Preparation and Characterisation of Activated Carbon from Palm Mixed Waste Treated with Trona Ore
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermogravimetric Analyses of OPW
2.2. Elemental Analysis of the Combine Palm Waste Activated Carbon
2.3. AC morphology and Surface Chemistry Mechanism
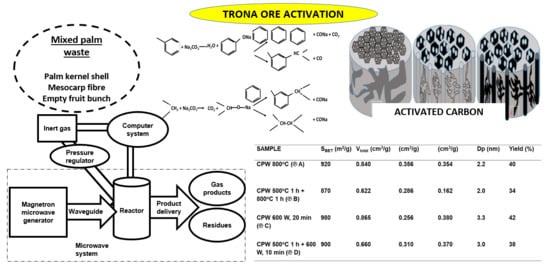
2.4. Effect of Process Parameters and Modes of Production on AC Characteristics
2.5. Overview of the Process and Resultant Challenges in the Use of Microwave
2.6. The Effectiveness of Trona Ore
3. Materials and Methods
3.1. Materials
3.2. Production of Activated Carbon
3.3. Characterisation of Trona Ore Activated Carbon (ToAC)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okoroigwe, E.C.; Ofomatah, A.C.; Oparaku, N.F.; Unachukwu, G.O. Production and evaluation of activated carbon from palm kernel shells (PKS) for economic and environmental sustainability. Int. J. Phys. Sci. 2013, 8, 1036–1041. [Google Scholar] [CrossRef] [Green Version]
- Suresh Kumar Reddy, K.; Al Shoaibi, A.; Srinivasakannan, C.A. Comparison of microstructure and adsorption characteristics of activated carbons by CO2 and H3PO4 activation from date palm pits. Xinxing Tan Cailiao New Carbon Mater. 2012, 27, 344–351. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Science of the Total Environment Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y.; Hua, X. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Shahbandeh, M. Palm oil: Global Production Volume. Available online: https://www.statista.com/statistics/613471/palm-oil-production-volume-worldwide/ (accessed on 8 April 2020).
- Chiew, Y.L.; Shimada, S. Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer–A case study of Malaysia. Biomass Bioenergy 2013, 51, 109–124. [Google Scholar] [CrossRef]
- Aghamohammadi, N.; Reginald, S.S.; Shamiri, A.; Zinatizadeh, A.A.; Wong, L.P.; Sulaiman, N.M.B.N. An investigation of sustainable power generation from oil palm biomass: A case study in Sarawak. Sustainability 2016, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Unpinit, T.; Poblarp, T.; Sailoon, N.; Wongwicha, P.; Thabuot, M. Fuel Properties of Bio-Pellets Produced from Selected Materials under Various Compacting Pressure. Energy Procedia Bangk. Thail. 2015, 79, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.S.; Kumar, S.; Arel, H.S. Sustainable concrete containing palm oil fuel ash as a supplementary cementitious material—A review. Renew. Sustain. Energy Rev. 2017, 80, 550–561. [Google Scholar] [CrossRef]
- Ahmmad, R.; Alengaram, U.J.; Jumaat, M.Z.; Sulong, N.H.R.; Yusuf, M.O.; Rehman, M.A. Feasibility study on the use of high volume palm oil clinker waste in environmental friendly lightweight concrete. Constr. Build. Mater. 2017, 135, 94–103. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Laginhas, C.; Carrott, M.M.L.R.; Carrott, P.J.M.; Amorós, J.E.C.; Gisbert, A.V.N. Surface and porous characterisation of activated carbons made from a novel biomass precursor, the esparto grass. Appl. Surf. Sci. 2013, 265, 919–924. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem. Eng. J. 2017, 314, 277–290. [Google Scholar] [CrossRef]
- Hesas, R.H.; Wan Daud, W.M.A.; Sahu, J.N.; Arami-Niya, A. The effects of a microwave heating method on the production of activated carbon from agricultural waste: A review. J. Anal. Appl. Pyrolysis 2013, 100, 1–11. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Liu, Z.; Lu, M.; Wang, C.; Li, H.; Ma, W.; Wang, S. Preparation and characterization of activated carbons from tobacco stem by chemical activation. J. Air Waste Manag. Assoc. 2017, 67, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Fujishige, M.; Yoshida, I.; Toya, Y.; Banba, Y.; Oshida, K.I.; Tanaka, Y.S.; Dulyaseree, P.; Wongwiriyapan, W.; Takeuchi, K. Preparation of activated carbon from bamboo-cellulose fiber and its use for EDLC electrode material. J. Environ. Chem. Eng. 2017, 5, 1801–1808. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Y.; Wang, W.; Yue, Q.; Yang, T. Comparative study on characterization of activated carbons prepared by microwave and conventional heating methods and application in removal of oxytetracycline (OTC). Chem. Eng. J. 2011, 171, 1446–1453. [Google Scholar] [CrossRef]
- Hidayu, A.R.; Muda, N. Preparation and Characterization of Impregnated Activated Carbon from Palm Kernel Shell and Coconut Shell for CO2 Capture. Procedia Eng. 2016, 148, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, N.A.; Yusup, S. Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption. J. Clean. Prod. 2017, 168, 474–486. [Google Scholar] [CrossRef]
- Liew, R.K.; Nam, W.L.; Chong, M.Y.; Phang, X.Y.; Su, M.H.; Yek, P.N.Y.; Ma, N.L.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Oil palm waste: An abundant and promising feedstock for microwave pyrolysis conversion into good quality biochar with potential multi-applications. Process Saf. Environ. Prot. 2018, 115, 57–69. [Google Scholar] [CrossRef]
- Sethupathi, S.; Bashir, M.J.; Akbar, Z.A.; Mohamed, A.R. Biomass-based palm shell activated carbon and palm shell carbon molecular sieve as gas separation adsorbents. Waste Manag. Res. 2015, 33, 303–312. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jewaratnam, J.; Ganesan, P. Prospect of hydrogen production from oil palm biomass by thermochemical process—A review. Int. J. Hydrogen Energy 2016, 41, 16637–16655. [Google Scholar] [CrossRef]
- Gourichon, H. Analysis of Incentives and Disincentives for Palm Oil in Nigeria; Technical Notes Series; MAFAP, FAO: Rome, Italy, 2013. [Google Scholar]
- Aziz, M.; Kurniawan, T. Enhanced utilization of palm oil mill wastes for power generation. Chem. Eng. Trans. 2016, 52, 727–732. [Google Scholar]
- Ooi, C.H.; Cheah, W.K.; Sim, Y.L.; Pung, S.Y.; Yeoh, F.Y. Conversion and characterization of activated carbon fiber derived from palm empty fruit bunch waste and its kinetic study on urea adsorption. J. Environ. Manag. 2017, 197, 199–205. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Zhang, J.; Yu, J. Formation of hollow carbon nanofibers on bio-char during microwave pyrolysis of palm kernel shell. Energy Convers. Manag. 2017, 148, 583–592. [Google Scholar] [CrossRef]
- Khanday, W.A.; Hameed, B.H. Zeolite-hydroxyapatite-activated oil palm ash composite for antibiotic tetracycline adsorption. Fuel 2018, 215, 499–505. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Microwave assisted preparation of activated carbon from pomelo skin for the removal of anionic and cationic dyes. Chem. Eng. J. 2011, 173, 385–390. [Google Scholar] [CrossRef]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N. Modeling and optimization by particle swarm embedded neural network for adsorption of zinc (II) by palm kernel shell based activated carbon from aqueous environment. J. Environ. Manag. 2017, 206, 178–191. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour. Technol. 2012, 104, 679–686. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, S.; Julkapli, N.M.; Bee, S.; Hamid, A. Functionalized Activated Carbon Derived from Biomass for Photocatalysis Applications Perspective. Int. J. Photoenergy 2015, 2015. [Google Scholar] [CrossRef]
- Xiao, H.; Peng, H.; Deng, S.; Yang, X.; Zhang, Y.; Li, Y. Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3 activation-Application in reactive black 5 adsorption from aqueous solution. Bioresour. Technol. 2012, 111, 127–133. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Wen, H.; Cai, Z.; He, C.; Wang, Z.; Yan, W. Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18. Powder Technol. 2018, 323, 230–237. [Google Scholar] [CrossRef]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes. C 2018, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Biliaminu, H.D.; Ibrahim, K.O. Non-Metallic Mineral Endownments in Nigeria; Raw Minerals Research and Development Council: Abuja, Nigeria, 2010. [Google Scholar]
- Zainal, N.H.; Aziz, A.A.; Idris, J.; Mamat, R.; Hassan, M.A.; Bahrin, E.K.; Abd-Aziz, S. Microwave-assisted pre-carbonisation of palm kernel shell produced charcoal with high heating value and low gaseous emission. J. Clean. Prod. 2017, 142, 2945–2949. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef]
- Xin-hui, D.; Srinivasakannan, C.; Jin-hui, P.; Li-bo, Z.; Zheng-yong, Z. Comparison of activated carbon prepared from Jatropha hull by conventional heating and microwave heating. Biomass Bioenergy 2011, 35, 3920–3926. [Google Scholar] [CrossRef]
- Lee, L.Z.; Ahmad Zaini, M.A. Metal chloride salts in the preparation of activated carbon and their hazardous outlook. Desalin. Water Treat. 2016, 57, 16078–16085. [Google Scholar] [CrossRef]
- Ahmed, M.J. Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments: Review. Process Saf. Environ. Prot. 2016, 102, 168–182. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y. A Review of Microwave-Assisted Reactions for Biodiesel Production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.S.; Wan Mahari, W.A.; Cheng, C.K.; Omar, R.; Chong, C.T.; Chase, H.A. Recovery of diesel-like fuel from waste palm oil by pyrolysis using a microwave heated bed of activated carbon. Energy 2016, 115, 791–799. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 126–1140. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Wang, Y.; Li, Q.; Zhao, P.; Chen, S. Physicochemical and adsorptive properties of activated carbons from Arundo donax Linn utilizing different iron salts as activating agents. J. Taiwan Inst. Chem. Eng. 2014, 45, 3007–3015. [Google Scholar] [CrossRef]
- Hussaro, K. preparation of activated carbon from palm oil shell by chemical activation with Na2CO3 and ZnCl2 as imprenated agents for H2S adsorption’. Am. J. Environ. Sci. 2014, 10, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Yakout, S.M.; Sharaf El-Deen, G. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef] [Green Version]
- Phuphuakrat, T.; Namioka, T.; Yoshikawa, K. Tar removal from biomass pyrolysis gas in two-step function of decomposition and adsorption. Appl. Energy 2010, 87, 2203–2211. [Google Scholar] [CrossRef]
- Salema, A.A.; Yeow, Y.K.; Ishaque, K.; Ani, F.N.; Afzal, M.T.; Hassan, A. Dielectric properties and microwave heating of oil palm biomass and biochar. Ind. Crops Prod. 2013, 50, 366–374. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Microwave heating of carbon-based solid materials. Carbon Lett. 2014, 15, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Wu, S.; Liu, B.; Yin, X.; Yang, Q. Catalytic effects of NaOH and Na2CO3 additives on alkali lignin pyrolysis and gasification. Appl. Energy 2012, 95, 22–30. [Google Scholar] [CrossRef]
- Chima, I.K.; Nwabinye, M.V. Economic Analysis of Household Waste Generation, Disposal and Management in Umuahia Metropolis, Abia. Int. J. Heal. Econ. Policy 2017, 2, 47–56. [Google Scholar]
- Bevan Nyakuma, B.; Johari, A.; Ahmad, A. Thermochemical analysis of palm oil wastes as fuel for biomass gasification. J. Teknol. Sci. Eng. 2013, 62, 73–76. [Google Scholar]
- Daoud, M.; Benturki, O.; Fontana, S.; Rogaume, Y.; Girods, P. Energy and matter balance of process of activated carbon production from Algerian agricultural wastes: Date palm rachis and jujube stones. Biomass Convers. Biorefinery 2019. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds actvated carbon, trona ore and impregnated mixed palm waste are available from the authors. |






| Sample/Process Parameters | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile | FC | Ash | C | H | N | O | S | |
| Raw CPW | 4.05 | 74.50 | 16.25 | 5.20 | 44.60 | 6.35 | 0.80 | 48.10 | 0.15 |
| CPW ℗ A: 800 °C-1 h | 5.25 | 12.95 | 73.56 | 8.24 | 74.20 | 2.88 | 1.10 | 21.70 | 0.12 |
| CPW ℗ B: 500 °C-1 h + 800 °C-1 h | 2.46 | 10.38 | 76.60 | 10.56 | 76.95 | 2.22 | 1.38 | 19.33 | 0.12 |
| CPW ℗ C: 600 W-20 min | 6.44 | 14.69 | 71.22 | 7.65 | 73.87 | 3.10 | 1.30 | 21.63 | 0.10 |
| CPW ℗ D: 500 °C-1 h + 600 W-10 min | 4.05 | 11.20 | 75.90 | 8.85 | 74.63 | 2.42 | 1.18 | 21.67 | 0.10 |
| Sample | Process Parameter | SBET (m2/g) | Vtotal (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | Dp (nm) | Yield (%) |
|---|---|---|---|---|---|---|---|
| PKS | ℗ A: 800 °C-1 h | 923 | 0.750 | 0.122 | 0.285 | 3.2 | 35 |
| MF | 1105 | 0.882 | 0.230 | 0.302 | 3.4 | 42 | |
| EFB | 845 | 0.645 | 0.234 | 0.285 | 2.3 | 30 | |
| CPW | 920 | 0.840 | 0.356 | 0.354 | 2.2 | 40 | |
| PKS | ℗ B: 500 °C-1 h + 800 °C-1 h | 650 | 0.745 | 0.108 | 0.230 | 2.8 | 30 |
| MF | 736 | 0.646 | 0.280 | 0.262 | 2.4 | 30 | |
| EFB | 820 | 0.568 | 0.250 | 0.145 | 2.5 | 24 | |
| CPW | 870 | 0.622 | 0.286 | 0.162 | 2.0 | 34 | |
| PKS | ℗ C: 600W-20 min | 1030 | 0.825 | 0.105 | 0.245 | 3.3 | 42 |
| MF | 1220 | 0.887 | 0.274 | 0.465 | 3.8 | 45 | |
| EFB | 735 | 0.640 | 0.222 | 0.346 | 3.1 | 37 | |
| CPW | 980 | 0.865 | 0.256 | 0.380 | 3.3 | 42 | |
| PKS | ℗ D: 500 °C-1 h + 600W-10 min | 670 | 0.542 | 0.089 | 0.200 | 3.1 | 37 |
| MF | 864 | 0.650 | 0.182 | 0.230 | 2.8 | 28 | |
| EFB | 810 | 0.712 | 0.234 | 0.242 | 3.0 | 28 | |
| CPW | 900 | 0.660 | 0.310 | 0.380 | 3.0 | 38 |
| PKS | MF | EFB | ||
|---|---|---|---|---|
| Proximate analysis (%w/w) | Moisture | 12 | 12.1 | 14.4 |
| Ash | 1.5 | 4.8 | 4.4 | |
| Volatiles | 70.6 | 72.9 | 73.7 | |
| Fixed carbon | 15.9 | 10.5 | 7.5 | |
| Ultimate analysis (%w/w) | C | 46 | 45.8 | 37.5 |
| H | 5.1 | 6.3 | 5.0 | |
| N | 0.4 | 0.9 | 0.4 | |
| S | 0.02 | 0.2 | 0.1 | |
| O * | 35 | 29.5 | 38 | |
| Lignocellulosic composition ** | Cellulose | 20.8 | 33.9 | 38.3 |
| Hemicellulose | 22.7 | 26.1 | 35.3 | |
| Lignin | 50.7 | 27.7 | 22.1 | |
| Thermal and energy properties | Organic content | 94.2 | 92 | 95.7 |
| Inorganic content | 5.8 | 8 | 4.3 | |
| Combustion rate, CR (X × 10−8 kg/s) ** | 4 | 4.2 | 3.8 | |
| Specific Heat, c, (J/kgK) ** | 3113 | 3231 | 2832 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E. Preparation and Characterisation of Activated Carbon from Palm Mixed Waste Treated with Trona Ore. Molecules 2020, 25, 5028. https://doi.org/10.3390/molecules25215028
Ukanwa KS, Patchigolla K, Sakrabani R, Anthony E. Preparation and Characterisation of Activated Carbon from Palm Mixed Waste Treated with Trona Ore. Molecules. 2020; 25(21):5028. https://doi.org/10.3390/molecules25215028
Chicago/Turabian StyleUkanwa, Kalu Samuel, Kumar Patchigolla, Ruben Sakrabani, and Edward Anthony. 2020. "Preparation and Characterisation of Activated Carbon from Palm Mixed Waste Treated with Trona Ore" Molecules 25, no. 21: 5028. https://doi.org/10.3390/molecules25215028