Effect of the Content and Ordering of the sp2 Free Carbon Phase on the Charge Carrier Transport in Polymer-Derived Silicon Oxycarbides
Abstract
:1. Introduction
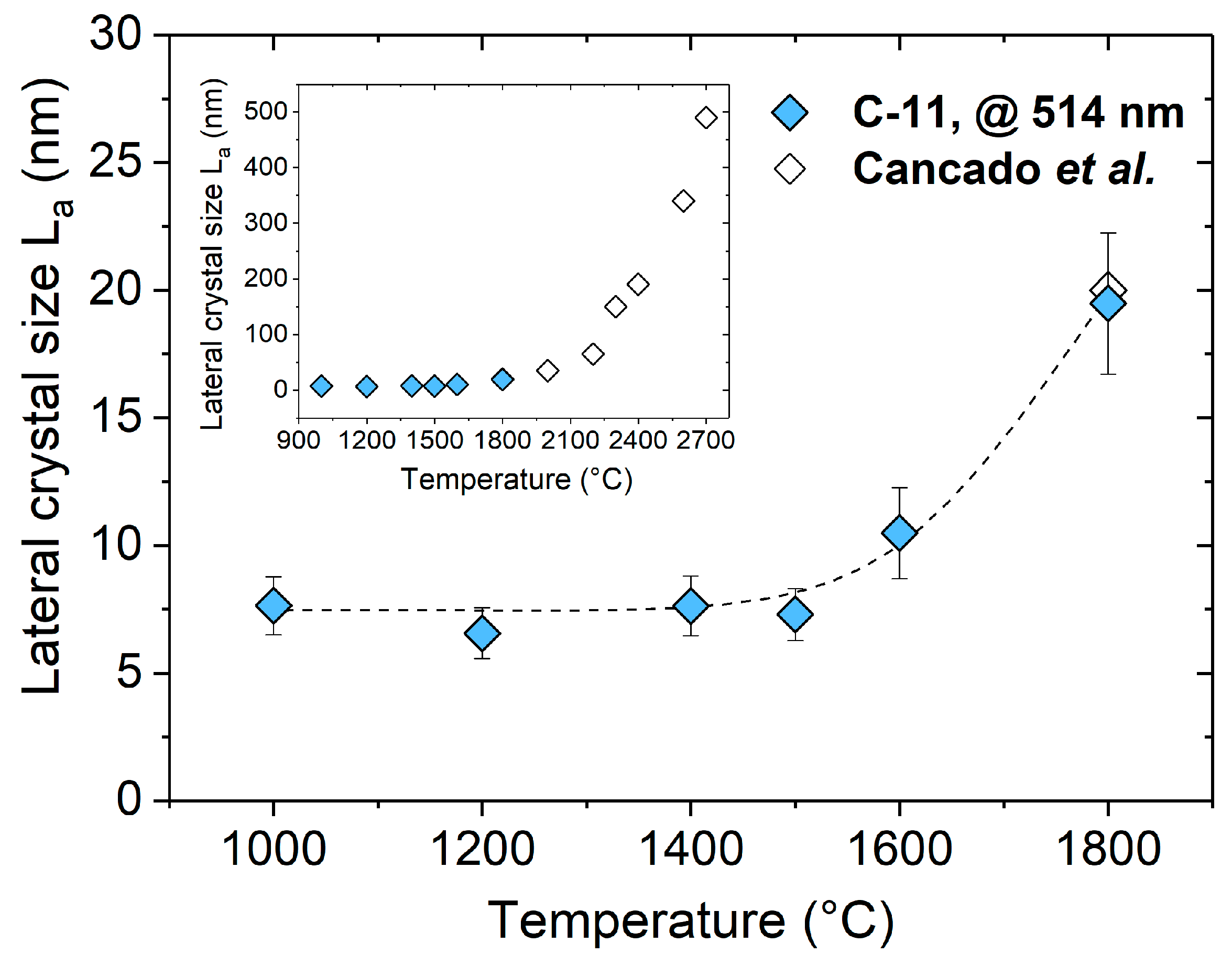
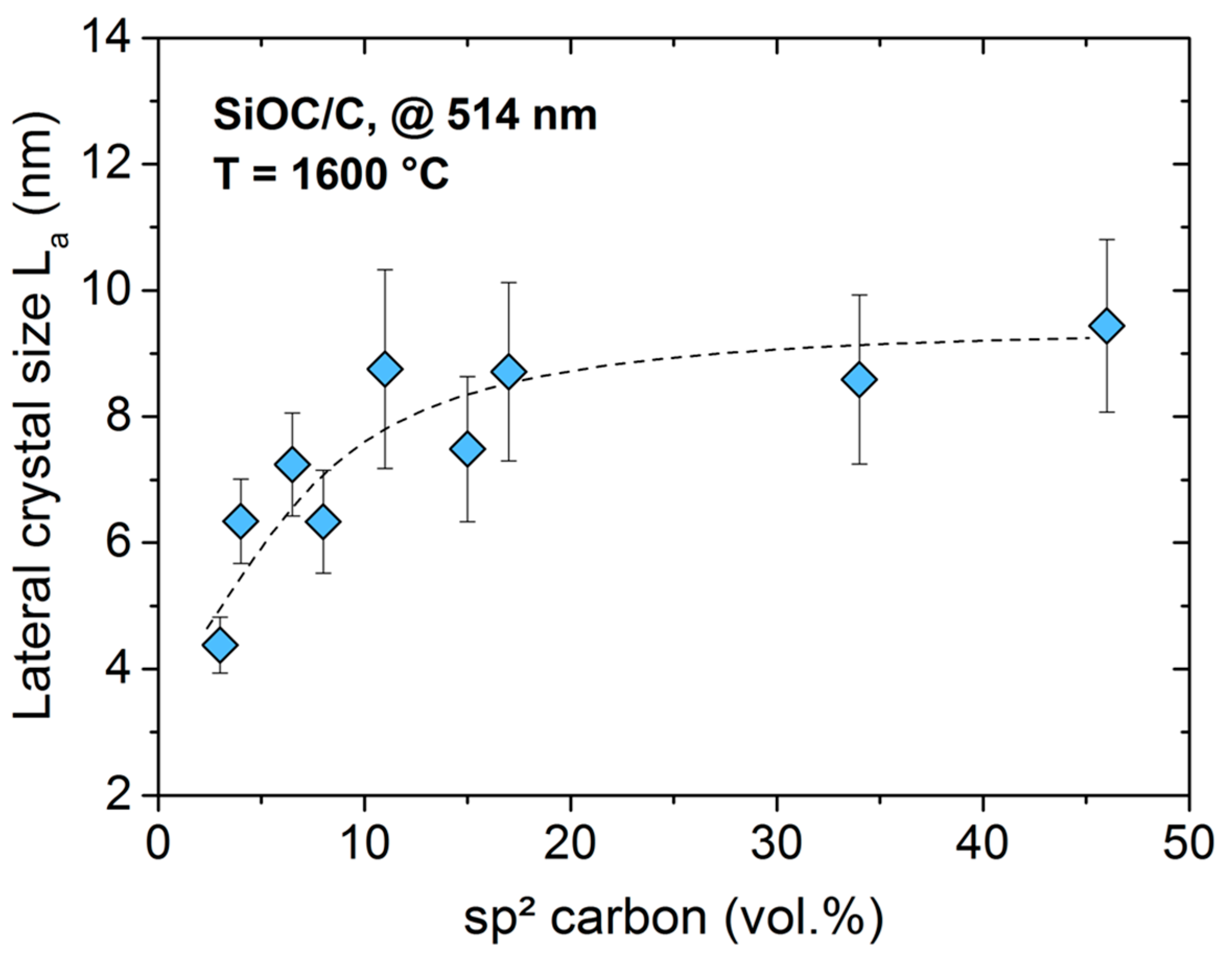
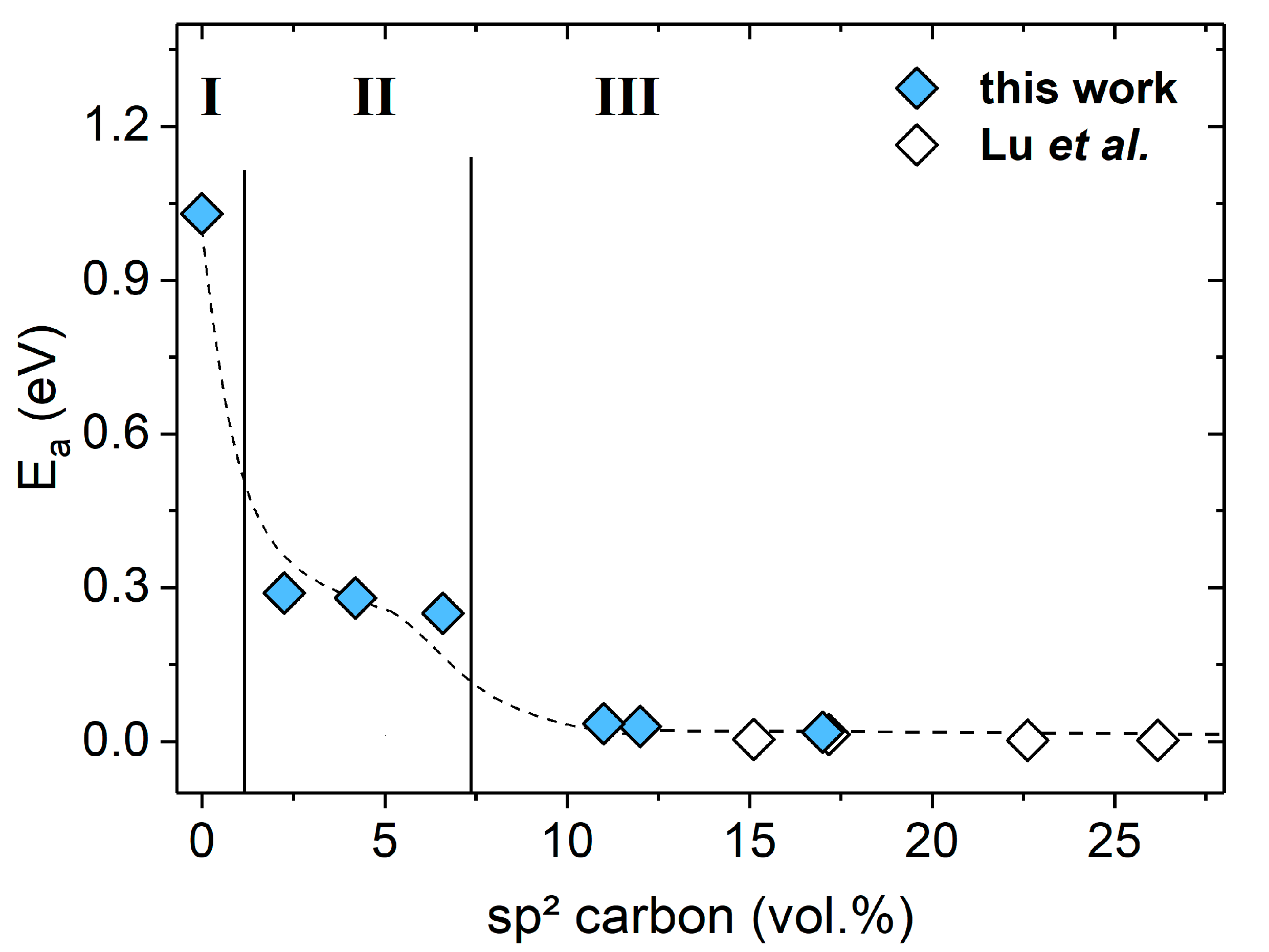
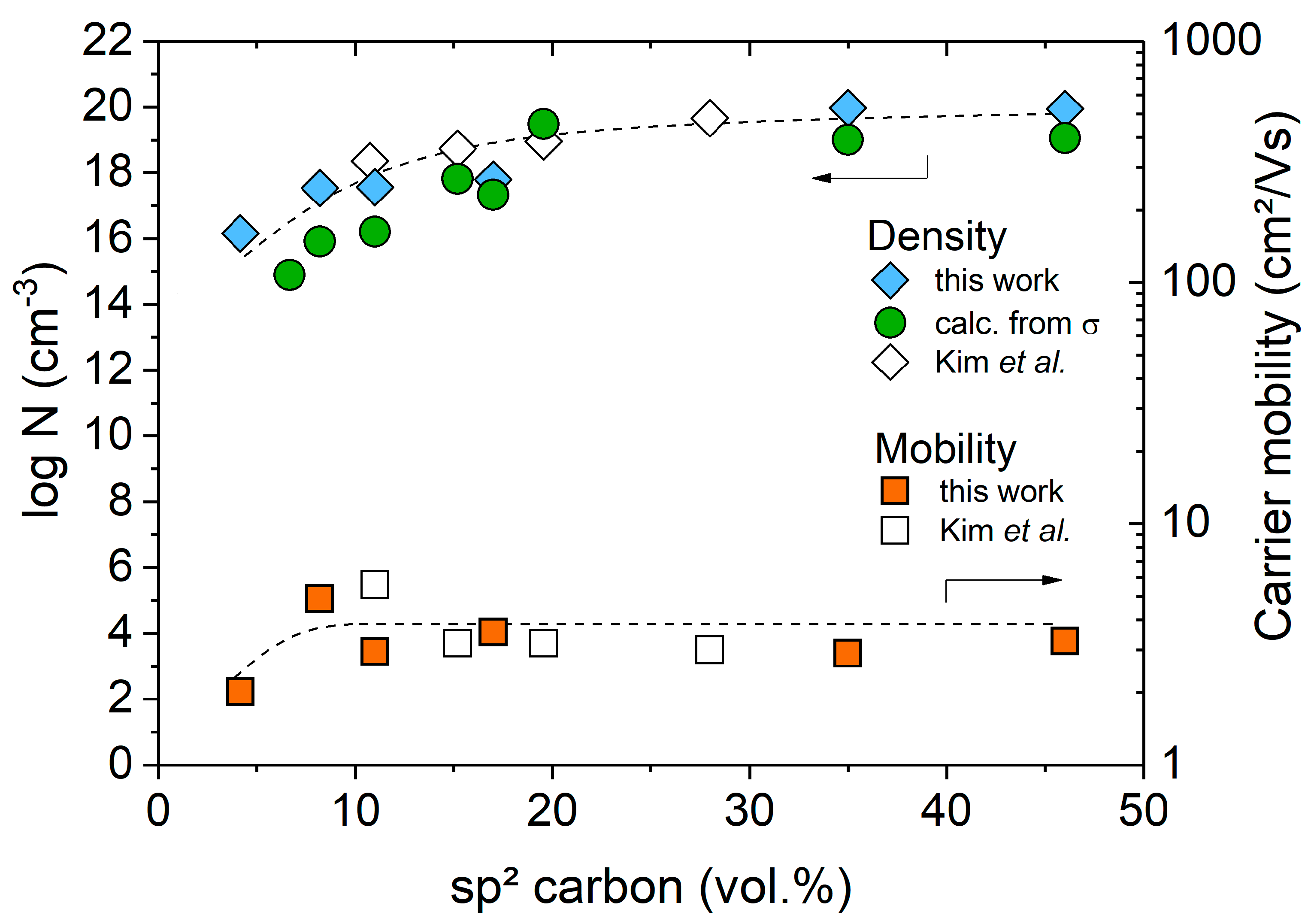
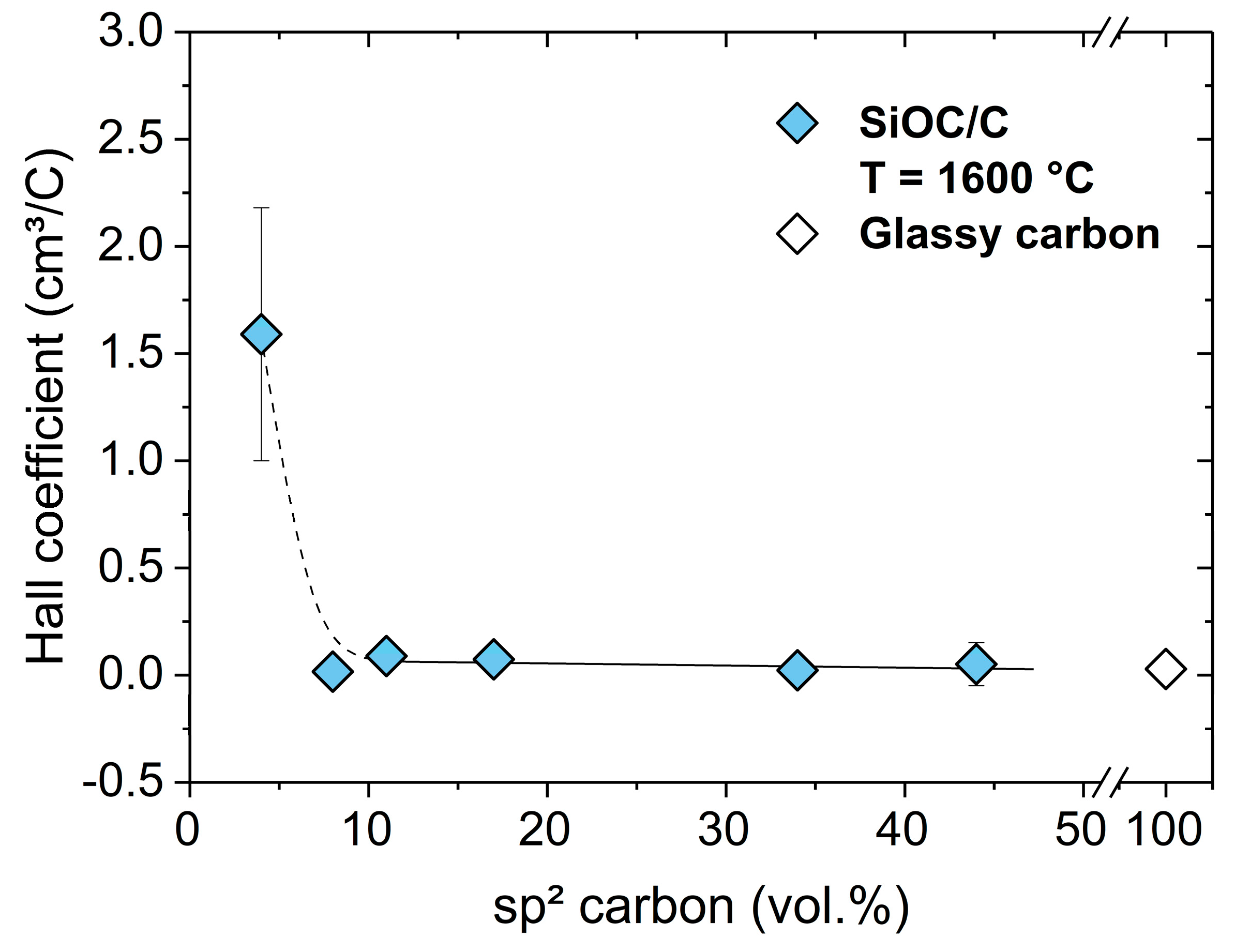
2. Results and Discussion
3. Materials and Methods
3.1. Materials Synthesis
3.2. Materials Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Widgeon, S.J.; Sen, S.; Mera, G.; Ionescu, E.; Riedel, R.; Navrotsky, A. 29Si and 13C Solid-State NMR Spectroscopic Study of Nanometer-Scale Structure and Mass Fractal Characteristics of Amorphous Polymer Derived Silicon Oxycarbide Ceramics. Chem. Mater. 2010, 22, 6221–6228. [Google Scholar] [CrossRef]
- Sen, S.; Widgeon, S.J.; Navrotsky, A.; Mera, G.; Tavakoli, A.; Ionescu, E.; Riedel, R. Carbon substitution for oxygen in silicates in planetary interiors. Proc. Natl. Acad. Sci. USA 2013, 110, 15904–15907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moysan, C.; Riedel, R.; Harshe, R.; Rouxel, T.; Augereau, F. Mechanical characterization of a polysiloxane-derived SiOC glass. J. Eur. Ceram. Soc. 2007, 27, 397–403. [Google Scholar] [CrossRef]
- Gonzalo-Juan, I.; Detsch, R.; Mathur, S.; Ionescu, E.; Boccaccini, A.R.; Riedel, R. Synthesis and In Vitro Activity Assessment of Novel Silicon Oxycarbide-Based Bioactive Glasses. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionescu, E.; Sen, S.; Mera, G.; Navrotsky, A. Structure, energetics and bioactivity of silicon oxycarbide-based amorphous ceramics with highly connected networks. J. Eur. Ceram. Soc. 2018, 38, 1311–1319. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Xie, F.; Gonzalo-Juan, I.; Riedel, R.; Ionescu, E.; Boccaccini, A.R. Silicon oxycarbide based materials for biomedical applications. Appl. Mater. Today 2019, 100482. [Google Scholar] [CrossRef]
- Xie, F.; Juan, I.G.; Arango-Ospina, M.; Riedel, R.; Boccaccini, A.R.; Ionescu, E. Apatite Forming Ability and Dissolution Behavior of Boron- and Calcium-Modified Silicon Oxycarbides in Comparison to Silicate Bioactive Glass. ACS Biomater. Sci. Eng. 2019, 5, 5337–5347. [Google Scholar] [CrossRef]
- Xie, F.T.; Gonzalo-Juan, I.; Breitzke, H.; Fasel, C.; Trapp, M.; Buntkowsky, G.; Kleebe, H.J.; Riedel, R.; Boccaccini, A.R.; Ionescu, E. Effect of Ca and B incorporation into silicon oxycarbide on its microstructure and phase composition. J. Am. Ceram. Soc. 2019, 102, 7645–7655. [Google Scholar] [CrossRef]
- Xie, F.; Ionescu, E.; Arango-Ospina, M.; Riedel, R.; Boccaccini, A.R.; Gonzalo-Juan, I. Facile Preparative Access to Bioactive Silicon Oxycarbides with Tunable Porosity. Materials 2019, 12, 3862. [Google Scholar] [CrossRef] [Green Version]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. A Si−O−C Composite anode: High capability and proposed mechanism of lithium storage associated with microstructural characteristics. ACS Appl. Mater. Interfaces 2010, 2, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Lithium insertion into carbon-rich SiOC ceramics: Influence of pyrolysis temperature on electrochemical properties. J. Power Sour. 2013, 244, 450–455. [Google Scholar] [CrossRef]
- Pradeep, V.S.; Graczyk-Zajac, M.; Riedel, R.; Soraru, G.D. New Insights in to the Lithium Storage Mechanism in Polymer Derived SiOC Anode Materials. Electrochimica Acta 2014, 119, 78–85. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Reinold, L.M.; Kaspar, J.; Sasikumar, P.V.W.; Soraru, G.D.; Riedel, R. New Insights into Understanding Irreversible and Reversible Lithium Storage within SiOC and SiCN Ceramics. Nanomaterials 2015, 5, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.M.; Reimers, J.N.; Fuller, E.W.; Dahn, J.R. Lithium insertion in pyrolyzed siloxane polymers. Solid State Ion. 1994, 74, 249–254. [Google Scholar] [CrossRef]
- Mazo, M.A.; Tamayo, A.; Caballero, A.C.; Rubio, J. Enhanced electrical and thermal conductivities of silicon oxycarbide nanocomposites containing carbon nanofibers. Carbon 2018, 138, 42–51. [Google Scholar] [CrossRef]
- Gurlo, A.; Ionescu, E.; Riedel, R.; Clarke, D.R. The Thermal Conductivity of Polymer-Derived Amorphous Si–O–C Compounds and Nano-Composites. J. Am. Ceram. Soc. 2016, 99, 281–285. [Google Scholar] [CrossRef]
- Stabler, C.; Reitz, A.; Stein, P.; Albert, B.; Riedel, R.; Ionescu, E. Thermal Properties of SiOC Glasses and Glass Ceramics at Elevated Temperatures. Materials 2018, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Riedel, R.; Toma, L.; Janssen, E.; Nuffer, J.; Melz, T.; Hanselka, H. Piezoresistive Effect in SiOC Ceramics for Integrated Pressure Sensors. J. Am. Ceram. Soc. 2010, 93, 920–924. [Google Scholar] [CrossRef]
- Toma, L.; Kleebe, H.-J.; Müller, M.M.; Janssen, E.; Riedel, R.; Melz, T.; Hanselka, H. Correlation Between Intrinsic Microstructure and Piezoresistivity in a SiOC Polymer-Derived Ceramic. J. Am. Ceram. Soc. 2012, 95, 1056–1061. [Google Scholar] [CrossRef]
- Roth, F.; Schmerbauch, C.; Ionescu, E.; Nicoloso, N.; Guillon, O.; Riedel, R. High-temperature piezoresistive C /SiOC sensors. J. Sens. Sens. Syst. 2015, 4, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Papendorf, B.; Ionescu, E.; Kleebe, H.-J.; Linck, C.; Guillon, O.; Nonnenmacher, K.; Riedel, R.; Wakai, F. High-Temperature Creep Behavior of Dense SiOC-Based Ceramic Nanocomposites: Microstructural and Phase Composition Effects. J. Am. Ceram. Soc. 2013, 96, 272–280. [Google Scholar] [CrossRef]
- Stabler, C.; Roth, F.; Narisawa, M.; Schliephake, D.; Heilmaier, M.; Lauterbach, S.; Kleebe, H.-J.; Riedel, R.; Ionescu, E. High-temperature creep behavior of a SiOC glass ceramic free of segregated carbon. J. Eur. Ceram. Soc. 2016, 36, 3747–3753. [Google Scholar] [CrossRef]
- Stabler, C.; Schliephake, D.; Heilmaier, M.; Rouxel, T.; Kleebe, H.-J.; Narisawa, M.; Riedel, R.; Ionescu, E. Influence of SiC/Silica and Carbon/Silica Interfaces on the High-Temperature Creep of Silicon Oxycarbide-Based Glass Ceramics: A Case Study. Adv. Eng. Mater. 2019, 21, 1800596. [Google Scholar] [CrossRef]
- Ionescu, E.; Balan, C.; Kleebe, H.-J.; Müller, M.M.; Guillon, O.; Schliephake, D.; Heilmaier, M.; Riedel, R. High-Temperature Creep Behavior of SiOC Glass-Ceramics: Influence of Network Carbon Versus Segregated Carbon. J. Am. Ceram. Soc. 2014, 97, 3935–3942. [Google Scholar] [CrossRef]
- Du, B.; He, C.; Qian, J.J.; Cai, M.; Wang, X.; Shui, A.Z. Electromagnetic wave absorbing properties of glucose-derived carbon-rich SiOC ceramics annealed at different temperatures. J. Am. Ceram. Soc. 2019, 102, 7015–7025. [Google Scholar] [CrossRef]
- Tamayo, A.; Peña-Alonso, R.; Rubio, J.; Raj, R.; Sorarù, G.D.; Oteo, J.L. Surface Energy of Sol Gel-Derived Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 2011, 94, 4523–4533. [Google Scholar] [CrossRef]
- Zhuo, R.; Colombo, P.; Pantano, C.; Vogler, E.A. Silicon oxycarbide glasses for blood-contact applications. Acta Biomater. 2005, 1, 583–589. [Google Scholar] [CrossRef]
- Narisawa, M.; Watase, S.; Matsukawa, K.; Dohmaru, T.; Okamura, K. White Si–O–C(–H) Particles with Photoluminescence Synthesized by Decarbonization Reaction on Polymer Precursor in a Hydrogen Atmosphere. Bull. Chem. Soc. Jpn. 2012, 85, 724–726. [Google Scholar] [CrossRef]
- Blum, Y.D.; MacQueen, D.B.; Kleebe, H.-J. Synthesis and characterization of carbon-enriched silicon oxycarbides. J. Eur. Ceram. Soc. 2005, 25, 143–149. [Google Scholar] [CrossRef]
- Kleebe, H.-J.; Blum, Y.D. SiOC ceramic with high excess free carbon. J. Eur. Ceram. Soc. 2008, 28, 1037–1042. [Google Scholar] [CrossRef]
- Rosenburg, F.; Ionescu, E.; Nicoloso, N.; Riedel, R. High-Temperature Raman Spectroscopy of Nano-Crystalline Carbon in Silicon Oxycarbide. Materials 2018, 11, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Crespiera, S.; Ionescu, E.; Kleebe, H.J.; Riedel, R. Pressureless synthesis of fully dense and crack-free SiOC bulk ceramics via photo-crosslinking and pyrolysis of a polysiloxane. J. Eur. Ceram. Soc. 2011, 31, 913–919. [Google Scholar] [CrossRef]
- Lu, K.; Erb, D.; Liu, M. Thermal stability and electrical conductivity of carbon-enriched silicon oxycarbide. J. Mater. Chem. C 2016, 4, 1829–1837. [Google Scholar] [CrossRef] [Green Version]
- Cordelair, J.; Greil, P. Electrical conductivity measurements as a microprobe for structure transitions in polysiloxane derived Si–O–C ceramics. J. Eur. Ceram. Soc. 2000, 20, 1947–1957. [Google Scholar] [CrossRef]
- Haluschka, C.; Engel, C.; Riedel, R. Silicon carbonitride ceramics derived from polysilazanes Part II. Investigation of electrical properties. J. Eur. Ceram. Soc. 2000, 20, 1365–1374. [Google Scholar] [CrossRef]
- Kim, K.J.; Eom, J.-H.; Kim, Y.-W.; Seo, W.-S. Electrical conductivity of dense, bulk silicon-oxycarbide ceramics. J. Eur. Ceram. Soc. 2015, 35, 1355–1360. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Zanella, C.; Bettotti, P.; Sorarù, G.D. Electrical Conductivity of SiOCN Ceramics by the Powder-Solution-Composite Technique. J. Am. Ceram. Soc. 2014, 97, 2525–2530. [Google Scholar] [CrossRef]
- Roth, F.; Waleska, P.; Hess, C.; Ionescu, E.; Nicoloso, N. UV Raman spectroscopy of segregated carbon in silicon oxycarbides. J. Ceram. Soc. Jpn. 2016, 124, 1042–1045. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Yu, Z.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2019, 100623. [Google Scholar] [CrossRef]
- Sorarù, G.D.; D’Andrea, G.; Campostrini, R.; Babonneau, F.; Mariotto, G. Structural Characterization and High-Temperature Behavior of Silicon Oxycarbide Glasses Prepared from Sol-Gel Precursors Containing Si-H Bonds. J. Am. Ceram. Soc. 1995, 78, 379–387. [Google Scholar] [CrossRef]
- Rouxel, T.; Soraru, G.-D.; Vicens, J. Creep Viscosity and Stress Relaxation of Gel-Derived Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 2001, 84, 1052–1058. [Google Scholar] [CrossRef]
- Bréquel, H.; Parmentier, J.; Sorarù, G.D.; Schiffini, L.; Enzo, S. Study of the phase separation in amorphous silicon oxycarbide glasses under heat treatment. Nanostruct. Mater. 1999, 11, 721–731. [Google Scholar] [CrossRef]
- Ionescu, E.; Terzioglu, C.; Linck, C.; Kaspar, J.; Navrotsky, A.; Riedel, R. Thermodynamic Control of Phase Composition and Crystallization of Metal-Modified Silicon Oxycarbides. J. Am. Ceram. Soc. 2013, 96, 1899–1903. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- López-Díaz, D.; Holgado, M.L.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Vázquez-Santos, M.B.; Geissler, E.; László, K.; Rouzaud, J.-N.; Martínez-Alonso, A.; Tascón, J.M.D. Comparative XRD, Raman, and TEM Study on Graphitization of PBO-Derived Carbon Fibers. J. Phys. Chem. C 2012, 116, 257–268. [Google Scholar] [CrossRef]
- Larouche, N.; Stansfield, B.L. Classifying nanostructured carbons using graphitic indices derived from Raman spectra. Carbon 2010, 48, 620–629. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Cattin, C.; Hubert, P. Piezoresistance in polymer nanocomposites with high aspect ratio particles. ACS Appl. Mater. Interfaces 2014, 6, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Mathew, M.; Schilling, T.; Oettel, M. Connectivity percolation in suspensions of hard platelets. Phys. Rev. E 2012, 85, 061407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batrouni, G.G.; Hansen, A.; Larson, B. Current distribution in the three-dimensional random resistor network at the percolation threshold. Phys. Rev. E 1996, 53, 2292–2297. [Google Scholar] [CrossRef] [Green Version]
- Clerc, J.P.; Podolskiy, V.A.; Sarychev, A.K. Precise determination of the conductivity exponent of 3D percolation using exact numerical renormalization. Eur. Phys. J. B 2000, 15, 507–516. [Google Scholar] [CrossRef]
- Vionnet-Menot, S.; Grimaldi, C.; Maeder, T.; Strässler, S.; Ryser, P. Tunneling-percolation origin of nonuniversality: Theory and experiments. Phys. Rev. B 2005, 71, 064201. [Google Scholar] [CrossRef] [Green Version]
- Balberg, I. Tunneling and nonuniversal conductivity in composite materials. Phys. Rev. Lett. 1987, 59, 1305–1308. [Google Scholar] [CrossRef]
- Balberg, I. Limits on the continuum-percolation transport exponents. Phys. Rev. B 1998, 57, 13351–13354. [Google Scholar] [CrossRef]
- Carcia, P.F.; Suna, A.; Childers, W.D. Electrical conduction and strain sensitivity in RuO2 thick film resistors. J. Appl. Phys. 1983, 54, 6002. [Google Scholar] [CrossRef]
- Renlund, G.M.; Prochazka, S.; Doremus, R.H. Silicon oxycarbide glasses: Part II. Structure and properties. J. Mater. Res. 1991, 6, 2723–2734. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Wang, K.; Li, X.; Liu, J.; An, L. Frequency-dependent conductive behavior of polymer-derived amorphous silicon carbonitride. Acta Mater. 2015, 89, 215–224. [Google Scholar] [CrossRef]
- Zhang, L.G.; Wang, Y.S.; Wei, Y.; Xu, W.X.; Fang, D.J.; Zhai, L.; Lin, K.C.; An, L.N. A Silicon Carbonitride Ceramic with Anomalously High Piezoresistivity. J. Am. Ceram. Soc. 2008, 91, 1346–1349. [Google Scholar] [CrossRef]
- Terauds, K.; Sanchez-Jimenez, P.E.; Raj, R.; Vakifahmetoglu, C.; Colombo, P. Giant piezoresistivity of polymer-derived ceramics at high temperatures. J. Eur. Ceram. Soc. 2010, 30, 2203–2207. [Google Scholar] [CrossRef]
- Engel, C. Elektrische Eigenschaften gefüllter Pyrolyse-Keramiken im System Si-O-C.; Shaker Verlag: Aachen, Germany, 2000. [Google Scholar]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Kawamura, K. Structure of Glassy Carbon. Nature 1971, 231, 175–176. [Google Scholar] [CrossRef]
- van Hapert, J.J. Hopping Conduction and Chemical Structure. A study on Silicon Suboxides. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2002. [Google Scholar]
- van Hapert, J.J.; Tomozeiu, N.; van Faassen, E.; Vredenberg, A.; Habraken, F. Charge transport through localized states in sputtered amorphous silicon suboxides. MRS Proc. 2001, 666, F3.6. [Google Scholar] [CrossRef]
- Dasgupta, D.; Demichelis, F.; Tagliaferro, A. Electrical conductivity of amorphous carbon and amorphous hydrogenated carbon. Philos. Mag. B 1991, 63, 1255–1266. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Kawamura, K. Polymeric Carbons—Carbon Fiber, Glass and Char; Cambridge University Press: New York, NY, USA, 1976. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Sugihara, K.; Spain, I.L.; Goldberg, H.A. Graphite Fibers and Filaments; Springer: Berlin, Germany, 1988. [Google Scholar]
- Robertson, J. Properties of diamond-like carbon. Surf. Coat. Technol. 1992, 50, 185–203. [Google Scholar] [CrossRef]
- Kim, K.J.; Eom, J.-H.; Koh, T.Y.; Kim, Y.-W.; Seo, W.-S. Effects of carbon addition on the electrical properties of bulk silicon-oxycarbide ceramics. J. Eur. Ceram. Soc. 2016, 36, 2705–2711. [Google Scholar] [CrossRef]
- Liu, C.-C.; Walters, A.B.; Vannice, M.A. Measurement of electrical properties of a carbon black. Carbon 1995, 33, 1699–1708. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Berezkin, V.I.; Kholodkevich, S.V.; Konstantinov, P.P. Hall effect in the natural glassy carbon of schungites. Phys. Solid State 1997, 39, 1590–1593. [Google Scholar] [CrossRef]
- Noda, T.; Inagaki, M.; Yamada, S. Glass-like carbons. J. Non-Cryst. Solids 1969, 1, 285–302. [Google Scholar] [CrossRef]
- Mrozowski, S.; Chaberski, A. Hall Effect and Magnetoresistivity in Carbons and Polycrystalline Graphites. Phys. Rev. 1956, 104, 74–83. [Google Scholar] [CrossRef]
- Klein, C.A.; Straub, W.D. Carrier Densities and Mobilities in Pyrolytic Graphite. Phys. Rev. 1961, 123, 1581–1583. [Google Scholar] [CrossRef]
- Baker, D.F.; Bragg, R.H. The electrical conductivity and Hall effect of glassy carbon. J. Non-Cryst. Solids 1983, 58, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Esquinazi, P.; Kruger, J.; Barzola-Quiquia, J.; Schonemann, R.; Herrmannsdorfer, T.; Garcia, N. On the low-field Hall coefficient of graphite. AIP Adv. 2014, 4, 117121. [Google Scholar] [CrossRef]
- van der Pauw, L.J. A Method of Measuring Specific Resistivity and Hall Effect of Discs of Arbitrary Shape. In Semiconductor Devices: Pioneering Papers; World Scientific: Singapore, 1991; pp. 174–182. [Google Scholar]





| Sample | Precursors | Ceramic Yield (wt %) | Ref. | |
|---|---|---|---|---|
| Sol-Gel Precursors | TREOS:MDES molar ratio | |||
| C-0 | 10:1 | 87.1 | [41,42,43] | |
| C-1 | 2:1 | 88.4 | ||
| C-2 | 1:2 | 86.6 | ||
| TREOS:TEMS molar ratio | ||||
| C-4 | 2:1 | 82.2 | this work | |
| C-6 | 1:1 | 80.7 | ||
| C-8 | 1:2 | 78.5 | ||
| TREOS:TEVS molar ratio | ||||
| C-13 | 1:1 | 85.0 | this work | |
| C-15 | 1:2 | 87.6 | ||
| C-18 | 1:3 | 88.2 | ||
| C-20 | 1:4 | 85.1 | ||
| Preceramic Polymers | C-11 | PMS MK | 81.0 | [44] |
| C-17 | SPR 212 | 85.6 | [12] | |
| C-36 | Silres 604 | 84.7 | this work | |
| C-46 | SPR 688 | 85.8 | [12] | |
| C-60 | SPR 684 | 82.0 | [12] |
| Sample | Element Content (wt %) | Empirical Formula SiO2(1 − x)Cx + y | Volume Fraction (vol.%) | ||||
|---|---|---|---|---|---|---|---|
| Si | O | C | SiO2 | SiC | C | ||
| C-0 | 51.78 | 40.88 | 7.03 | SiO1.39C0.31 | 83.2 | 16.8 | - |
| C-1 | 50.97 | 42.14 | 6.89 | SiO1.45C0.28 + 0.04C | 84.27 | 14.55 | 1.18 |
| C-8 | 48.38 | 38.69 | 12.93 | SiO1.42C0.29 + 0.31C | 77.42 | 14.38 | 8.20 |
| C-11 | 46.65 | 39.40 | 13.95 | SiO1.48C0.26 + 0.44C | 76.75 | 12.24 | 11.01 |
| C-17 | 49.13 | 26.44 | 24.43 | SiO0.94C0.53 + 0.63C | 54.49 | 27.83 | 17.68 |
| C-36 | 36.53 | 27.90 | 35.57 | SiO1.34C0.33 + 1.95C | 51.91 | 11.66 | 36.43 |
| C-46 | 13.72 | 38.23 | 48.05 | SiO0.63C0.69 + 2.25C | 26.86 | 26.71 | 46.42 |
| C-60 | 27.96 | 16.93 | 55.11 | SiO1.06C0.47 + 4.13C | 30.19 | 12.28 | 57.53 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenburg, F.; Balke, B.; Nicoloso, N.; Riedel, R.; Ionescu, E. Effect of the Content and Ordering of the sp2 Free Carbon Phase on the Charge Carrier Transport in Polymer-Derived Silicon Oxycarbides. Molecules 2020, 25, 5919. https://doi.org/10.3390/molecules25245919
Rosenburg F, Balke B, Nicoloso N, Riedel R, Ionescu E. Effect of the Content and Ordering of the sp2 Free Carbon Phase on the Charge Carrier Transport in Polymer-Derived Silicon Oxycarbides. Molecules. 2020; 25(24):5919. https://doi.org/10.3390/molecules25245919
Chicago/Turabian StyleRosenburg, Felix, Benjamin Balke, Norbert Nicoloso, Ralf Riedel, and Emanuel Ionescu. 2020. "Effect of the Content and Ordering of the sp2 Free Carbon Phase on the Charge Carrier Transport in Polymer-Derived Silicon Oxycarbides" Molecules 25, no. 24: 5919. https://doi.org/10.3390/molecules25245919






