Marine-Derived Compounds and Prospects for Their Antifungal Application
Abstract
:1. Introduction
2. Marine Natural Products as New Antifungal Candidates
2.1. Sponge-Derived Compounds
2.2. Bacteria-Derived Compounds
2.3. Fungi-Derived Compounds
2.4. Sea Cucumber-Derived Compounds
3. Prospects for the Application of Marine Antifungal-Derived Compounds
3.1. New Mechanisms of Action
3.2. Anti-Virulence Therapy
3.3. Combination of Antifungal and Non-Antifungal Agents
3.3.1. Combination with Efflux Pumps Inhibitors
3.3.2. Combination with Compounds that Induce the Reactive Oxygen Species Formation
3.4. Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berbee, M.L.; James, T.Y.; Strullu-Derrien, C. Early Diverging Fungi: Diversity and Impact at the Dawn of Terrestrial Life. Annu. Rev. Microbiol. 2017, 71, 41–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossart, H.P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, G.J.; Sole, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Barlocher, F. Fungi in freshwaters: Ecology, physiology and biochemical potential. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef] [PubMed]
- Hassett, B.T.; Borrego, E.J.; Vonnahme, T.R.; Rama, T.; Kolomiets, M.V.; Gradinger, R. Arctic marine fungi: Biomass, functional genes, and putative ecological roles. ISME J. 2019, 13, 1484–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterflinger, K.; Tesei, D.; Zakharova, K. Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecol. 2012, 5, 453–462. [Google Scholar] [CrossRef]
- Bennett, J.W. Mycotechnology: The role of fungi in biotechnology. J. Biotechnol. 1998, 66, 101–107. [Google Scholar] [CrossRef]
- Kavanagh, K. Fungi: Biology and Applications, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; ISBN 978-1-119-37432-9. [Google Scholar]
- Soares-Costa, A.; Nakayama, D.G.; Andrade Lde, F.; Catelli, L.F.; Bassi, A.P.; Ceccato-Antonini, S.R.; Henrique-Silva, F. Industrial PE-2 strain of Saccharomyces cerevisiae: From alcoholic fermentation to the production of recombinant proteins. New Biotechnol. 2014, 31, 90–97. [Google Scholar] [CrossRef]
- Elabboubi, M.; Bennani, L.; Ainane, A.; Charaf, S.; Bouhadi, M.; Hamed, M.; Kouali, E.; Talbi, M.; Cherroud, S.; Tarik, A. Treatment of mycoses by essential oils: Mini Review. J. Anal. Sci. Appl. Biotechnol. 2019, 1, 35–40. [Google Scholar]
- Fuentefria, A.M.; Pippi, B.; Dalla Lana, D.F.; Donato, K.K.; de Andrade, S.F. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2018, 66, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Bruggemann, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinetti, M.; Posteraro, B.; Lass-Florl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, I.; Ayadi, A. Epidemiology of antifungal susceptibility: Review of literature. J. Mycol. Med. 2018, 28, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Monteiro, C.; Maia, M.; Faria, M.A.; Lopes, V.; Lameiras, C.; Pinheiro, D. Aspergillus Species and Antifungals Susceptibility in Clinical Setting in the North of Portugal: Cryptic Species and Emerging Azoles Resistance in A. fumigatus. Front. Microbiol. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.R.; Cadena, J.; Patterson, T.F. Overview of antifungal agents. Clin. Chest Med. 2009, 30, 203–215. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef]
- Ngo, H.X.; Garneau-Tsodikova, S.; Green, K.D. A complex game of hide and seek: The search for new antifungals. Med. Chem. Comm. 2016, 7, 1285–1306. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current Status and Future Prospects of Marine Natural Products (MNPs) as Antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine Microbial-Derived Molecules and Their Potential Use in Cosmeceutical and Cosmetic Products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; El-Sayed Hamed, A.N.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017, 126, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and Antifungal Compounds from Marine Fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, I.; Kim, S.K. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, Y.; Zhang, W.; MM Franco, C. Distribution of Saponins in the Sea Cucumber Holothuria lessoni; the Body Wall Versus the Viscera, and Their Biological Activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef] [Green Version]
- Angawi, R.F.; Bavestrello, G.; Calcinai, B.; Dien, H.A.; Donnarumma, G.; Tufano, M.A.; Paoletti, I.; Grimaldi, E.; Chianese, G.; Fattorusso, E.; et al. Aurantoside J: A New Tetramic Acid Glycoside from Theonella swinhoei. Insights into the Antifungal Potential of Aurantosides. Mar. Drugs 2011, 9, 2809–2817. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Subramani, R.; Feussner, K.D.; Aalbersberg, W. Aurantoside K, a New Antifungal Tetramic Acid Glycoside from a Fijian Marine Sponge of the Genus Melophlus. Mar. Drugs 2012, 10, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.B.; Liu, X.F.; Xu, Y.; Gan, J.H.; Jiao, W.H.; Shen, Y.; Lin, H.W. Woodylides A-C, New Cytotoxic Linear Polyketides from the South China Sea Sponge Plakortis simplex. Mar. Drugs 2012, 10, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Shaala, L.A.; Mohamed, G.A.; Badr, J.M.; Bamanie, F.H.; Ibrahim, S.R.M. Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei. Mar. Drugs 2014, 12, 1911–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Lang, J.-H.; Jiao, W.-H.; Wang, R.-P.; Peng, Y.; Song, S.-J.; Zhang, B.-H.; Lin, H.-W. Formamido-diterpenes from the South China Sea Sponge Acanthella cavernosa. Mar. Drugs 2012, 10, 1445–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.H.; Gan, J.H.; Hu, W.Z.; Lin, H.W. Aaptamine Derivatives with Antifungal and Anti-HIV-1 Activities from the South China Sea Sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Calabro, K.; Kalahroodi, E.L.; Rodrigues, D.; Diaz, C.; Cruz, M.; Cautain, B.; Laville, R.; Reyes, F.; Perez, T.; Soussi, B.; et al. Poecillastrosides, Steroidal Saponins from the Mediterranean Deep-Sea Sponge Poecillastra compressa (Bowerbank, 1866). Mar. Drugs 2017, 15, 199. [Google Scholar] [CrossRef] [Green Version]
- El Amraoui, B.; El Wahidi, M.; Fassouane, A. In vitro screening of antifungal activity of marine sponge extracts against five phytopathogenic fungi. Springerplus 2014, 3, 629. [Google Scholar] [CrossRef] [Green Version]
- El-Amraoui, B.; Biard, J.F.; Fassouane, A. Haliscosamine: A new antifungal sphingosine derivative from the Moroccan marine sponge Haliclona viscosa. Springerplus 2013, 2, 252. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Subramani, R.; Aalbersberg, W. Three bioactive sesquiterpene quinones from the Fijian marine sponge of the genus Hippospongia. Nat. Prod. Res. 2013, 27, 1488–1491. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gomez, C.; Diaz, C.; de la Cruz, M.; Perez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New Ikarugamycin Derivatives with Antifungal and Antibacterial Properties from Streptomyces zhaozhouensis. Mar. Drugs 2014, 13, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Zhang, J.; He, S.; Yan, X. New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade. Mar. Drugs 2017, 15, 132. [Google Scholar] [CrossRef] [Green Version]
- MacMillan, J.B.; Ernst-Russell, M.A.; de Ropp, J.S.; Molinski, T.F. Lobocyclamides A-C, Lipopeptides from a Cryptic Cyanobacterial Mat Containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Piotrowski, J.S.; Hou, Y.; Braun, D.; Deshpande, R.; McIlwain, S.; Ong, I.M.; Myers, C.L.; Guzei, I.A.; Westler, W.M.; et al. Forazoline A: Marine-Derived Polyketide with Antifungal In Vivo Efficacy. Angew. Chem. Int. Ed. Engl. 2014, 53, 11583–11586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpiński, T.M. Marine Macrolides with Antibacterial and/or Antifungal Activity. Mar. Drugs 2019, 17, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, M.; Sugita, T.; Kinoshita, K.; Koyama, K. Macrolides from a Marine-Derived Fungus, Penicillium meleagrinum var. viridiflavum, Showing Synergistic Effects with Fluconazole against Azole-Resistant Candida albicans. J. Nat. Prod. 2016, 79, 1208–1212. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Identification and Bioactivity of Compounds from the Fungus Penicillium sp. CYE-87 Isolated from a Marine Tunicate. Mar. Drugs 2015, 13, 1698–1709. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.B.; Wang, X.L.; Xu, W.H.; Zhang, Y.X.; Qian, Y.-S.; Zhang, J.P.; Lu, X.L.; Liu, X.Y. Eutypellenoids A-C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2018, 16, 284. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.-L.; Wang, D.; Tian, X.-Y.; Cao, F.; Li, Y.-Q.; Zhang, C.-S. Anti-Phytopathogenic and Cytotoxic Activities of Crude Extracts and Secondary Metabolites of Marine-Derived Fungi. Mar. Drugs 2018, 16, 36. [Google Scholar] [CrossRef] [Green Version]
- Haga, A.; Tamoto, H.; Ishino, M.; Kimura, E.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Pyridone Alkaloids from a Marine-Derived Fungus, Stagonosporopsis cucurbitacearum, and Their Activities against Azole-Resistant Candida albicans. J. Nat. Prod. 2013, 76, 750–754. [Google Scholar] [CrossRef]
- Liu, F.; Cai, X.L.; Yang, H.; Xia, X.K.; Guo, Z.Y.; Yuan, J.; Li, M.F.; She, Z.G.; Lin, Y.C. The Bioactive Metabolites of the Mangrove Endophytic Fungus Talaromyces sp. ZH-154 Isolated from Kandelia candel (L.) Druce. Planta Med. 2010, 76, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mándi, A.; Li, X.M.; Meng, L.H.; Kurtán, T.; Wang, B.G. Peniciadametizine A, a Dithiodiketopiperazine with a Unique Spiro[furan-2,7’-pyrazino [1,2-b] [1,2] oxazine] Skeleton, and a Related Analogue, Peniciadametizine B, from the Marine Sponge-Derived Fungus Penicillium adametzioides. Mar. Drugs 2015, 13, 3640–3652. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Lin, X.; Han, L.; Ma, J.; Ma, Q.; Zhong, J.; Liu, Y.; Sun, T.; Wang, J.; Huang, X. New Metabolites and Bioactive Chlorinated Benzophenone Derivatives Produced by a Marine-Derived Fungus Pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.H.; Zou, Z.R.; Yi, Y.H.; Han, H.; Li, L.; Pan, M.X. Variegatusides: New Non-Sulphated Triterpene Glycosides from the Sea Cucumber Stichopus variegates Semper. Mar. Drugs 2014, 12, 2004–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattab, R.A.; Elbandy, M.; Lawrence, A.; Paget, T.; Rae-Rho, J.; Binnaser, Y.S.; Ali, I. Extraction, Identification and Biological Activities of Saponins in Sea Cucumber Pearsonothuria graeffei. Combinatorial Chem. High. Throughput Screen 2018, 21, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Tidgewell, K.; Clark, B.R.; Gerwick, W.H. The Natural Products Chemistry of Cyanobacteria. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; Volume 2, pp. 141–188. ISBN 978-140-108-045382-045388. [Google Scholar]
- Singh, P.; Yadav, R.; Pandey, S.; Bhunia, S.S. Past, Present, and Future of Antifungal Drug Development. In Topics in Medicinal Chemistry; Bernstein, P.R., Garner, A.L., Georg, G.I., Lowe, J.A., Meanwell, N.A., Saxena, A.K., Supuran, C.T., Zhang, A., Eds.; Springer: Cham, Switzerland, 2016; Volume 29, pp. 125–167. ISBN 978-123-319-78254-78256. [Google Scholar]
- Pettit, R.K.; Pettit, G.R.; Hazen, K.C. Specific Activities of Dolastatin 10 and Peptide Derivatives against Cryptococcus neoformans. Antimicrob. Agents Chemother. 1998, 42, 2961–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila, T.; Romo, J.A.; Pierce, C.G.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Targeting Candida albicans filamentation for antifungal drug development. Virulence 2017, 8, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Sun, C.; Zhang, C.; Song, S.; Sun, X.; Ju, J.; Deng, Y. Efficacy of Compounds Isolated from Streptomyces olivaceus against the Morphogenesis and Virulence of Candida albicans. Mar. Drugs 2019, 17, 442. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Costa, E.; Silva, S.; Tavaria, F.; Pintado, M. Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans. Pathogens 2014, 3, 908–919. [Google Scholar] [CrossRef]
- Inamdar, N.; Mourya, V.K.; Tiwari, A. Carboxymethyl Chitosan and Its Applications. Ad. Mat. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Kurniasih, M.; Cahyati, T.; Dewi, R.S. Carboxymethyl chitosan as an antifungal agent on gauze. Int. J. Biol. Macromol. 2018, 119, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.H.; Ma, Y.Y.; Ding, Y.; Chen, X.Q.; Gao, G.X. An insight into new strategies to combat antifungal drug resistance. Drug Des. Devel. Ther. 2018, 12, 3807–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Y.; Liu, A.; Zheng, Y.; Ye, B. In vitro damage of Candida albicans biofilms by chitosan. Exp. Ther. Med. 2014, 8, 929–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, D.G. Novel Approaches for Efficient Antifungal Drug Action. J. Microbiol. Biotechnol. 2018, 28, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, Y.; Chen, X.; Gao, Y.; Li, H.; Sun, S. Combination of fluconazole with non-antifungal agents: A promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int. J. Antimicrob. Agents 2014, 43, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting Antimicrobial Drug Resistance with Marine Natural Products. Int. J. Antimicrob. Agents 2020, 56, 106005. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Lamping, E.; Adachi, K.; Takano, Y.; Kawabata, K.; Shizuri, Y.; Niimi, M.; Uehara, Y. Inhibition of fungal ABC transporters by unnarmicin A and unnarmicin C, novel cyclic peptides from marine bacterium. Biochem. Biophys. Res. Commun. 2007, 364, 990–995. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Pierce, C.G.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. From Biology to Drug Development: New Approaches to Combat the Threat of Fungal Biofilms. Microbiol. Spectr. 2015, 3, 373–388. [Google Scholar] [CrossRef] [Green Version]
- Bink, A.; Vandenbosch, D.; Coenye, T.; Nelis, H.; Cammue, B.P.; Thevissen, K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 2011, 55, 4033–4037. [Google Scholar] [CrossRef] [Green Version]
- De Cremer, K.; De Brucker, K.; Staes, I.; Peeters, A.; Van den Driessche, F.; Coenye, T.; Cammue, B.P.; Thevissen, K. Stimulation of superoxide production increases fungicidal action of miconazole against Candida albicans biofilms. Sci. Rep. 2016, 6, 27463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delattin, N.; Cammue, B.P.; Thevissen, K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans Biofilms Produce Antifungal-Tolerant Persister Cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal Susceptibility of Candida Biofilms: Unique Efficacy of Amphotericin B Lipid Formulations and Echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, B.; Cheng, S.; Clancy, C.J.; Nguyen, M.H. Caspofungin Kills Candida albicans by Causing both Cellular Apoptosis and Necrosis. Antimicrob. Agents Chemother. 2013, 57, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibane, V.S.; Kock, J.L.; Ells, R.; van Wyk, P.W.; Pohl, C.H. Effect of marine polyunsaturated fatty acids on biofilm formation of Candida albicans and Candida dubliniensis. Mar. Drugs 2010, 8, 2597–2604. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula, E.S.A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, M.; Kalaivani, R.; Manikandan, S.; Kumaraguru, A.K. Metallic silver nanoparticle: A therapeutic agent in combination with antifungal drug against human fungal pathogen. Bioprocess. Biosyst. Eng. 2013, 36, 407–415. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]




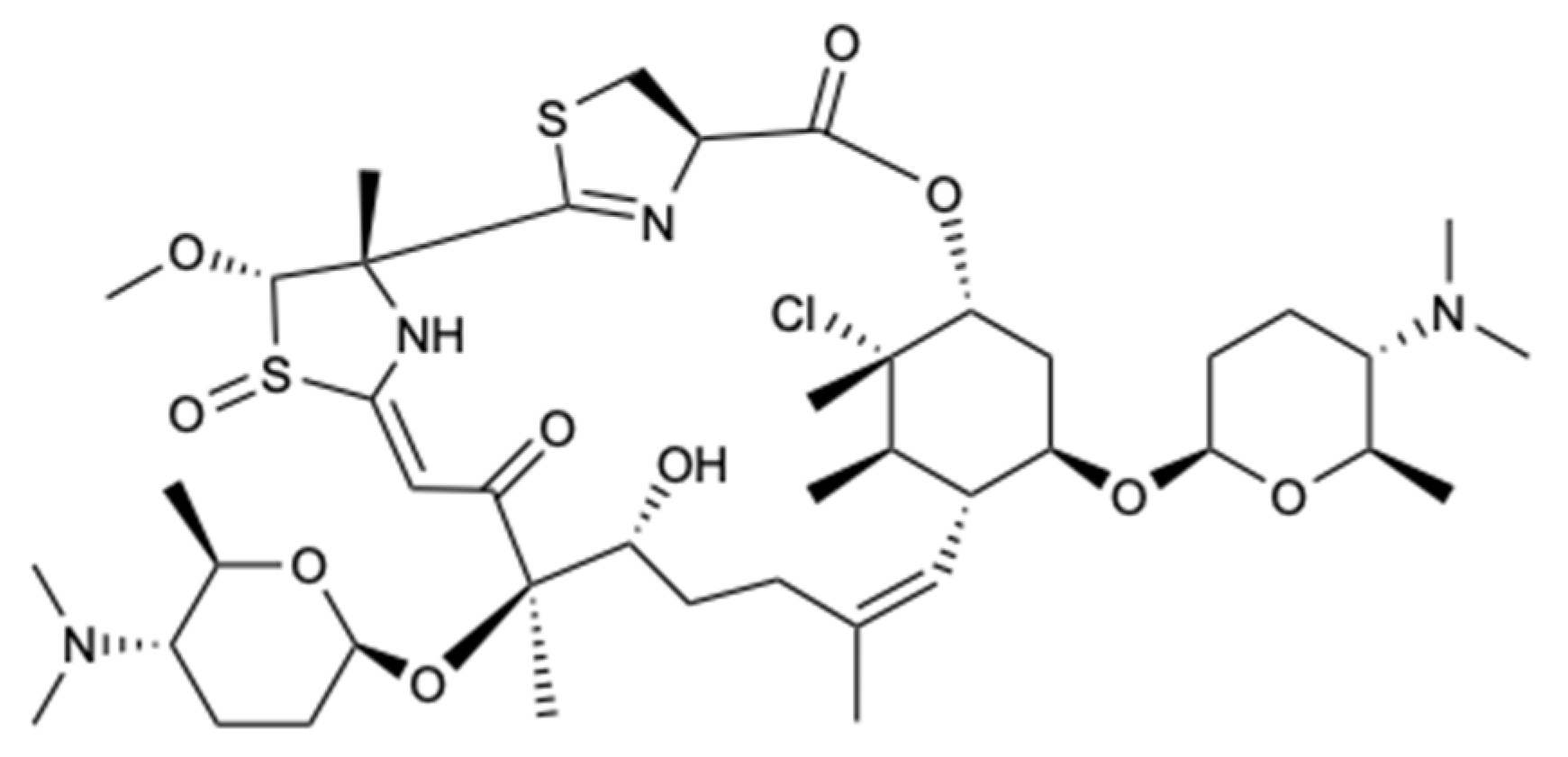


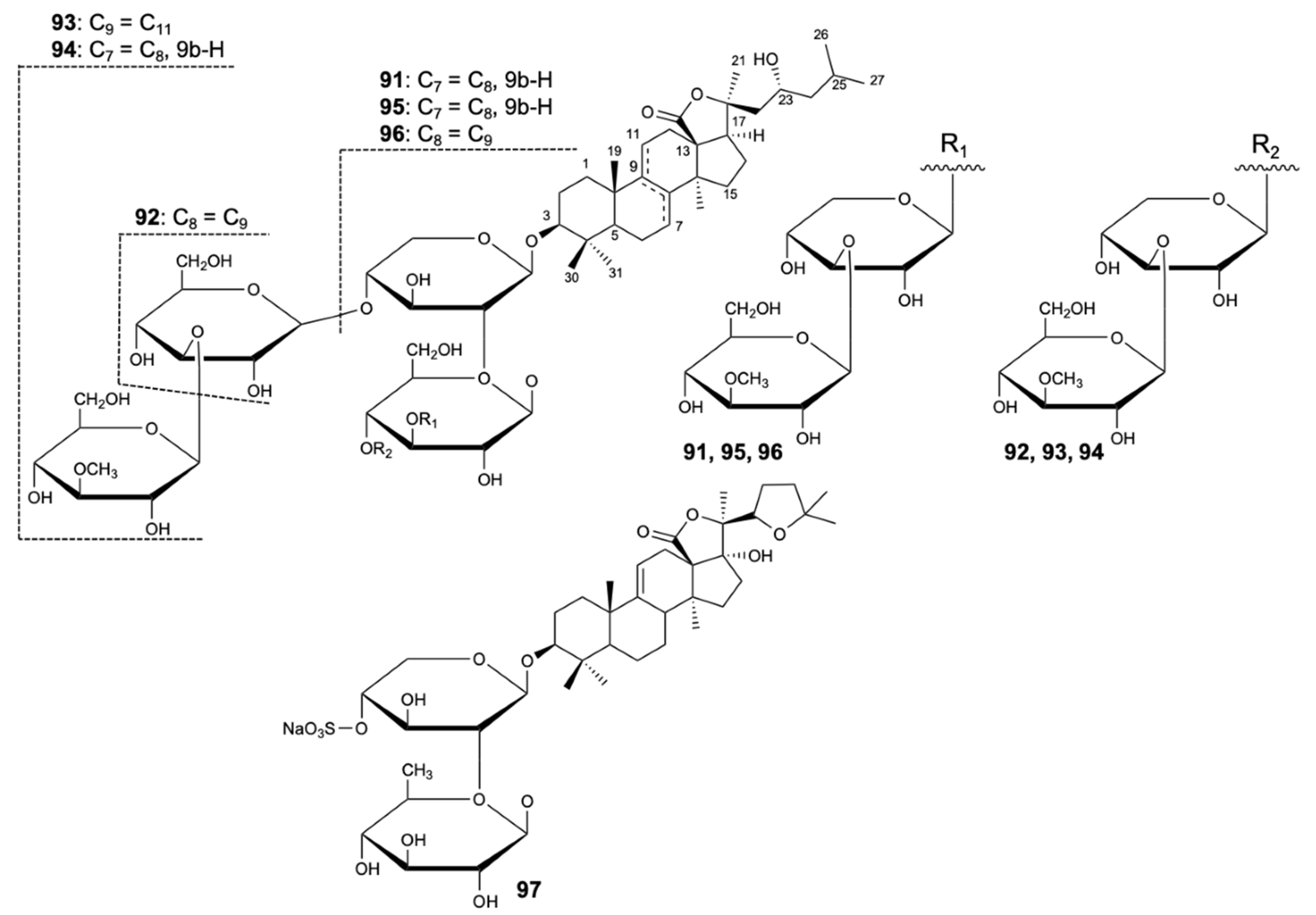



| Compound | Chemical Class | Source | Activity | References |
|---|---|---|---|---|
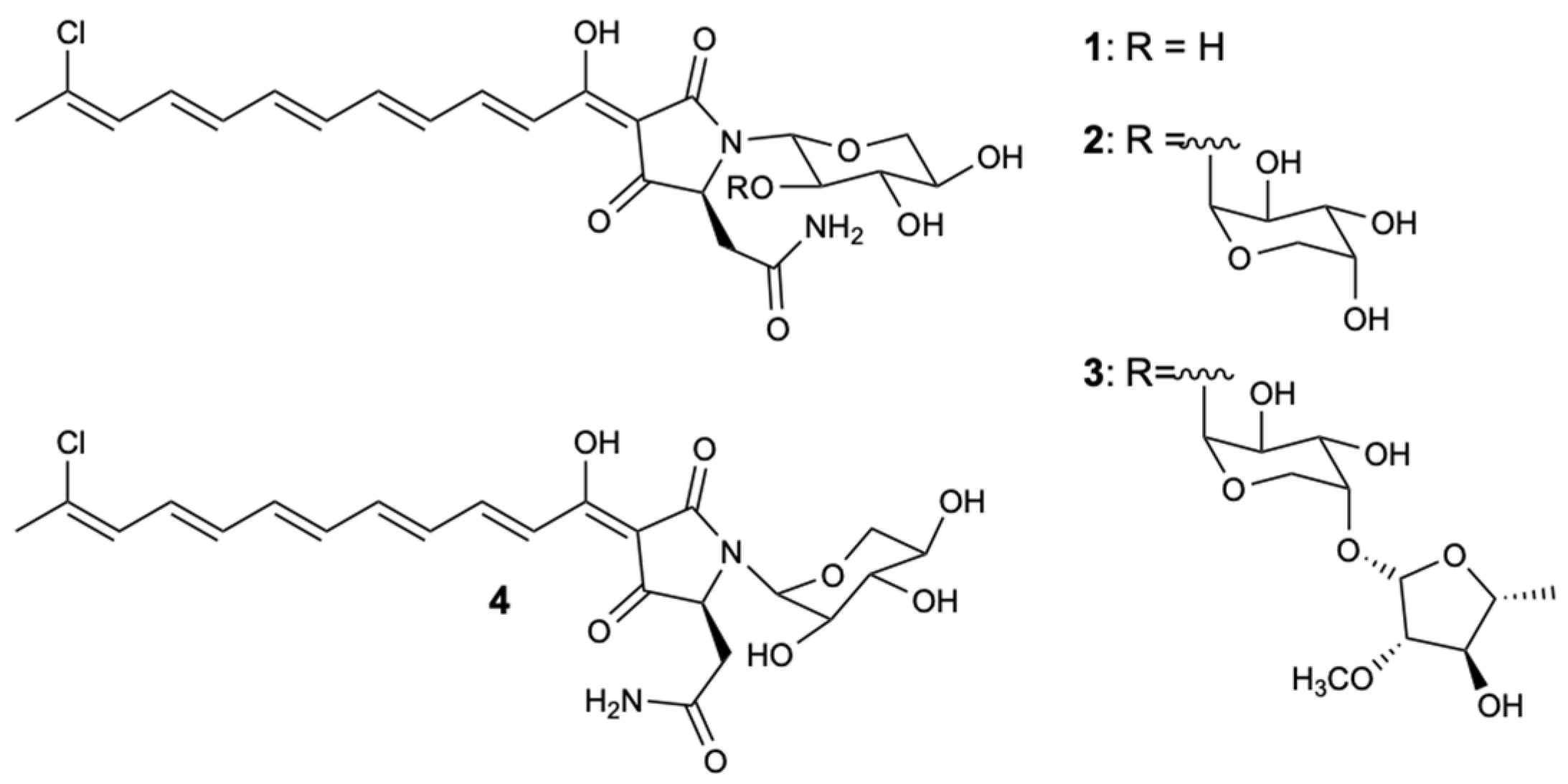
| Aurantoside G (1) | Peptide | Sponge Theonella swinhoei | C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis (MIC90 8, 8, 4, and 4 mg/L) | [30] |
| Aurantoside I (3) | Peptide | Sponge Theonella swinhoei | C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and F. solani (MIC90 0.5, 0.125, 0.5, 0.5, and 2 mg/L) | [30] |
| Aurantoside K (5) | Peptide | Sponge Meophlus sp. | AmB-R and WT C. albicans (MIC 31.25 and 1.95 mg/L); C. neoformans, A. niger, Rhizopus sporangia, Penicillium sp., and Sordaria sp. (Ø inhibition 14, 28, 21, 31, and 29 mm) | [31] |
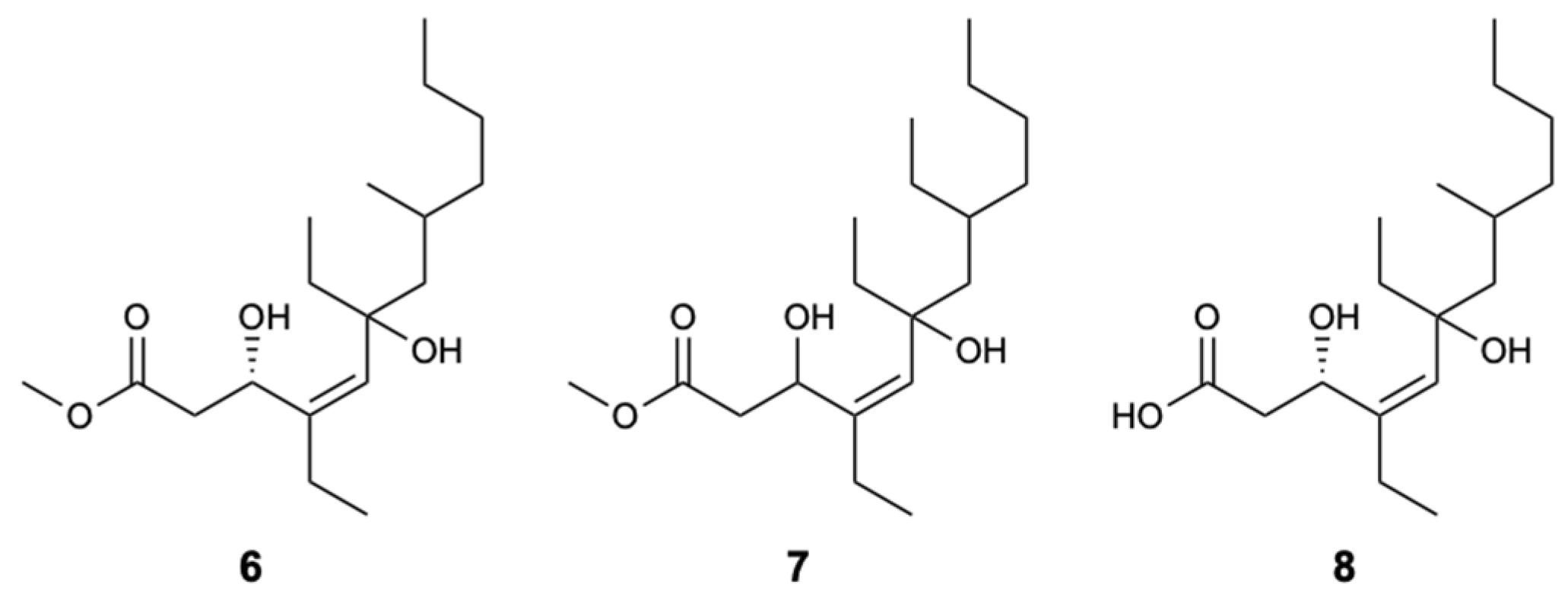
| Woodylide A (6) | Polyketide | Sponge Plakortis simplex | C. neoformans (IC50 3.67 mg/L) C. albicans, N. gypsea, and T. rubrum (MIC 32 mg/L) | [32] |
| Woodylide C (8) | Polyketide | Sponge Plakortis simplex | C. neoformans (IC50 10.85 mg/L); N. gypsea and T. rubrum (MIC 32 mg/L) | [32] |
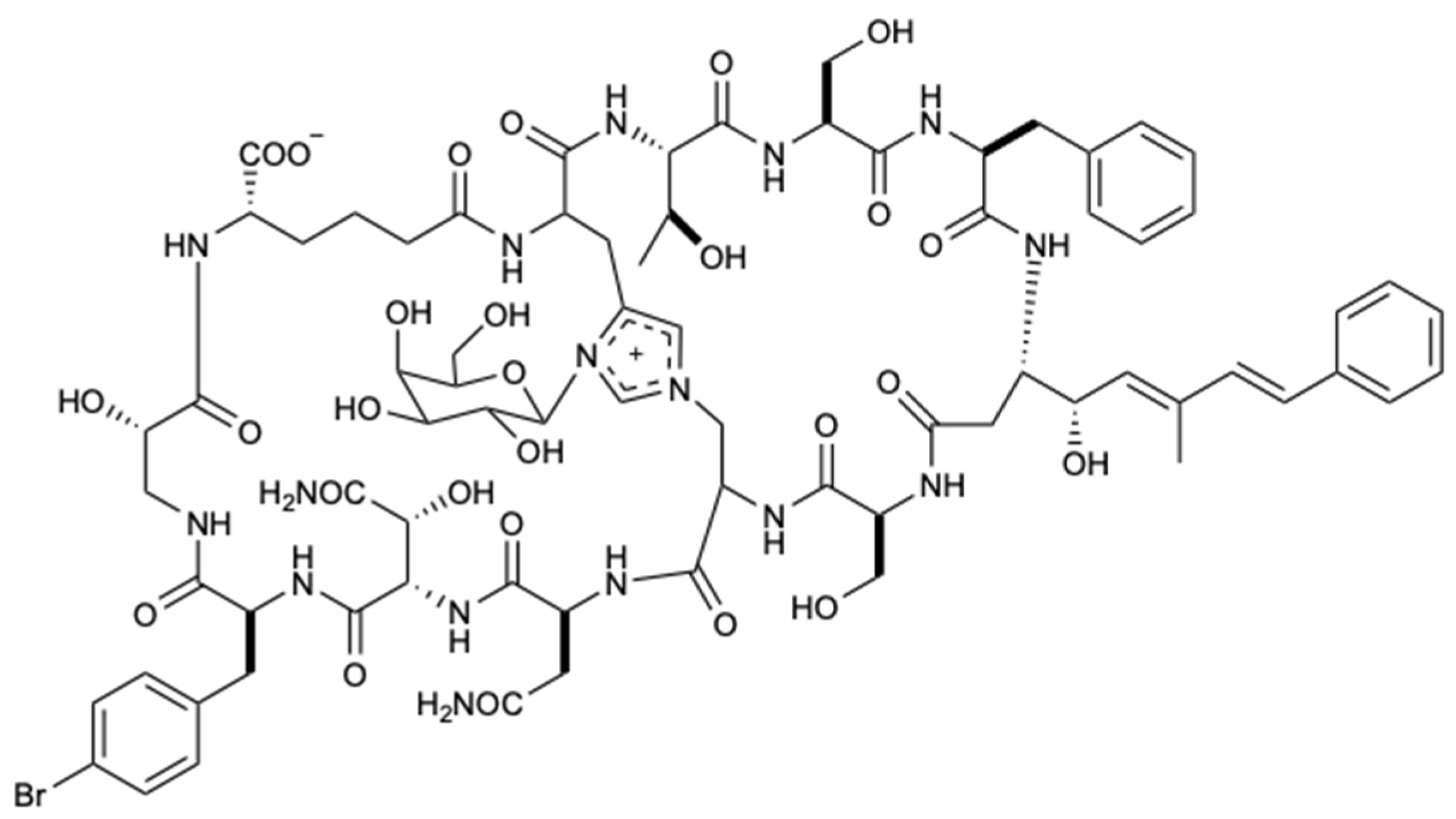
| Theonellamide G (9) | Peptide | Sponge Theonella swinhoei | AmB-R and WT C. albicans (IC50 4.49 and 2.0 µM) | [33] |
| 15-Formamido-kalihinene (18) | Terpene | Sponge Acanthella cavernosa | N. gypsea and T. rubrum (MIC 8 and 32 mg/L) | [34] |
| 10-Formamido-kalihinene (19) | Terpene | Sponge Acanthella cavernosa | C. albicans, C. neoformans, N. gypsea, and T. rubrum (MIC 8, 8, 8, and 4 mg/L) | [34] |
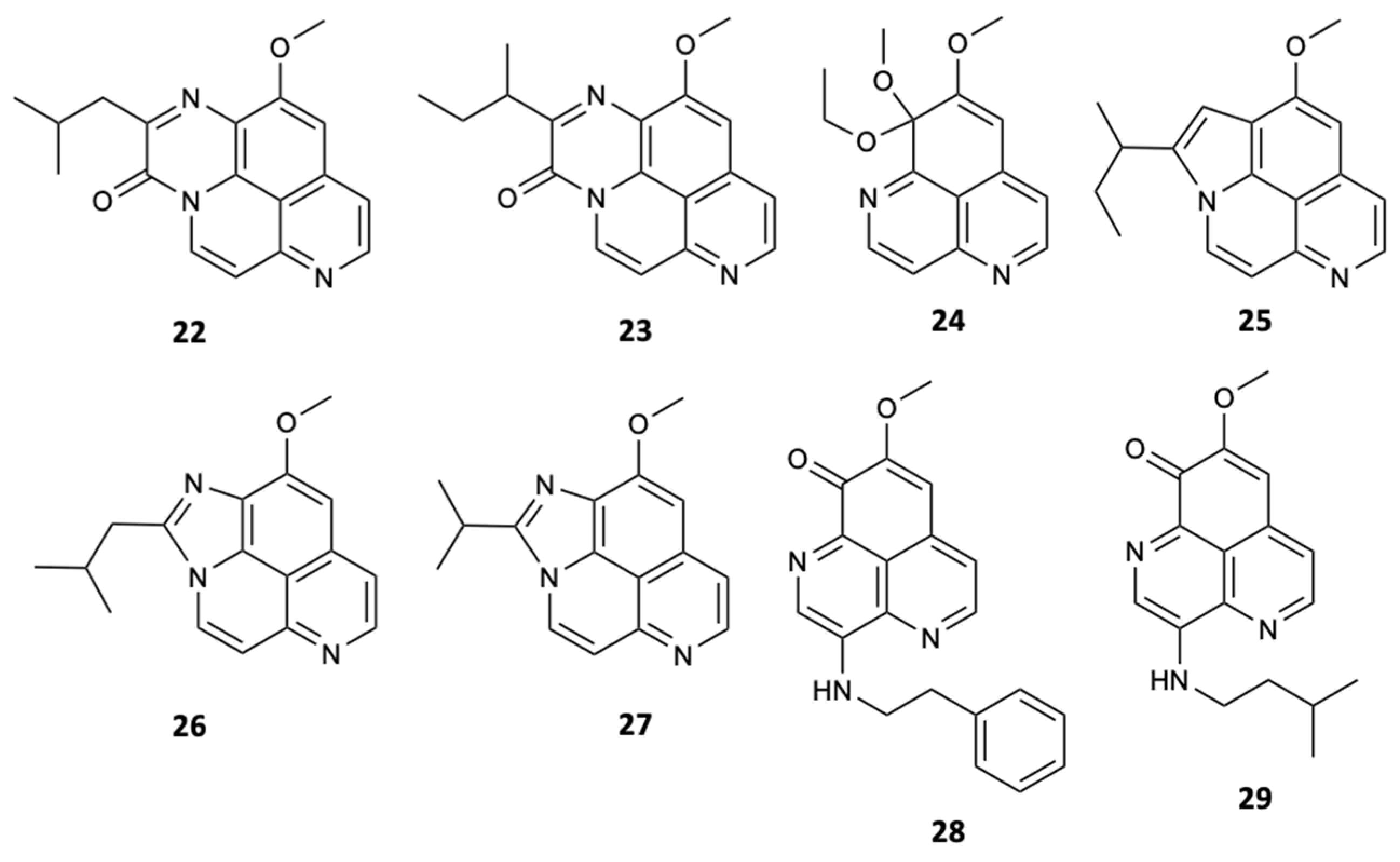
| Aaptamine (24) | Alkaloid | Sponge Aaptos aaptos | C. parapsilosis (MIC 32 mg/L) | [35] |
| Aaptamine (25) | Alkaloid | Sponge Aaptos aaptos | N. gypsea (MIC 64 mg/L) | [35] |
| Aaptamine (26) | Alkaloid | Sponge Aaptos aaptos | N. gypsea (MIC 64 mg/L) | [35] |
| Aaptamine (27) | Alkaloid | Sponge Aaptos aaptos | N. gypsea (MIC 64 mg/L) | [35] |
| Aaptamine (28) | Alkaloid | Sponge Aaptos aaptos | C. albicans, C. parapsilosis, C. neoformans, N. gypsea, and T. rubrum (MIC 32, 64, 32, 16, and 4 mg/L) | [35] |
| Aaptamine (29) | Alkaloid | Sponge Aaptos aaptos | C. neoformans, N. gypsea, and T. rubrum (MIC 64, 32, and 8 mg/L) | [35] |
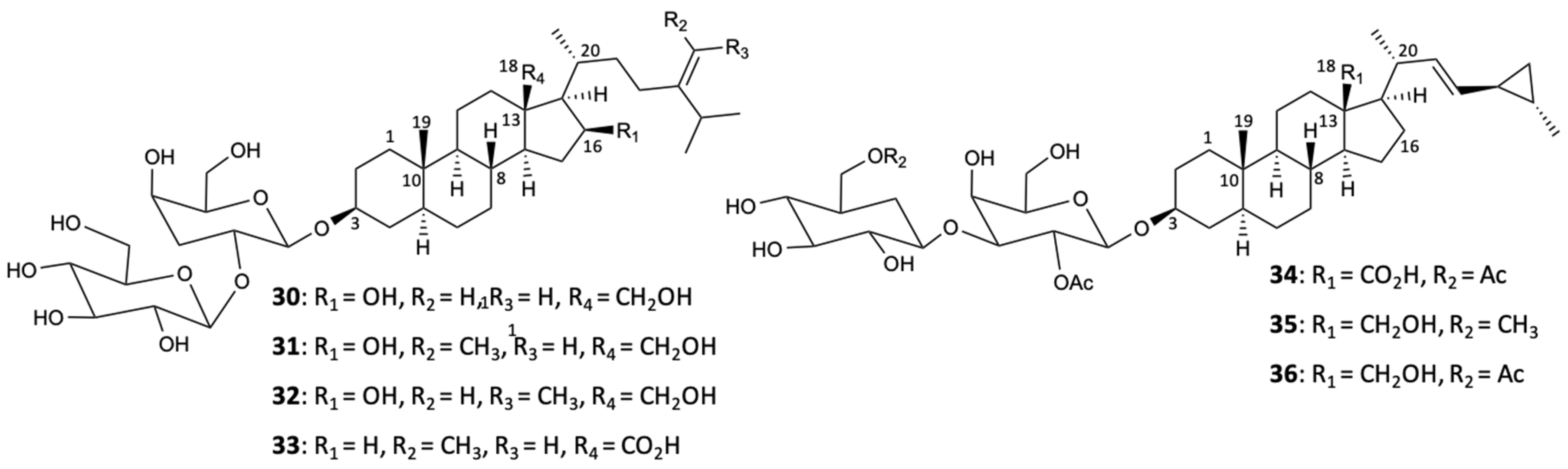
| Poecillastroside D (33) | Steroid | Sponge Poecillastra compressa | A. fumigatus (MIC90 6 mg/L) | [36] |
| Poecillastroside E (34) | Steroid | Sponge Poecillastra compressa | A. fumigatus (MIC90 24 mg/L) | [36] |
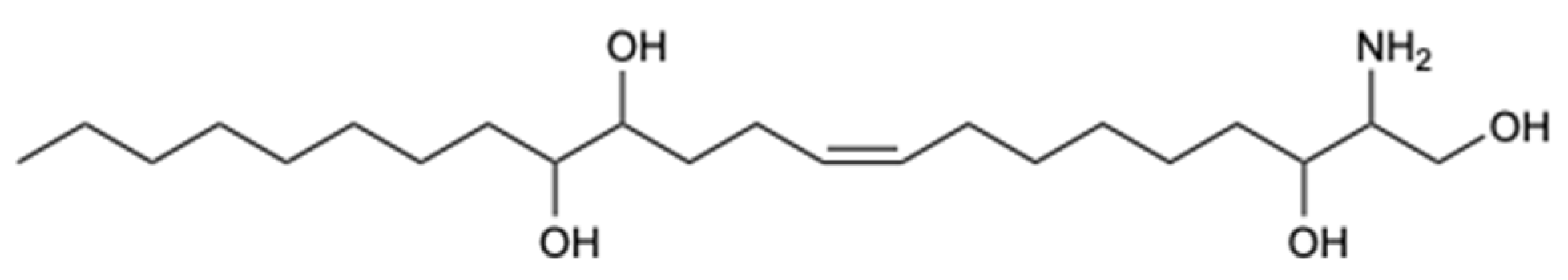
| Haliscosamine (37) | Polyketide | Sponge Haliclona viscosa | C. albicans, C. tropicalis, and C. neoformans (MIC90 0.4–0.8, 0.4–0.8, and 0.2–0.4 mg/L) | [38] |
| Epi-ilimaquinone (38) | Polyketide | Sponge Hippospongia sp. | AmB-R C. albicans (MIC 125 mg/L) | [39] |
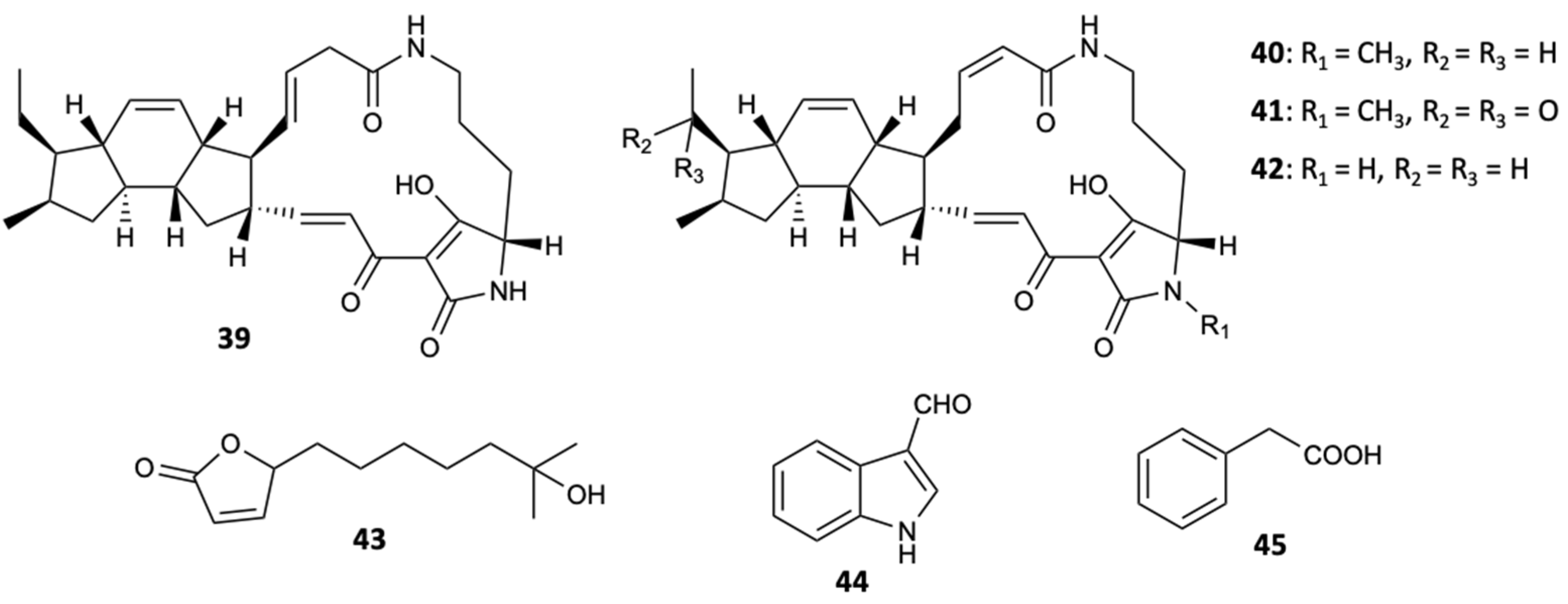
| Isoikarugamycin (39) | Polyketide | Bacteria Streptomyces zhaozhouensis | C. albicans and A. fumigatus (MIC 2–4 and 4–8 mg/L) | [40] |
| 28-N-methylikaguramycin (40) | Polyketide | Bacteria Streptomyces zhaozhouensis | C. albicans and A. fumigatus (MIC 4 and 4–8 mg/L) | [40] |
| Ikarugamycin (42) | Polyketide | Bacteria Streptomyces zhaozhouensis | C. albicans and A. fumigatus (MIC 4 and 4–8 mg/L) | [40] |
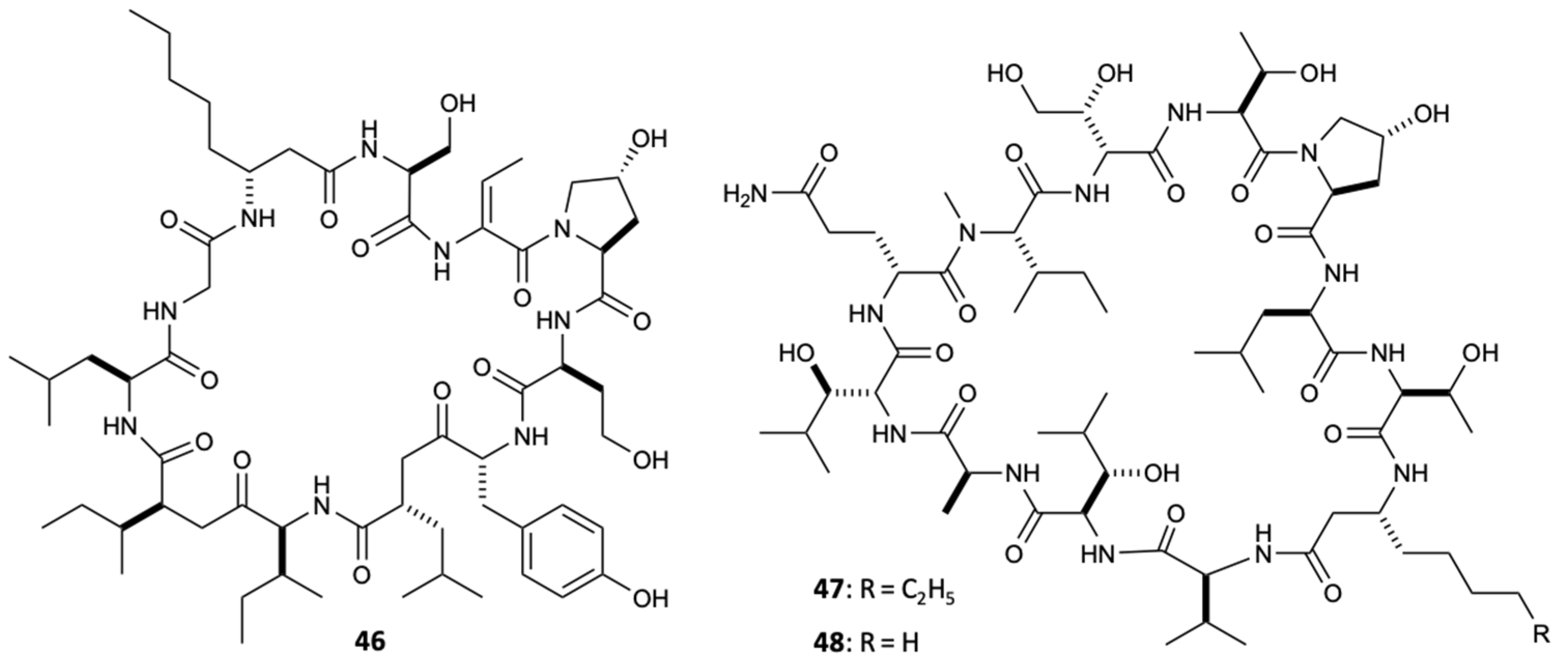
| Lobocyclamide A (46) | Peptide | Cyanobacterium Lyngbya confervoides | C. albicans (MIC 91 mg/L) Synergism with mixture of 46 and 47 against C. albicans (MIC 10–30 mg/L) | [42] |
| Lobocyclamide B (47) | Peptide | Cyanobacterium Lyngbya confervoides | C. albicans (MIC 30-100 mg/L) Synergism with mixture of 46 and 47 against C. albicans (MIC 10–30 mg/L) | [42] |
| Forazoline A (49) | Polyketide | Bacteria Actinomadura spp. | C. albicans (MIC < 16 mg/L); Synergism with AmB | [43] |
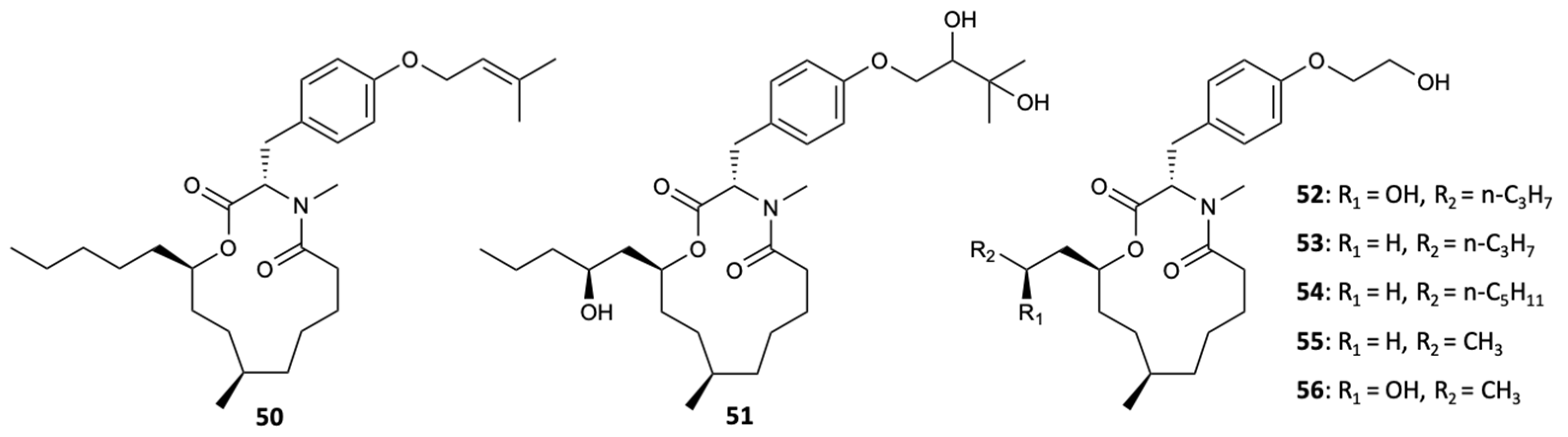
| PF1163A (52) | Polyketide | Fungus Penicillium meleagrinum var. viridiflavum | Azole-resistant C. albicans (MIC 1 mg/L) Synergism with fluconazole against the azole-resistant C. albicans | [45] |
| PF1163B (53) | Polyketide | Fungus Penicillium meleagrinum var. viridiflavum | Azole-resistant C. albicans (MIC 2 mg/L) Synergism with fluconazole against the azole-resistant C. albicans | [45] |
| PF1163H (55) | Polyketide | Fungus Penicillium meleagrinum var. viridiflavum | Azole-resistant C. albicans (MIC 16 mg/L) Synergism with fluconazole against the azole-resistant C. albicans | [45] |
| PF1163F (56) | Polyketide | Fungus Penicillium meleagrinum var. viridiflavum | Azole-resistant C. albicans (MIC 8 mg/L) Synergism with fluconazole against the azole-resistant C. albicans | [45] |
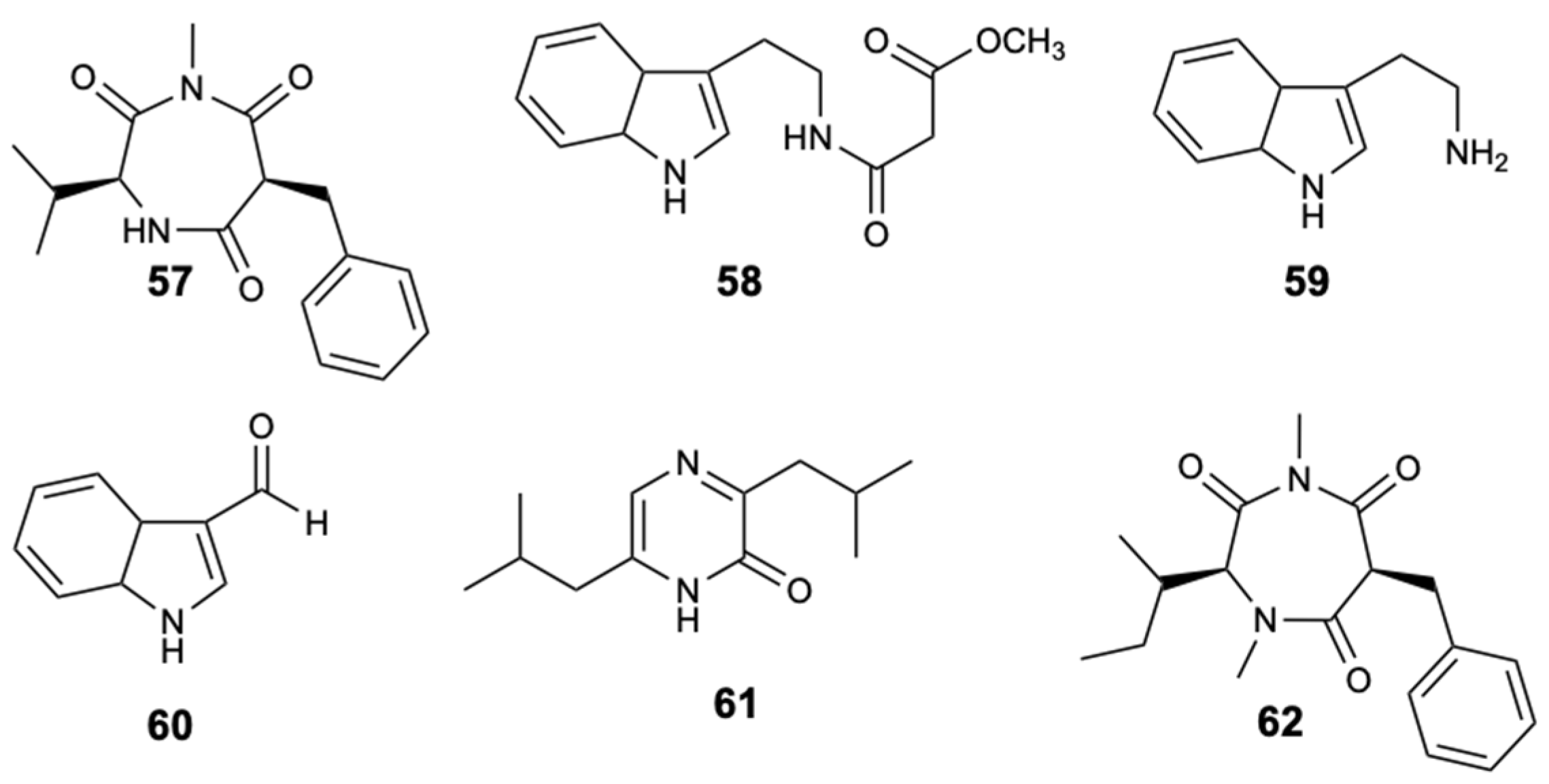
| Terretrione D (57) | Alkaloid | Fungus Penicillium sp. CYE-87 | C. albicans (Ø inhibition 17 mm and MIC 32 mg/L) | [46] |
| Terretrione C (62) | Alkaloid | Fungus Penicillium sp. CYE-87 | C. albicans (Ø inhibition 19 mm and MIC 32 mg/L) | [46] |
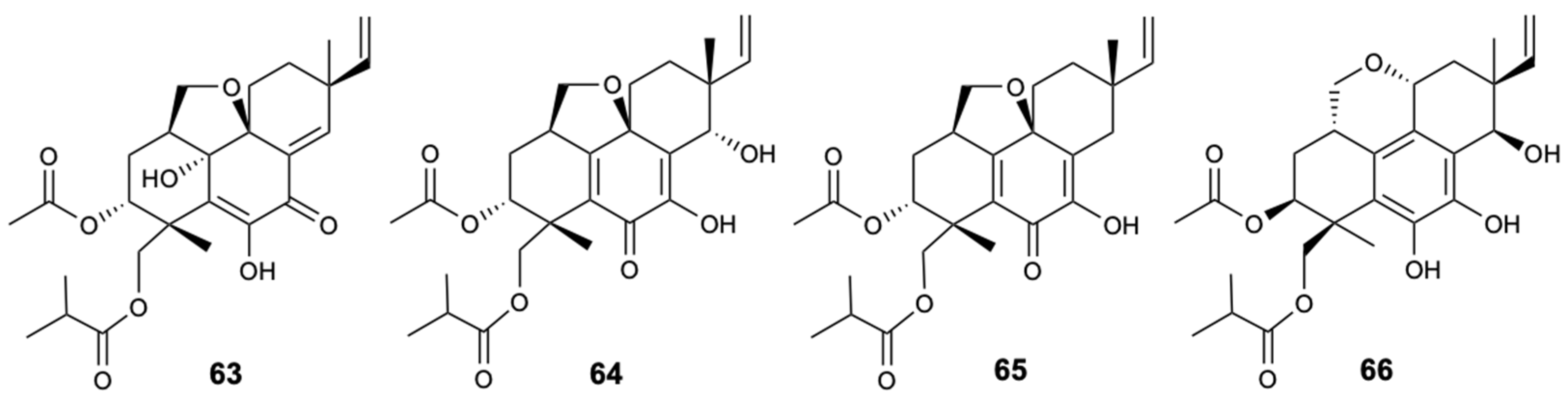
| Eutypellenoid A (63) | Terpene | Fungus Eutypella sp. D-1 | C. albicans, C. glabrata, C. parapsilosis and C. tropicalis (MIC 8, 16, 8, and 32 mg/L) | [47] |
| Anthraquinone (67) | Polyketide | Fungus Fusarium equiseti | Pestallozzia theae (MIC 31.3 mg/L) | [48] |
| Anthraquinone (68) | Polyketide | Fungus Fusarium equiseti | Pestallozzia theae (MIC 31.3 mg/L) | [48] |
| Stemphyperlenol (69) | Polyketide | Fungus Alternaria sp. | Pestallozzia theae and Alternaria brassicicola (MIC 7.81 and 125 mg/L) | [48] |
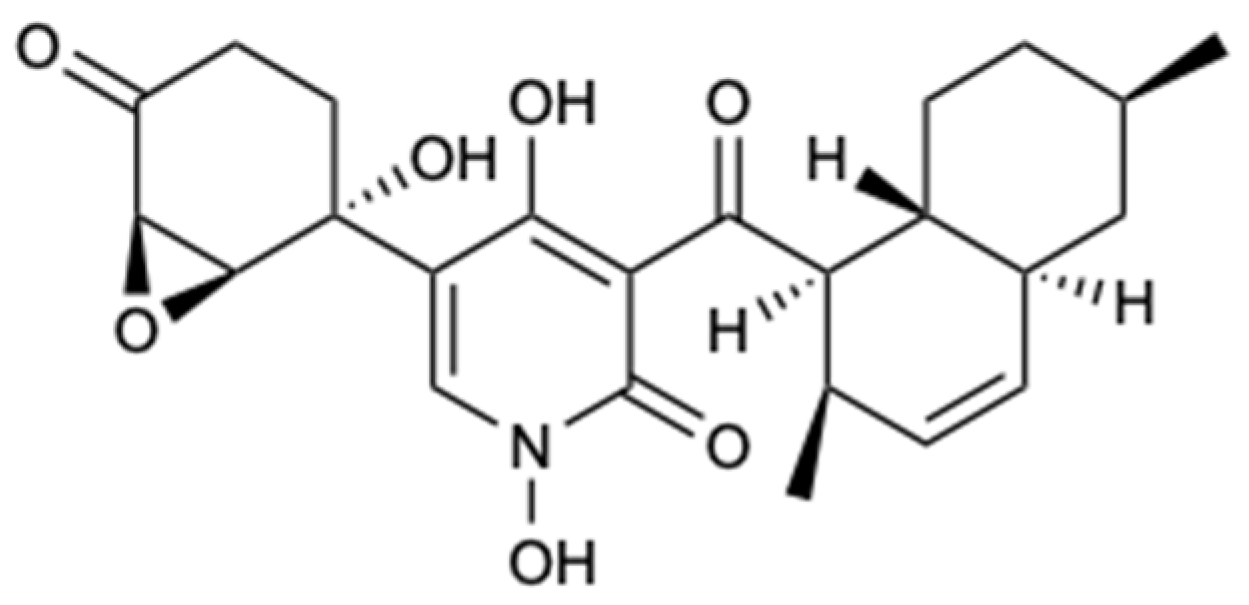
| Didymellamide A (71) | Alkaloid | Fungus Stagonosporopsis cucurbitacearum | Azole-resistant C. albicans J2-36, azole-sensitive C. albicans J1-97, C. glabrata J-92, and C. neoformans Mpu-B (MIC 3.1, 3.1, 3.1, and 1.6 mg/L) | [49] |
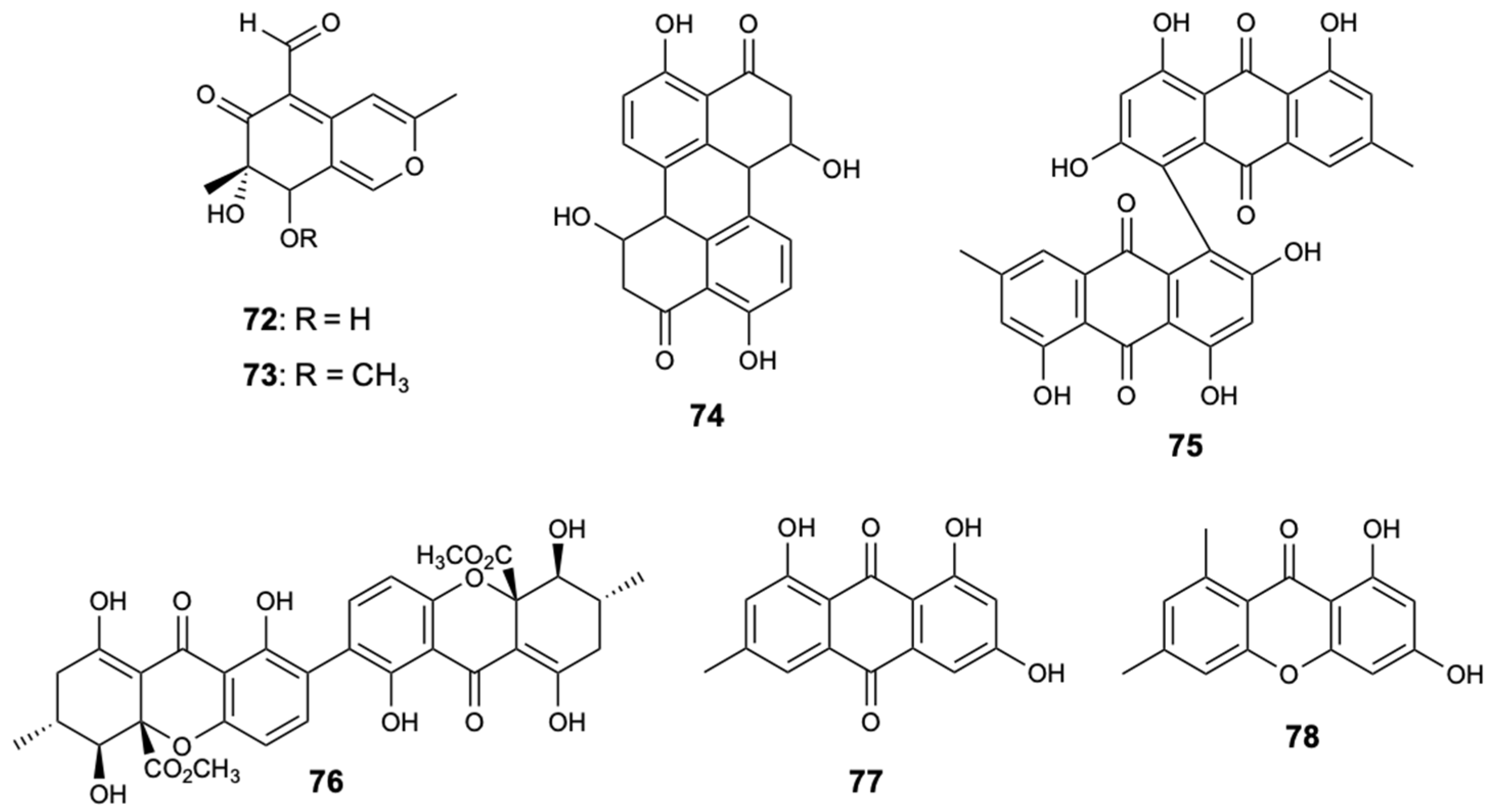
| Secalonic acid A (76) | Polyketide | Fungus Talaromyces sp. ZH-154 | C. albicans, A. niger and F. oxysporum f. sp. cubense (MIC 6.25, 6.25, and 12.5 mg/L) | [50] |
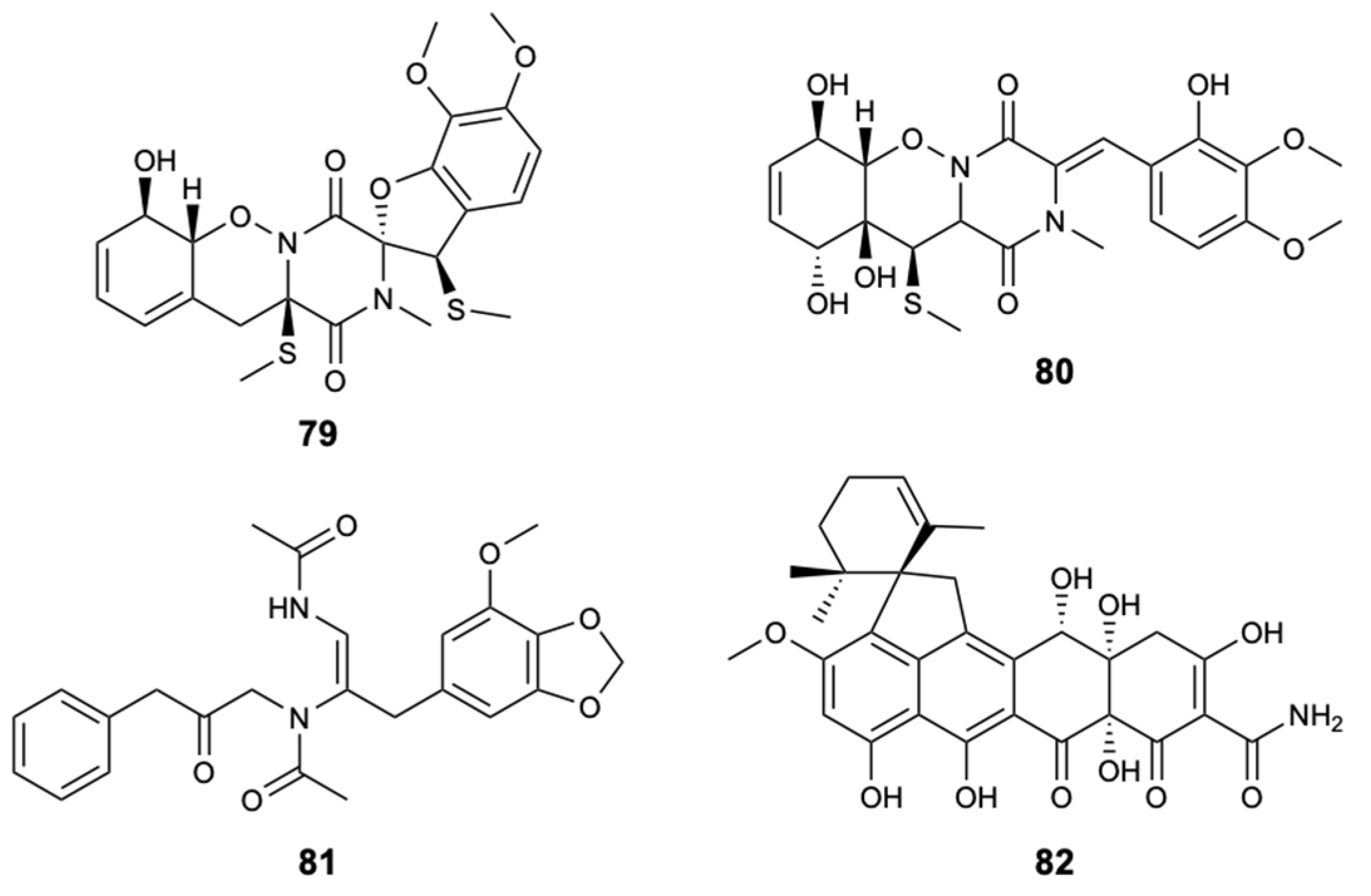
| Peniciadametizine A (79) | Polyketide | Fungus Penicillium adametzioides AS-53 | Alternaria brassicae (MIC 4.0 mg/L) | [51] |
| Peniciadametizine B (80) | Polyketide | Fungus Penicillium adametzioides AS-53 | Alternaria brassicae (MIC 32 mg/L) | [51] |
| Pestaloisocoumarin A (83) | Lactone | Fungus Pestalotiopsis heterocornis | C. albicans, C. parapsilosis, and C. neoformans (MIC 100 mg/L) | [52] |
| Pestaloisocoumarin B (84) | Lactone | Fungus Pestalotiopsis heterocornis | C. neoformans (MIC 100 mg/L) | [52] |
| Gamahorin (87) | Lactone | Fungus Pestalotiopsis heterocornis | C. parapsilosis and C. neoformans (MIC 100 mg/L) | [52] |
| Variegatuside D (92) | Terpene | Sea cucumber Stichopus variegates | C. albicans, C. parapsilosis, C. tropicalis, C. pseudotropicalis, C. neoformans, and N. gypsea (MIC80 3.4, 3.4, 13.6, 3.4, 6.8, and 3.4 mg/L) | [53] |
| Variegatuside E (93) | Terpene | Sea cucumber Stichopus variegates | C. albicans, C. parapsilosis, C. tropicalis, C. pseudotropicalis, C. neoformans, and N. gypsea (MIC80 25, 12.5, 12.5, 12.5, 12.5, and 12.5 mg/L) | [53] |
| Holothurin A (98) | Terpene | Sea cucumber Pearsontrhuria graeffei | C. albicans (24 h LC50 = 10 mg/L) | [54] |
| Echinoside A (99) | Terpene | Sea cucumber Pearsontrhuria graeffei | C. albicans (24 h LC50 = 10 mg/L) | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, J.; Nakayama, D.G.; Sousa, E.; Pinto, E. Marine-Derived Compounds and Prospects for Their Antifungal Application. Molecules 2020, 25, 5856. https://doi.org/10.3390/molecules25245856
Cardoso J, Nakayama DG, Sousa E, Pinto E. Marine-Derived Compounds and Prospects for Their Antifungal Application. Molecules. 2020; 25(24):5856. https://doi.org/10.3390/molecules25245856
Chicago/Turabian StyleCardoso, Joana, Darlan Gonçalves Nakayama, Emília Sousa, and Eugénia Pinto. 2020. "Marine-Derived Compounds and Prospects for Their Antifungal Application" Molecules 25, no. 24: 5856. https://doi.org/10.3390/molecules25245856







