Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol
Abstract
:1. Introduction
2. Resources and Synthesis
2.1. Resources
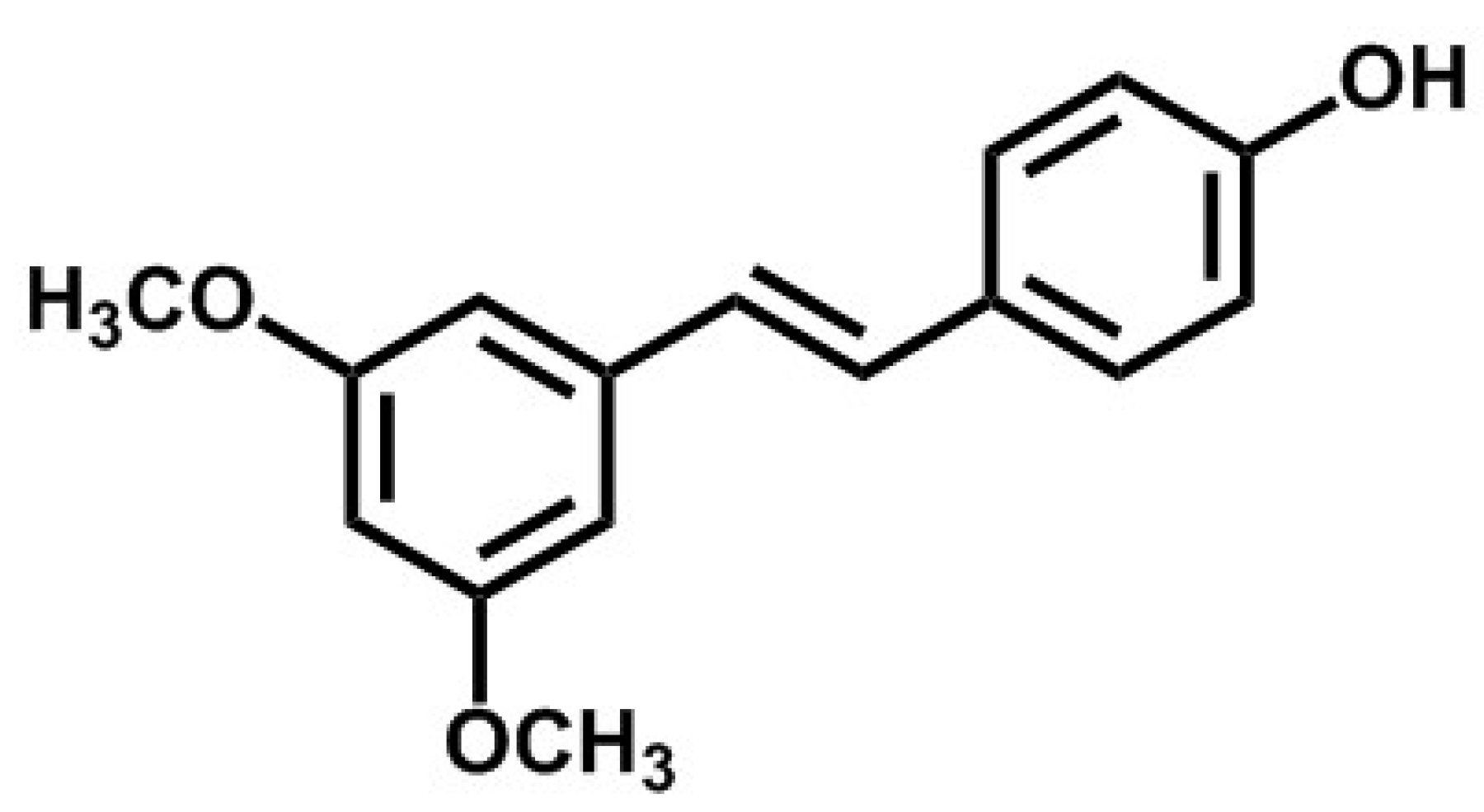
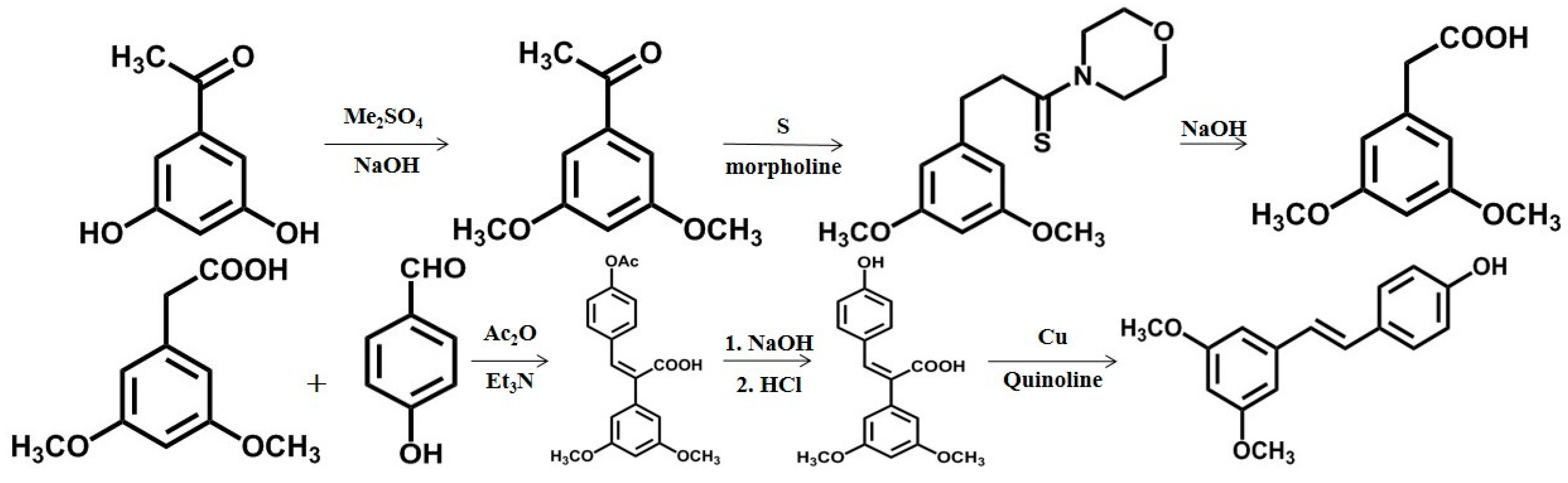
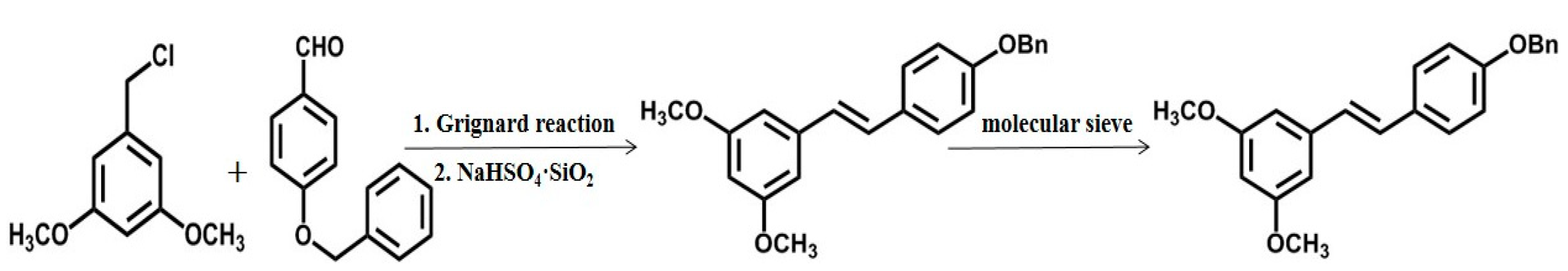
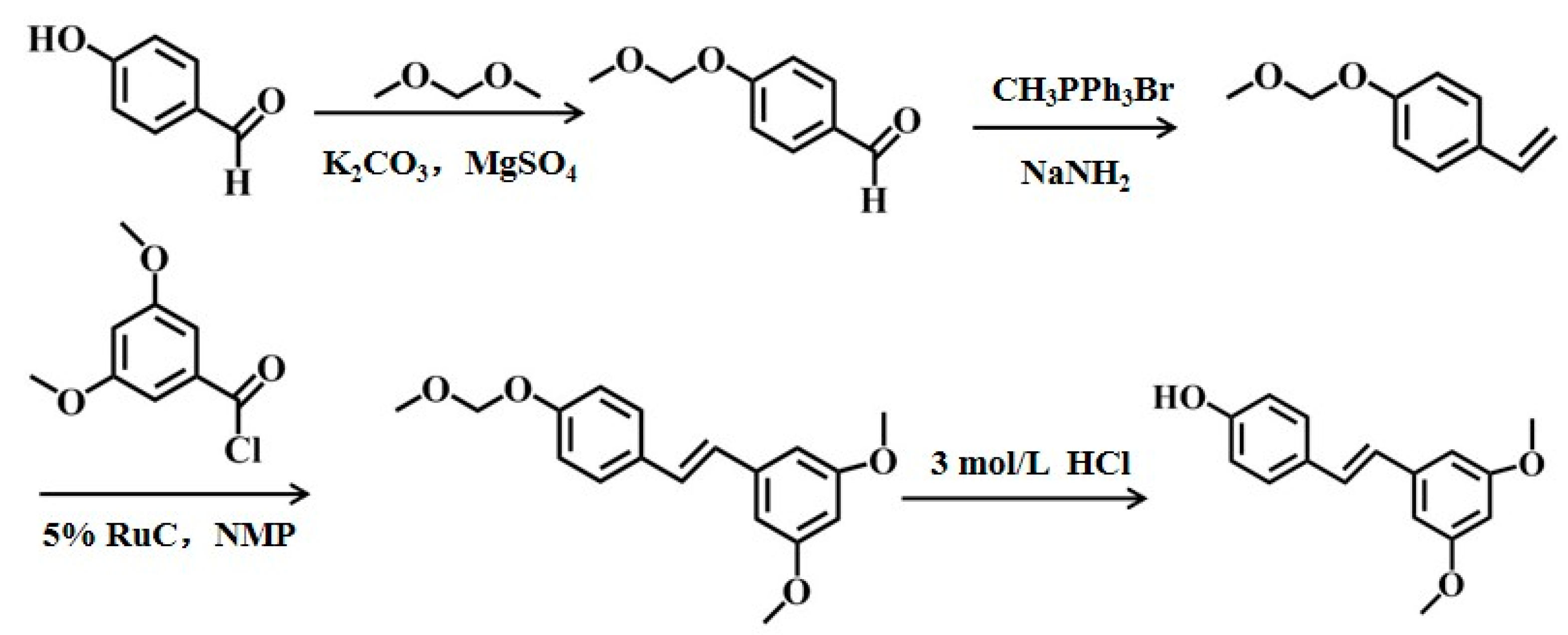
2.2. Chemical Synthesis and Biosynthesis of Pterostilbene
3. Pharmacokinetics
3.1. Absorption
3.2. Distribution
3.3. Metabolism
3.4. Excretion
4. Bioactivities of Pterostilbene
4.1. Anti-Tumor Activity
4.2. Anti-Inflammation Activity
4.3. Neuroprotective Activity
4.3.1. Cerebral Ischemia
4.3.2. Alzheimer’s Disease
4.3.3. Others
4.4. Antioxidation Activity
4.5. Lipid-Lowering Activity
4.5.1. Serum Lipids
4.5.2. Liver Steatosis
4.5.3. Obesity
4.6. Hypoglycemic Activity
4.7. Others
4.7.1. Antifungal Activity
4.7.2. Antiviral Activity
4.7.3. Antipsychotic Activity
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur. J. Pharmacol. 2016, 789, 229–243. [Google Scholar] [CrossRef]
- Paul, B.; Masih, I.; Deopujari, J.; Charpentier, C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J. Ethnopharmacol. 1999, 68, 71–76. [Google Scholar] [CrossRef]
- Ding, X.; Mei, W.; Huang, S.; Wang, H.; Zhu, J.; Hu, W.; Ding, Z.; Tie, W.; Peng, S.; Dai, H. Genome survey sequencing for the characterization of genetic background of Dracaena cambodiana and its defense response during dragon’s blood formation. PLoS ONE 2018, 13, e0209258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.P.; Cai, Y.; Phillipson, J.D. Studies on the anti-tumour, anti-bacterial, and wound-healing properties of dragon’s blood. Planta Medica 1994, 60, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Li, Y.J.; Li, X.; Wang, X.; Li, Y.; Zhang, X.; Dai, R.J.; Meng, W.W.; Wang, H.L.; Ma, H.; et al. Dragon’s blood may have radioprotective effects in radiation-induced rat brain injury. Radiat. Res. 2012, 178, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Gupta, R.K. Bioprotective properties of Dragon’s blood resin: In vitro evaluation of antioxidant activity and antimicrobial activity. BMC Complement. Altern. Med. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Fan, C.; Yu, L.; Yang, Y.; Jiang, S.; Ma, Z.; Hu, W.; Li, T.; Yang, Z.; Tian, T.; et al. Pterostilbene exerts an anti-inflammatory effect via regulating endoplasmic reticulum stress in endothelial cells. Cytokine 2016, 77, 88–97. [Google Scholar] [CrossRef]
- Amarnath Satheesh, M.; Pari, L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J. Pharm. Pharmacol. 2006, 58, 1483–1490. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J.; et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol. Res. 2019, 145, 104265. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Nagmani, R.; Feller, D.R.; Yokoyama, W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J. Agric. Food Chem. 2005, 53, 3403–3407. [Google Scholar] [CrossRef] [PubMed]
- Bhakkiyalakshmi, E.; Shalini, D.; Sekar, T.V.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br. J. Pharmacol. 2014, 171, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Ho, C.T.; Chen, Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Rimando, A.M.; Lee, H.J.; Ji, Y.; Reddy, B.S.; Suh, N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. (Phila.) 2009, 2, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Roberti, M.; Pizzirani, D.; Meli, M.; Dusonchet, L.; Gebbia, N.; Abbadessa, V.; Crosta, L.; et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int. J. Biochem. Cell Biol. 2005, 37, 1709–1726. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Zhang, X.; Chen, B.; Deng, Y.; Li, Y. UPLC-MS method for quantification of pterostilbene and its application to comparative study of bioavailability and tissue distribution in normal and Lewis lung carcinoma bearing mice. J. Pharm. Biomed. Anal. 2015, 114, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeandet, P.; Sobarzo-Sánchez, E.; Silva, A.; Clément, C.; Nabavi, S.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M.; et al. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020, 39, 107461. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Fernandez, M.; Pico, Y.; Manes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of safety from a human clinical trial with pterostilbene. J. Toxicol. 2013, 2013, 463595. [Google Scholar] [CrossRef]
- Kambiranda, D.M.; Basha, S.M.; Stringer, S.J.; Obuya, J.O.; Snowden, J.J. Multi-year Quantitative Evaluation of Stilbenoids Levels Among Selected Muscadine Grape Cultivars. Molecules 2019, 24, 981. [Google Scholar] [CrossRef] [Green Version]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Shuchun, Z.; Yifeng, Y.; Yue, Z. Progress in synthetic methods of stilbene compounds. Chin. J. Org. Chem. 2013, 33, 1851–1873. (In Chinese) [Google Scholar]
- Chunfen, X.; Hongyi, S.; Yu, C.; Zeliang, L.; Yong, Z. Synthesis of natural products pterostilbene and (E)-3,5,3′- trimethoxy-4O-hydroxy stilbene. Nat. Prod. Res. Dev. 2010, 22, 548–552. (In Chinese) [Google Scholar]
- Peddikotla, P.; Chittiboyina, A.G.; Khan, I.A. Synthesis of Pterostilbene by Julia Olefination. Synthetic Commun. 2013, 43, 3217–3223. [Google Scholar] [CrossRef]
- Xiaoling, L.; Guodong, H.; Yangqun, H.; Liang, C. Preparation of pterostilbene. Chin. J. Pharm. 2014, 45, 9. (In Chinese) [Google Scholar]
- Yubai, S.; Cheng, L.; Shaohua, M. A new process for synthesis of trans stilbene compound pterostilbene. Chin. J. Pharm. 2016, 47, 4. (In Chinese) [Google Scholar]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, K.T.; Kang, S.Y.; Hong, Y.S. De novo biosynthesis of pterostilbene in an Escherichia coli strain using a new resveratrol O-methyltransferase from Arabidopsis. Microb Cell Fact 2017, 16, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeandet, P.; Sobarzo-Sánchez, E.; Clément, C.; Nabavi, S.; Habtemariam, S.; Nabavi, S.; Cordelier, S. Engineering stilbene metabolic pathways in microbial cells. Biotechnol. Adv. 2018, 36, 2264–2283. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Vogt, M.; Bott, M.; Marienhagen, J. Functional expression of plant-derived O-methyltransferase, flavanone 3-hydroxylase, and flavonol synthase in Corynebacterium glutamicum for production of pterostilbene, kaempferol, and quercetin. J. Biotechnol. 2017, 258, 190–196. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Tsai, M.L.; Nagabhushanam, K.; Wang, Y.J.; Wu, C.H.; Ho, C.T.; Pan, M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef]
- Nutakul, W.; Sobers, H.S.; Qiu, P.; Dong, P.; Decker, E.A.; McClements, D.J.; Xiao, H. Inhibitory Effects of Resveratrol and Pterostilbene on Human Colon Cancer Cells: A Side-by-Side Comparison. J. Agric. Food Chem. 2011, 59, 10964–10970. [Google Scholar] [CrossRef] [Green Version]
- Azzolini, M.; Mattarei, A.; La Spina, M.; Fanin, M.; Chiodarelli, G.; Romio, M.; Zoratti, M.; Paradisi, C.; Biasutto, L. New natural amino acid-bearing prodrugs boost pterostilbene’s oral pharmacokinetic and distribution profile. Eur. J. Pharm. Biopharm. 2017, 115, 149–158. [Google Scholar] [CrossRef]
- Yeo, S.C.; Ho, P.C.; Lin, H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: The impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013, 57, 1015–1025. [Google Scholar] [CrossRef]
- Azzolini, M.; La Spina, M.; Mattarei, A.; Paradisi, C.; Zoratti, M.; Biasutto, L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol. Nutr. Food Res. 2014, 58, 2122–2132. [Google Scholar] [CrossRef]
- Shao, X.; Chen, X.; Badmaev, V.; Ho, C.-T.; Sang, S. Structural identification of mouse urinary metabolites of pterostilbene using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2010, 24, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.; Garcia, A.; Meyskens, F. Differences in the glucuronidation of resveratrol and pterostilbene: Altered enzyme specificity and potential gender differences. Drug Metab. Pharmacok. 2014, 29, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Albassam, A.A.; Frye, R.F. Effect of pterostilbene on in vitro drug metabolizing enzyme activity. Saudi Pharm. J. 2019, 27, 406–412. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Z.; Xia, Y.; Wang, Z.; Wang, X.; Wang, S.; Wang, Z.; Liu, Y. Pterostilbene supplements carry the risk of drug interaction via inhibition of UDP-glucuronosyltransferases (UGT) 1A9 enzymes. Toxicol. Lett. 2020, 320, 46–51. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yanez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. PTR 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Lin, H.S.; Yue, B.D.; Ho, P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 1308–1315. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Li, M.; Li, S.; Zhao, H. Targeting cancer stem cells and signaling pathways by resveratrol and pterostilbene. BioFactors (Oxf. Engl.) 2018, 44, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Tollefsbol, T.O. Pterostilbene down-regulates hTERT at physiological concentrations in breast cancer cells: Potentially through the inhibition of cMyc. J. Cell Biochem. 2018, 119, 3326–3337. [Google Scholar] [CrossRef]
- Huynh, T.T.; Lin, C.M.; Lee, W.H.; Wu, A.T.; Lin, Y.K.; Lin, Y.F.; Yeh, C.T.; Wang, L.S. Pterostilbene suppressed irradiation-resistant glioma stem cells by modulating GRP78/miR-205 axis. J. Nutr. Biochem. 2015, 26, 466–475. [Google Scholar] [CrossRef]
- Pan, M.H.; Chang, Y.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J. Agric. Food Chem. 2007, 55, 7777–7785. [Google Scholar] [CrossRef]
- Chen, R.J.; Tsai, S.J.; Ho, C.T.; Pan, M.H.; Ho, Y.S.; Wu, C.H.; Wang, Y.J. Chemopreventive effects of pterostilbene on urethane-induced lung carcinogenesis in mice via the inhibition of EGFR-mediated pathways and the induction of apoptosis and autophagy. J. Agric. Food Chem. 2012, 60, 11533–11541. [Google Scholar] [CrossRef]
- Mak, K.K.; Wu, A.T.; Lee, W.H.; Chang, T.C.; Chiou, J.F.; Wang, L.S.; Wu, C.H.; Huang, C.Y.; Shieh, Y.S.; Chao, T.Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef]
- McCormack, D.; Schneider, J.; McDonald, D.; McFadden, D. The antiproliferative effects of pterostilbene on breast cancer in vitro are via inhibition of constitutive and leptin-induced Janus kinase/signal transducer and activator of transcription activation. Am. J. Surg. 2011, 202, 541–544. [Google Scholar] [CrossRef]
- Su, C.-M.; Lee, W.-H.; Wu, A.T.H.; Lin, Y.-K.; Wang, L.-S.; Wu, C.-H.; Yeh, C.-T. Pterostilbene inhibits triple-negative breast cancer metastasis via inducing microRNA-205 expression and negatively modulates epithelial-to-mesenchymal transition. J. Nutr. Biochem. 2015, 26, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Kim, J.S.; Cho, S.M.; Lee, H.J.; Ahn, K.S.; Kim, S.H.; Lee, E.O. Urokinase-type plasminogen activator expression and Rac1/WAVE-2/Arp2/3 pathway are blocked by pterostilbene to suppress cell migration and invasion in MDA-MB-231 cells. Bioorg. Med. Chem. Lett. 2014, 24, 1176–1179. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the -catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Jeong, J.H.; Ryu, J.H.; Kim, W.Y. ATM/CHK/p53 Pathway Dependent Chemopreventive and Therapeutic Activity on Lung Cancer by Pterostilbene. PLoS ONE 2016, 11, e0162335. [Google Scholar] [CrossRef]
- Guo, L.; Tan, K.; Wang, H.; Zhang, X. Pterostilbene inhibits hepatocellular carcinoma through p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol. Rep. 2016, 36, 3233–3240. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.L.; Shen, Y.; Xu, J.P.; Han, K.; Zhou, Y.; Yang, S.; Yin, J.Y.; Min, D.L.; Hu, H.Y. Pterostilbene suppresses human endometrial cancer cells in vitro by down-regulating miR-663b. Acta Pharmacol. Sin. 2017, 38, 1394–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, L.; Wu, Y.; Lv, C.; Li, X.; Cao, X.; Yang, M.; Feng, D.; Luo, Z. Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway. Toxicology 2013, 304, 120–131. [Google Scholar] [CrossRef]
- Chen, R.J.; Ho, C.T.; Wang, Y.J. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol. Nutr. Food Res. 2010, 54, 1819–1832. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Y.; Fan, C.; Di, S.; Hu, W.; Jiang, S.; Li, T.; Ma, Z.; Chao, D.; Feng, X.; et al. Pterostilbene Inhibits the Growth of Human Esophageal Cancer Cells by Regulating Endoplasmic Reticulum Stress. Cell Physiol. Biochem. 2016, 38, 1226–1244. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, S.Y.; Wang, S.Y.; Lin, J.A.; Yen, G.C. Pterostilbene Enhances Cytotoxicity and Chemosensitivity in Human Pancreatic Cancer Cells. Biomolecules 2020, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef]
- Ko, C.; Lin, C.; Chen, M.; Yang, S.; Chiou, H.; Hsieh, M. Pterostilbene induce autophagy on human oral cancer cells through modulation of Akt and mitogen-activated protein kinase pathway. Oral Oncol. 2015, 51, 593–601. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Cheng, L.; Chang, W.; Kao, Y.; Chang, M.; Wang, B.; Cheng, H. Pterostilbene prevents AKT-ERK axis-mediated polymerization of surface fibronectin on suspended lung cancer cells independently of apoptosis and suppresses metastasis. J. Hematol. Oncol. 2017, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ruzhi, D.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci. Rep. 2016, 6, 19408. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Koh, Y.C.; Li, S.; Chen, P.Y.; Wu, J.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Prevention of Vascular Inflammation by Pterostilbene via Trimethylamine-N-Oxide Reduction and Mechanism of Microbiota Regulation. Mol. Nutr. Food Res. 2019, 63, e1900514. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, L.; Yue, L.; Wang, B.; Li, X.; Guo, H.; Ma, Y.; Yao, C.; Gao, L.; Deng, J.; et al. Pterostilbene Attenuates Early Brain Injury Following Subarachnoid Hemorrhage via Inhibition of the NLRP3 Inflammasome and Nox2-Related Oxidative Stress. Mol. Neurobiol. 2017, 54, 5928–5940. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.M.; Ma, A.; Zhang, Y.L.; Chen, Y.Y.; Zhou, H.; Li, W.J.; Jin, X. Orally administrated pterostilbene attenuates acute cerebral ischemia-reperfusion injury in a dose- and time-dependent manner in mice. Pharmacol. Biochem. Behav. 2015, 135, 199–209. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Luo, J.; Wang, X.; Guo, H.; Feng, D.; Zhao, L.; Bai, H.; Song, M.; Liu, X.; et al. Pterostilbene Attenuates Astrocytic Inflammation and Neuronal Oxidative Injury After Ischemia-Reperfusion by Inhibiting NF-kappaB Phosphorylation. Front. Immunol. 2019, 10, 2408. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Li, Y.; Fan, C.; Jiang, S.; Zhao, L.; Di, S.; Xin, Z.; Wang, B.; Wu, G.; et al. HO-1 Signaling Activation by Pterostilbene Treatment Attenuates Mitochondrial Oxidative Damage Induced by Cerebral Ischemia Reperfusion Injury. Mol. Neurobiol. 2016, 53, 2339–2353. [Google Scholar] [CrossRef]
- Sun, J.; Huo, H.; Song, Y.; Zheng, J.; Zhao, Y.; Huang, W.; Wang, Y.; Zhu, J.; Tu, P.; Li, J. Method development and application for multi-component quantification in rats after oral administration of Longxuetongluo Capsule by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2018, 156, 252–262. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, G.; Miao, F.; Ding, L.; Mou, Y.; Wang, L.; Su, G.; Chen, G.; Yang, J.; Wu, C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 92–102. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, J.; Wei, Y.; Li, J. Effects of piceatannol and pterostilbene against beta-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016, 7, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.; Nirwane, A.; Majumdar, A. Pterostilbene ameliorates intracerebroventricular streptozotocin induced memory decline in rats. Cogn. Neurodyn. 2017, 11, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, H.; Yue, L.; Li, X.; Zhao, L.; Yang, X.; Wang, X.; Yang, Y.; Qu, Y. Neuroprotective effects of pterostilbene against oxidative stress injury: Involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Res. 2016, 1643, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- Li, D.; Song, T.; Yang, L.; Wang, X.; Yang, C.; Jiang, Y. Neuroprotective actions of pterostilbene on hypoxic-ischemic brain damage in neonatal rats through upregulation of heme oxygenase-1. Int. J. Dev. Neurosci. 2016, 54, 22–31. [Google Scholar] [CrossRef]
- Song, Z.; Han, S.; Pan, X.; Gong, Y.; Wang, M. Pterostilbene mediates neuroprotection against oxidative toxicity via oestrogen receptor alpha signalling pathways. J. Pharm. Pharmacol. 2015, 67, 720–730. [Google Scholar] [CrossRef]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene Activates the Nrf2-Dependent Antioxidant Response to Ameliorate Arsenic-Induced Intracellular Damage and Apoptosis in Human Keratinocytes. Front. Pharmacol. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Rong, H. Pterostilbene impact on retinal endothelial cells under high glucose environment. Int. J. Clin. Exp. Pathol. 2015, 8, 12589–12594. [Google Scholar]
- Nagao, K.; Jinnouchi, T.; Kai, S.; Yanagita, T. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J. Nutr. Biochem. 2017, 43, 151–155. [Google Scholar] [CrossRef]
- La Spina, M.; Galletta, E.; Azzolini, M.; Gomez Zorita, S.; Parrasia, S.; Salvalaio, M.; Salmaso, A.; Biasutto, L. Browning Effects of a Chronic Pterostilbene Supplementation in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2019, 20, 5377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, L.; Milton-Laskibar, I.; Hijona, E.; Bujanda, L.; Rimando, A.M.; Portillo, M.P. Effects of pterostilbene in brown adipose tissue from obese rats. J. Physiol. Biochem. 2016, 73, 457–464. [Google Scholar] [CrossRef]
- Aguirre, L.; Palacios-Ortega, S.; Fernandez-Quintela, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients 2019, 11, 961. [Google Scholar]
- Gomez-Zorita, S.; Trepiana, J.; Fernandez-Quintela, A.; Gonzalez, M.; Portillo, M.P. Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue. Int. J. Mol. Sci. 2018, 19, 2654. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.; Portillo, M. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J. Agric. Food Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Belles, C.; Briot, A.; Fernandez-Quintela, A.; Portillo, M.P.; Carpene, C. Pterostilbene Inhibits Lipogenic Activity similar to Resveratrol or Caffeine but Differently Modulates Lipolysis in Adipocytes. Phytother. Res. 2017, 31, 1273–1282. [Google Scholar] [CrossRef]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef]
- Etxeberria, U.; Hijona, E.; Aguirre, L.; Milagro, F.I.; Bujanda, L.; Rimando, A.M.; Martinez, J.A.; Portillo, M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017, 61, 61. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Satheesh, M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci. 2006, 79, 641–645. [Google Scholar] [CrossRef]
- Elango, B.; Dornadula, S.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene Ameliorates Streptozotocin-Induced Diabetes through Enhancing Antioxidant Signaling Pathways Mediated by Nrf2. Chem. Res. Toxicol. 2016, 29, 47–57. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Aguirre, L.; Macarulla, M.; Rimando, A.; Portillo, M. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: Involvement of skeletal muscle and liver. Food Funct. 2015, 6, 1968–1976. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Long, S.R.; Teng, W.; Ge, H.; Wang, Y.; Yu, S.; Xue, Y.; Zhang, Y.; Li, X.; et al. Antidiabetic effects of pterostilbene through PI3K/Akt signal pathway in high fat diet and STZ-induced diabetic rats. Eur. J. Pharmacol. 2019, 859, 172526. [Google Scholar] [CrossRef]
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.S.; Tan, K.S. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci. Rep. 2020, 10, 9072. [Google Scholar] [CrossRef]
- Chan, C.N.; Trinite, B.; Levy, D.N. Potent Inhibition of HIV-1 Replication in Resting CD4 T Cells by Resveratrol and Pterostilbene. Antimicrob. Agents Chemother. 2017, 61, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pflieger, A.; Waffo Teguo, P.; Papastamoulis, Y.; Chaignepain, S.; Subra, F.; Munir, S.; Delelis, O.; Lesbats, P.; Calmels, C.; Andreola, M.L.; et al. Natural stilbenoids isolated from grapevine exhibiting inhibitory effects against HIV-1 integrase and eukaryote MOS1 transposase in vitro activities. PLoS ONE 2013, 8, e81184. [Google Scholar] [CrossRef]
- Al Rahim, M.; Rimando, A.M.; Silistreli, K.; El-Alfy, A.T. Anxiolytic action of pterostilbene: Involvement of hippocampal ERK phosphorylation. Planta Med. 2013, 79, 723–730. [Google Scholar] [CrossRef]
- Yang, L.; Ran, Y.; Quan, Z.; Wang, R.; Yang, Q.; Jia, Q.; Zhang, H.; Li, Y.; Peng, Y.; Liang, J.; et al. Pterostilbene, an active component of the dragon’s blood extract, acts as an antidepressant in adult rats. Psychopharmacology 2019, 236, 1323–1333. [Google Scholar] [CrossRef]



| Subject | Mode of Administration | Dose (mg/kg) | AUC (mg h/L) | V (L/kg) | CL (mL/min/kg) | T1/2 (min) | Reference |
|---|---|---|---|---|---|---|---|
| C57 BL/6 mice | iv. | 10 | 26.7 ± 8.2 | Vc 0.674 ± 0.12 | (0.014 ± 0.003) × 103 | 34.5 ± 1.0 | [16] |
| C57 BL/6 mice | ig. | 14 | 4.43 ± 2.0 | Vc 4.9 ± 1.9 | (0.027 ± 0.008) × 103 | 102± 19.2 | [16] |
| C57 BL/6 mice | ig. | 28 | 11.9 ± 2.1 | Vc 3.76 ± 0.85 | (0.012 ± 0.008) × 103 | 87.8 ± 29.2 | [16] |
| C57 BL/6 mice | ig. | 56 | 39.4 ± 9.7 | Vc 1.57 ± 0.27 | (0.030 ± 0.005) × 103 | 56.9 ± 13.9 | [16] |
| SD rat | iv. | 2.5 | (35.6 ± 5.1)/60 | Vc 2.85 ± 0.50 | 68.2 ± 9.8 | 93.9 ± 22.3 | [35] |
| SD rat | iv. | 5 | (135,650 ± 8944)/(60 × 103) | Vc (1267 ± 364)/103 | 37.0 ± 2.5 | 96.6 ± 23.7 | [43] |
| SD rat | iv. | 10 | (168.7 ± 28.6)60 | Vc 3.03 ± 0.88 | 59.1 ± 8.8 | 155.1 ± 64.1 | [35] |
| SD rat | iv. | 11.2 | 4009/103 | Vss 5.30 | 2.7 × 103/60 | 2.9 × 60 | [17] |
| SD rat | iv. | 20 | 17.5 ± 6.6 | Vd 2.41 ± 1.13 | (0.960 ± 0.025) × 103/60 | (1.73 ± 0.87) × 60 | [39] |
| SD rat | iv. | 25 | (689.2 ± 124.1)/60 | Vc 2.19 ± 0.17 | 36.4 ± 7.8 | 150.8 ± 15.9 | [35] |
| Wistar rat | iv. | 22.5 | (38.8 ± 5.3) × 256.3/103 | Vss 6.1 ± 1.0 | (2.3 ± 0.3) × 103/60 | (1.8 ± 0.3) × 60 | [36] |
| Signaling Pathway | Model | Dose | Reference |
|---|---|---|---|
| EGFR, Akt/mTOR, Stat3, ERK1/2, and NFκB pathways | urethane-caused lung tumor | 250 mg/kg | [48] |
| microRNA 448 circuit | MDA-MB-231 cells were cocultured with M2 TAM and were subcutaneously injected into the left flank of NOD/SCID mice | 5 mg/kg | [49] |
| JAK/STAT3 signaling pathway | Breast cancer cell lines (MDA-231 and ZR-751) | 75 μM | [50] |
| Src/Fak signaling pathway | MDA-MB-231-bearing NOD/SCID mice | 10 mg/kg | [51] |
| Rac1/WAVE/Arp2/3 pathway | MDA-MB-231 cells | 10 μM | [52] |
| β-catenin/p65 downstream signaling pathway | F344 rats were given two AOM injections subcutaneously | 0.004% in the diet for 45 weeks | [53] |
| ATM/CHK/p53 pathway | non-small cell lung cancer cell (A549) | 21 μM | [54] |
| GRP78 signaling pathway | human glioblastoma cell lines GBM8401 and U87MG | 2.99, 1.42 μM | [46] |
| p53/SOD2/ROS pathway | HepG2 cells | 100 μM | [55] |
| miR-663b/BCL2L14 signaling pathway | HTB-111 and Ishikawa cells | 71.64 nM, 74.34 µM | [56] |
| JAK2/STAT3 signaling pathway | human osteosarcoma cell line, SOSP-9607 | 1.81 µM | [57] |
| AKT/mTOR/p70S6K and ERK1/2 pathways | T24 human bladder cancer cell | 66.58 ± 1.84 µM | [58] |
| Fas/FasL pathway | human AGS gastric carcinoma cells (CCRC 60102) | 50.7 μM | [47] |
| ERS signaling pathway | Human EC109 and TE1 esophageal cancer cells | 150 μM | [59] |
| RAGE/PI3K/Akt signaling pathway | MIA PaCa-2 and MIA PaCa-2GEMR cells (GEM-resistant cells) | 41.8, 42.0 µM | [60] |
| signal transducer and activator of transcription 3 signaling pathway | HeLa, CaSki, and SiHa cervical cancer adherent cells | 32.67, 14.83, 34.17 µM | [61] |
| Model | Dose | Reference |
|---|---|---|
| Human keratinocytes, mouse epidermal cells | 3.75, 7.5, 15 μM | [80] |
| human retinal endothelial cells | 1.0 mM | [81] |
| Streptozotocin–nicotinamide-induced type 2 diabetes mellitus in Wistar rats | 40 mg/kg for 6 weeks | [8] |
| Model | Dose | Reference |
|---|---|---|
| H4IIEC3 cells | 100 µM | [11] |
| Obese Otsuka Long–Evans Tokushima fatty rats | 0.5% diet for 4 weeks | [82] |
| 3T3-L1 mature adipocytes | 5 µM | [83] |
| Mice fed an obesogenic high-fat diet | 352 µmol/kg/d for 30 weeks | [83] |
| Genetic obesity Zucker (fa/fa) rats | 15, 30 mg/kg body weight/day for 6 weeks | [84,85] |
| Wistar rats fed an obesogenic diet | 15, 30 mg/kg body weight/day for 6 weeks | [86,87] |
| 3T3-F442A preadipocytes | 1–10 μM | [88] |
| 3T3-L1 preadipocytes | 5–40 μM | [89] |
| Genetic obesity Zucker (fa/fa) rats | 15 mg/kg body weight/day for 6 weeks | [90] |
| Model | Dose | Reference |
|---|---|---|
| Streptozotocin-nicotinamide-induced diabetic male albino Wistar rats | 10, 20, 40 mg/kg for 2, 4, 6 weeks | [91] |
| Islet β cells of INS-1E rats induced by streptozotocin | 4, 8 μM | [12] |
| Moderate diabetic mice with glycosuria and hyperglycemia | 5 mg/kg for 5 weeks | [11] |
| Insulin resistance associated Wistar rats with obesity feeding | 15 mg/kg body weight/d for 6 weeks | [93] |
| Diabetes was induced in rats by streptozotocin and a high-sugar and high-fat diet | 20, 40 and 80 mg/kg/d for 8 weeks | [94] |
| Model | Dose | Reference |
|---|---|---|
| Conidia of Botrytis cinerea | 60 μg/mL | [2] |
| Fusobacterium nucleatum | 0.02 mg/mL | [95] |
| HIV-1 infection in resting CD4 T cells | 5 μM | [96] |
| Transformed fibroblast cell line | 10 μM | [97] |
| Elevated plus maze test mice | 2 mg/kg | [98] |
| Chronic unexpected stressed model rats | 2.5 mg/L | [99] |
| In Vivo or In Vitro | Model | Comparison | Mechanism | Reference |
|---|---|---|---|---|
| In vitro | Multidrug resistant HL60-R (human myeloid cell line expressing P-glycoprotein) | AC50 of pterostilbene is 85 ± 11, resveratrol has almost no effect on inducing apoptosis | Caspase-independent pathway | [15] |
| In vitro | HT-29 human adenocarcinoma cell line | Pterostilbene (IC50, 22.4 μmol/L), resveratrol (IC50, 43.8 μmol/L) | P38 mitogen-activated protein kinase cascade | [14] |
| In vivo | Mouse susceptibility No. 8 (SAMP8) AD model | Pterostilbene was a more effective cognitive and cellular stress regulator than resveratrol | The increasing of the expression of peroxisome proliferator-activated receptor α | [10] |
| In vivo | Wistar rats fed an obesogenic diet | Pterostilbene is more effective than resveratrol at a dose of 15 mg/kg/d | The decrease of lipogenesis in adipose tissue and the increase of fatty acid oxidation in liver | [88] |
| In vivo | Wistar rats fed an obesogenic diet | 15 mg/kg body weight/d was not as effective as pterostilbene in reducing serum glucose levels | The increase of liver glucokinase activity and skeletal muscle glucose uptake | [93] |
| In vitro | Fusobacterium nucleatum | The minimum inhibitory concentration was more than 60 times lower than that of resveratrol | Inducing the leakage of cell contents, which resulted in the loss of bacterial cell vitality | [95] |
Sample Availability: Samples of the compounds pterostilbene and resveratrol are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. https://doi.org/10.3390/molecules25215166
Liu Y, You Y, Lu J, Chen X, Yang Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules. 2020; 25(21):5166. https://doi.org/10.3390/molecules25215166
Chicago/Turabian StyleLiu, Yeju, Yuyang You, Juan Lu, Xi Chen, and Zhihong Yang. 2020. "Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol" Molecules 25, no. 21: 5166. https://doi.org/10.3390/molecules25215166








