The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line
Abstract
1. Introduction
2. Results
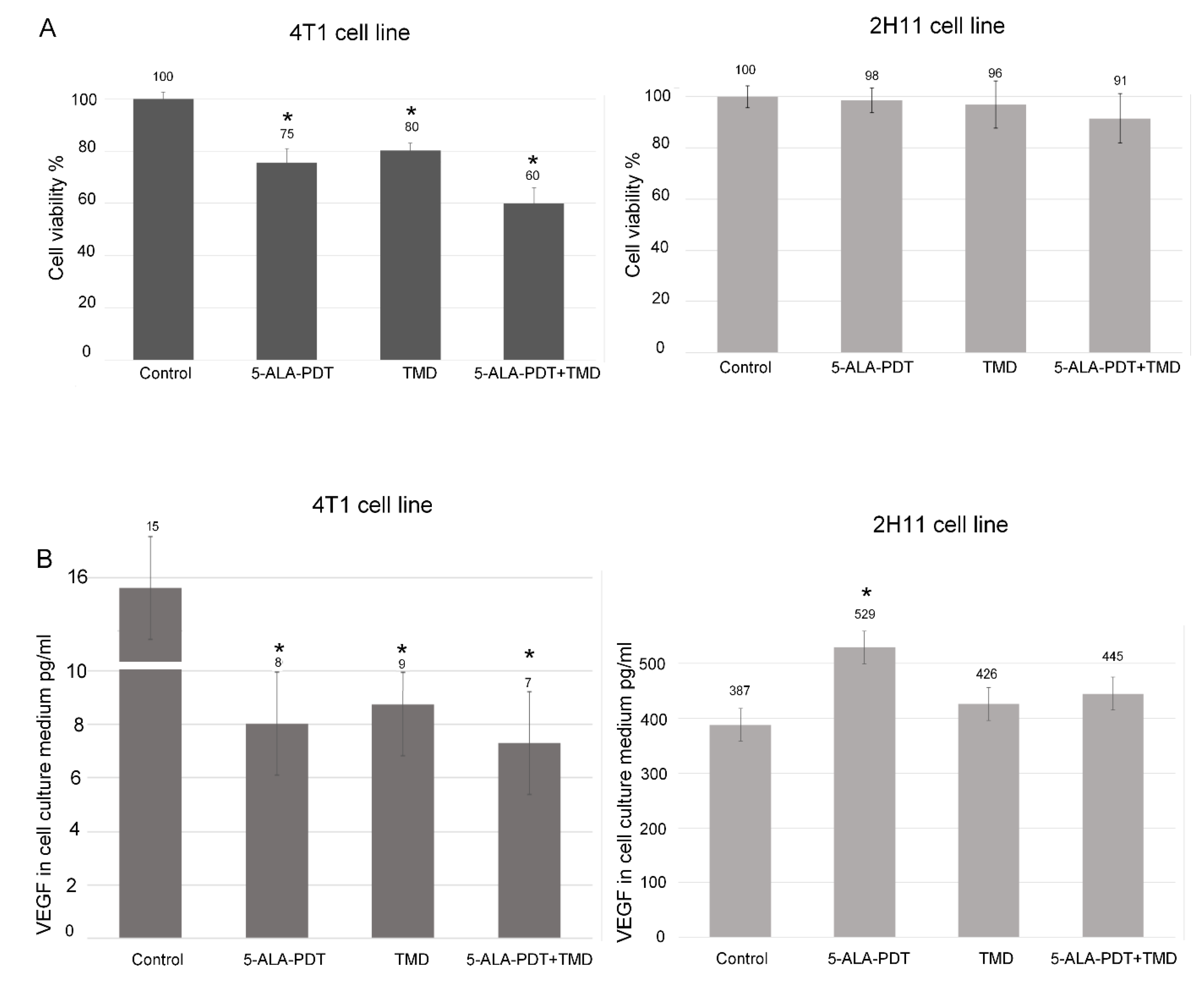
2.1. MTT Assay
2.2. Apoptosis Assay
2.3. ELISA Assay
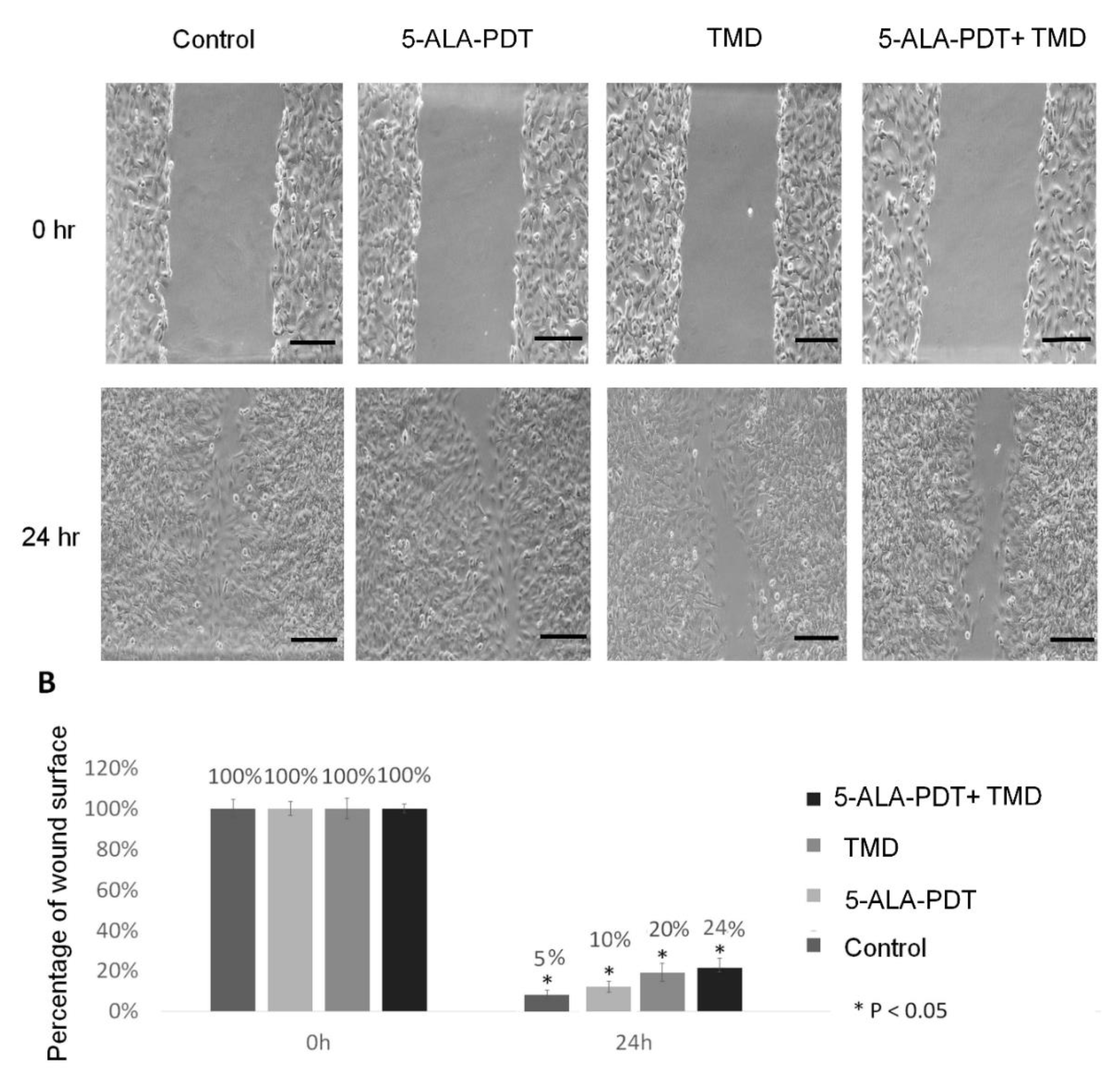
2.4. Wound Healing Assay
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. 5-ALA-PDT + Thalidomide (TMD) Therapy Protocol
4.3. MTT Assay
4.4. Apoptosis Assay
4.5. ELISA Assay
4.6. Wound Healing Assay
4.7. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 2019, 20510–20517. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Wang, W.; Moriyama, L.T.; Bagnato, V.S. Photodynamic therapy induced vascular damage: An overview of experimental PDT. Laser Phys. Lett. 2012, 10, 23001. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Z.; Ma, Z.; Liu, J.; Han, G.; Ma, F.; Jia, N.; Miao, Z.; Zhang, W.; Sheng, C.; et al. Design and synthesis of novel water-soluble amino acid derivatives of chlorin p6 ethers as photosensitizer. Chin. Chem. Lett. 2019, 30, 247–249. [Google Scholar] [CrossRef]
- Liu, H.; Daly, L.; Rudd, G.; Khan, A.P.; Mallidi, S.; Liu, Y.; Cuckov, F.; Hasan, T.; Celli, J.P. Development and evaluation of a low-cost, portable, LED-based device for PDT treatment of early-stage oral cancer in resource-limited settings. Lasers Surg. Med. 2018, 51, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Pavličková, V.; Jurášek, M.; Rimpelová, S.; Záruba, K.; Sedlák, D.; Šimková, M.; Kodr, D.; Staňková, E.; Fähnrich, J.; Rottnerová, Z.; et al. Oxime-based 19-nortestosterone-pheophorbide: A conjugate: Bimodal controlled release concept for PDT. J. Mater. Chem. B 2019, 7, 5465–5477. [Google Scholar] [CrossRef]
- kyung Oh, E.; Jin, S.-E.; Kim, J.-K.; Park, J.-S.; Park, Y.; Kim, C.-K. Retained topical delivery of 5-aminolevulinic acid using cationic ultradeformable liposomes for photodynamic therapy. Eur. J. Pharm. Sci. 2011, 44, 149–157. [Google Scholar] [CrossRef]
- Xu, J.; Gao, J.; Wei, Q. Combination of Photodynamic Therapy with Radiotherapy for Cancer Treatment. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Sun, G.; Anderson, M.A.; Gorospe, E.C.; Leggett, C.L.; Lutzke, L.S.; Song, L.M.W.K.; Levy, M.; Wang, K.K. Synergistic effects of photodynamic therapy with HPPH and gemcitabine in pancreatic cancer cell lines. Lasers Surg. Med. 2012, 44, 755–761. [Google Scholar] [CrossRef]
- Osaki, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; Okamoto, Y. Antimalarial Drugs Enhance the Cytotoxicity of 5-Aminolevulinic Acid-Based Photodynamic Therapy against the Mammary Tumor Cells of Mice In Vitro. Molecules 2019, 24, 3891. [Google Scholar] [CrossRef]
- Tomanová, P.; Rimpelová, S.; Jurášek, M.; Buděšínský, M.; Vejvodová, L.; Ruml, T.; Kmoníčková, E.; Drašar, P.B. Trilobolide-porphyrin conjugates: On synthesis and biological effects evaluation. Steroids 2015, 97, 8–12. [Google Scholar] [CrossRef]
- Filho, A.L.; Lopes, J.M.; Schmitt, F.C. Angiogenesis and Breast Cancer. J. Oncol. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor Activity of Thalidomide in Refractory Multiple Myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef]
- de Souza, C.M.; e Silva, A.C.A.; de Jesus Ferraciolli, C.; Moreira, G.V.; Campos, L.C.; dos Reis, D.C.; Lopes, M.T.P.; Ferreira, M.A.N.D.; Andrade, S.P.; Cassali, G.D. Combination therapy with carboplatin and thalidomide suppresses tumor growth and metastasis in 4T1 murine breast cancer model. Biomed. Pharmacother. 2014, 68, 51–57. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, F.; Hsieh, T.-C.; Wu, J.; Wu, E. Thalidomide—A Notorious Sedative to a Wonder Anticancer Drug. Curr. Med. Chem. 2013, 20, 4102–4108. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Anand, S.; Rollakanti, K.R.; Brankov, N.; Brash, D.E.; Hasan, T.; Maytin, E. V Fluorouracil Enhances Photodynamic Therapy of Squamous Cell Carcinoma via a p53-Independent Mechanism that Increases Protoporphyrin {IX} levels and Tumor Cell Death. Mol. Cancer Ther. 2017, 16, 1092–1101. [Google Scholar] [CrossRef]
- Ali, S.; Muhammad, S.; Khurshid, A.; Ikram, M.; Maqsood, M.; Fisher, C.; Cathcart, J.; Lilge, L. Effective phthalocyanines mediated photodynamic therapy with doxorubicin or methotrexate combination therapy at sub-micromolar concentrations in vitro. Photodiagnosis Photodyn. Ther. 2018, 22, 51–64. [Google Scholar] [CrossRef]
- Uehara, M.; Inokuchi, T.; Sano, K.; ZuoLin, W. Expression of vascular endothelial growth factor in mouse tumours subjected to photodynamic therapy. Eur. J. Cancer 2001, 37, 2111–2115. [Google Scholar] [CrossRef]
- Gomer, C.J.; Ferrario, A.; von Tiehl, K.; Shwartz, M.A.; Gill, P.S.; Rucker, N. Combining photodynamic therapy with antiangiogenic therapy. In Photodynamic Therapy; Royal Society of Chemistry: London, UK, 2003; pp. 119–126. [Google Scholar]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Benz, P.M.; Ding, Y.; Stingl, H.; Loot, A.E.; Zink, J.; Wittig, I.; Popp, R.; Fleming, I. AKAP12 deficiency impairs VEGF-induced endothelial cell migration and sprouting. Acta Physiol. 2019, 228, e13325. [Google Scholar] [CrossRef]
- Bailey-Downs, L.C.; Thorpe, J.E.; Disch, B.C.; Bastian, A.; Hauser, P.J.; Farasyn, T.; Berry, W.L.; Hurst, R.E.; Ihnat, M.A. Development and Characterization of a Preclinical Model of Breast Cancer Lung Micrometastatic to Macrometastatic Progression. PLoS ONE 2014, 9, e98624. [Google Scholar] [CrossRef]
- Kaur, P.; Nagaraja, G.M.; Zheng, H.; Gizachew, D.; Galukande, M.; Krishnan, S.; Asea, A. A mouse model for triple-negative breast cancer tumor-initiating cells (TNBC-TICs) exhibits similar aggressive phenotype to the human disease. BMC Cancer 2012, 12, 120. [Google Scholar] [CrossRef] [PubMed]

Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zduniak, K.; Gdesz-Birula, K.; Woźniak, M.; Duś-Szachniewicz, K.; Ziółkowski, P. The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line. Molecules 2020, 25, 5184. https://doi.org/10.3390/molecules25215184
Zduniak K, Gdesz-Birula K, Woźniak M, Duś-Szachniewicz K, Ziółkowski P. The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line. Molecules. 2020; 25(21):5184. https://doi.org/10.3390/molecules25215184
Chicago/Turabian StyleZduniak, Krzysztof, Katarzyna Gdesz-Birula, Marta Woźniak, Kamila Duś-Szachniewicz, and Piotr Ziółkowski. 2020. "The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line" Molecules 25, no. 21: 5184. https://doi.org/10.3390/molecules25215184
APA StyleZduniak, K., Gdesz-Birula, K., Woźniak, M., Duś-Szachniewicz, K., & Ziółkowski, P. (2020). The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line. Molecules, 25(21), 5184. https://doi.org/10.3390/molecules25215184




