. 2020. "A FRET-ICT Dual-Modulated Ratiometric Fluorescence Sensor for Monitoring and Bio-Imaging of Cellular Selenocysteine" Molecules 25, no. 21: 4999. https://doi.org/10.3390/molecules25214999




