Electrohydrodynamic Processing of Potato Protein into Particles and Fibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrical Conductivity of the Potato Protein Solutions
2.2. Effect of Solvents on the Size and Morphology of Electrosprayed/Spun Potato Protein Particles and Fibers
2.2.1. Water
2.2.2. Water:Ethanol
2.2.3. Water:Glycerol
2.2.4. Hexafluoro-2-Propanol (HFIP)
2.3. Encapsulation and Release of Vitamin B12 from Potato Protein Fibers
3. Materials and Methods
3.1. Materials
3.2. Conductivity of the Solutions
3.3. Electrospray and Electrospinning
3.4. Scanning Electron Microscopy (SEM)
3.5. Release of Vitamin B12
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waglay, A.; Karboune, S. Potato Proteins. Adv. Potato Chem. Technol. 2016, 75–104. [Google Scholar] [CrossRef]
- Alting, A.C.; Pouvreau, L.; Giuseppin, M.L.F.; van Nieuwenhuijzen, N.H. Potato Proteins; Woodhead Publishing Limited: Sawston, UK, 2011; Volume 2008, ISBN 9780857093639. [Google Scholar]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Stounbjerg, L.C.; Vestergaard, C.; Andreasen, B.; Ipsen, R. Associative phase separation of potato protein and anionic polysaccharides. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 104–112. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Larsen, L.B.; Hammershøj, M. Foam and emulsion properties of potato protein isolate and purified fractions. Food Hydrocoll. 2018, 74, 367–378. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Soladoye, O.P. Towards potato protein utilisation: Insights into separation, functionality and bioactivity of patatin. Int. J. Food Sci. Technol. 2020, 55, 2314–2322. [Google Scholar] [CrossRef]
- David, S.; Livney, Y.D. Potato protein based nanovehicles for health promoting hydrophobic bioactives in clear beverages. Food Hydrocoll. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato protein- based carriers for enhancing bioavailability of astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Mendes, A.C.L.; Stephansen, K.B.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Jacobsen, C.; Garcia-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of Electrospinning for Encapsulation of Sensitive Bioactive Compounds and Applications in Food. Annu. Rev. Food Sci. Technol. 2018, 9, 1–25. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Mendes, A.C.; Jacobsen, C.; Chronakis, I.S. Biopolymers for the Nano-microencapsulation of Bioactive Ingredients by Electrohydrodynamic Processing. Polym. Food Appl. 2018, 447–479. [Google Scholar]
- Lim, L.T.T.; Mendes, A.C.; Chronakis, I.S. Electrospinning and Electrospraying Technologies for Food Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 88, ISBN 9780128160732. [Google Scholar]
- Moreno, J.A.S.; Mendes, A.C.; Stephansen, K.; Engwer, C.; Goycoolea, F.M.; Boisen, A.; Nielsen, L.H.; Chronakis, I.S. Development of electrosprayed mucoadhesive chitosan microparticles. Carbohydr. Polym. 2018, 190, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Masuda, A.; Aiba, T. In vivo application of chitosan to improve bioavailability of cyanocobalamin, a form of vitamin B12, following intraintestinal administration in rats. Int. J. Pharm. 2015, 483, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, K.; Sridhar, R.; Sundarrajan, S.; Venugopal, J.R.; Ramakrishna, S. Vitamin B12 loaded polycaprolactone nanofibers: A novel transdermal route for the water soluble energy supplement delivery. Int. J. Pharm. 2013, 444, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Zadeh, B.S.M.; Moghimipour, E. Preparation and Characterization of Cyanocobalamin (Vit B12) Microemulsion Properties and Structure for Topical and Transdermal Application. Iran J. Basic Med. Sci. 2013, 16, 865–872. [Google Scholar]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Sha, S.; Yin, H.; Zhang, H.; Wang, Y.; Zhao, B.; Song, F. Analysis of the tendency for the electronic conductivity to change during alcoholic fermentation. Sci. Rep. 2019, 9, 5512. [Google Scholar] [CrossRef]
- Nakagawa, H.; Oyama, T. Molecular Basis of Water Activity in Glycerol-Water Mixtures. Front. Chem. 2019, 7, 731. [Google Scholar] [CrossRef] [Green Version]
- Aceituno-Medina, M.; Lopez-Rubio, A.; Mendoza, S.; Lagaron, J.M. Development of novel ultrathin structures based in amaranth (Amaranthus hypochondriacus) protein isolate through electrospinning. Food Hydrocoll. 2013, 31, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Sharif, N.; Golmakani, M.; Niakousari, M.; Ghorani, B.; Lopez-Rubio, A. Food-grade gliadin microstructures obtained by electrohydrodynamic processing. Food Res. Int. 2019, 116, 1366–1373. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Electrospun zein nanofibers incorporating cyclodextrins. Carbohydr. Polym. 2012, 90, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. 2016, 35, 139–162. [Google Scholar] [CrossRef]
- Van Koningsveld, G.A.; Gruppen, H.; De Jongh, H.H.J.; Wijngaards, G.; Van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. Effects of Ethanol on Structure and Solubility of Potato Proteins and the Effects of Its Presence during the Preparation of a Protein Isolate. J. Agric. Food Chem. 2002, 50, 2947–2956. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Fathi, M. Application of Cellulosic Nanofibers in Food Science Using Electrospinning and Its Potential Risk. Compr. Rev. Food Sci. Food Saf. 2015, 14, 269–284. [Google Scholar] [CrossRef]
- Ibili, H.; Dasdemir, M. Investigation of electrohydrodynamic atomization (electrospraying) parameters’ effect on formation of poly(lactic acid) nanoparticles. J. Mater. Sci. 2019, 54, 14609–14623. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Zaeim, D.; Jamab, M.S.; Ghorani, B.; Kadkhodaee, R.; Tromp, R. Electrospray-assisted drying of live probiotics in acacia gum microparticles matrix. Carbohydr. Polym. 2018, 183, 183–191. [Google Scholar] [CrossRef]
- Stephansen, K.; Chronakis, I.S.; Jessen, F. Bioactive electrospun fish sarcoplasmic proteins as a drug delivery system. Colloids Surf. B Biointerfaces 2014, 122, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Srikar, R.; Yarin, A.L.; Megaridis, C.M.; Gemeinhart, R.A. Mechanistic Examination of Protein Release from Polymer Nanofibers. Mol. Pharm. 2009, 6, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Srikar, R.; Yarin, A.L.; Megaridis, C.M.; Bazilevsky, A.V.; Kelley, E. Desorption-Limited Mechanism of Release from Polymer Nanofibers. Langmuir 2008, 24, 965–974. [Google Scholar] [CrossRef]
- Stephansen, K.; García-Díaz, M.; Jessen, F.; Chronakis, I.S.; Nielsen, H.M. Bioactive protein-based nanofibers interact with intestinal biological components resulting in transepithelial permeation of a therapeutic protein. Int. J. Pharm. 2015, 495, 58–66. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]

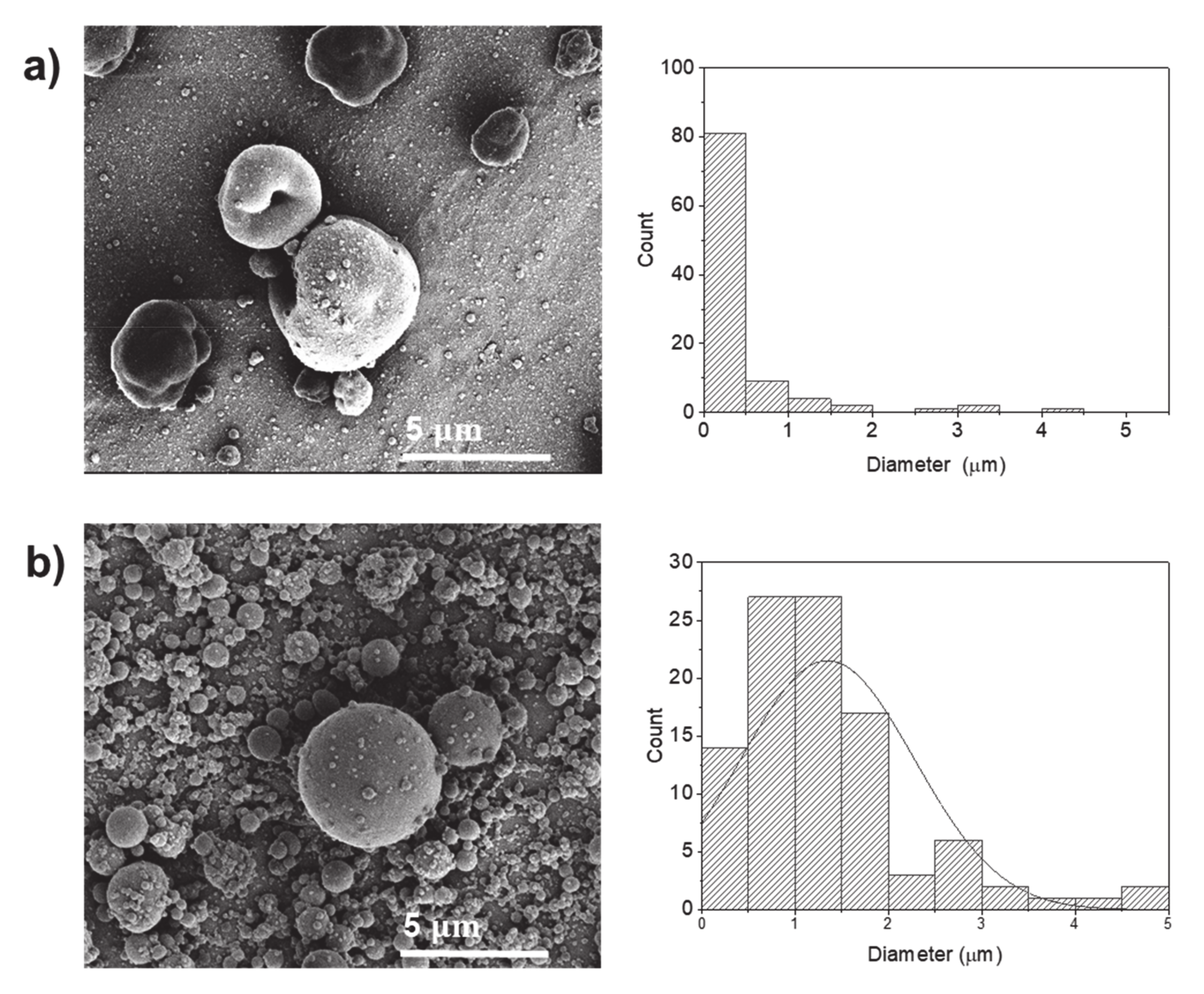


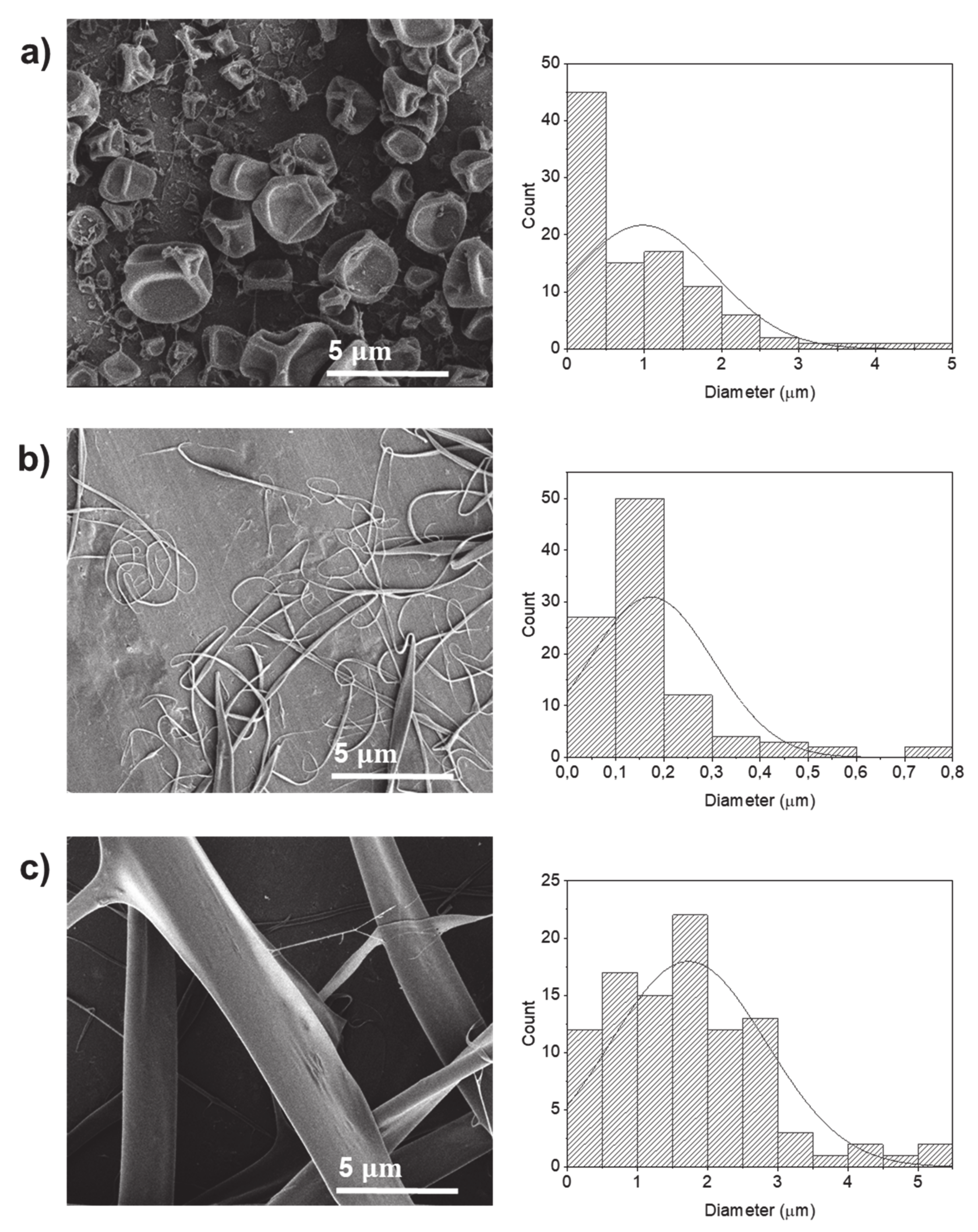
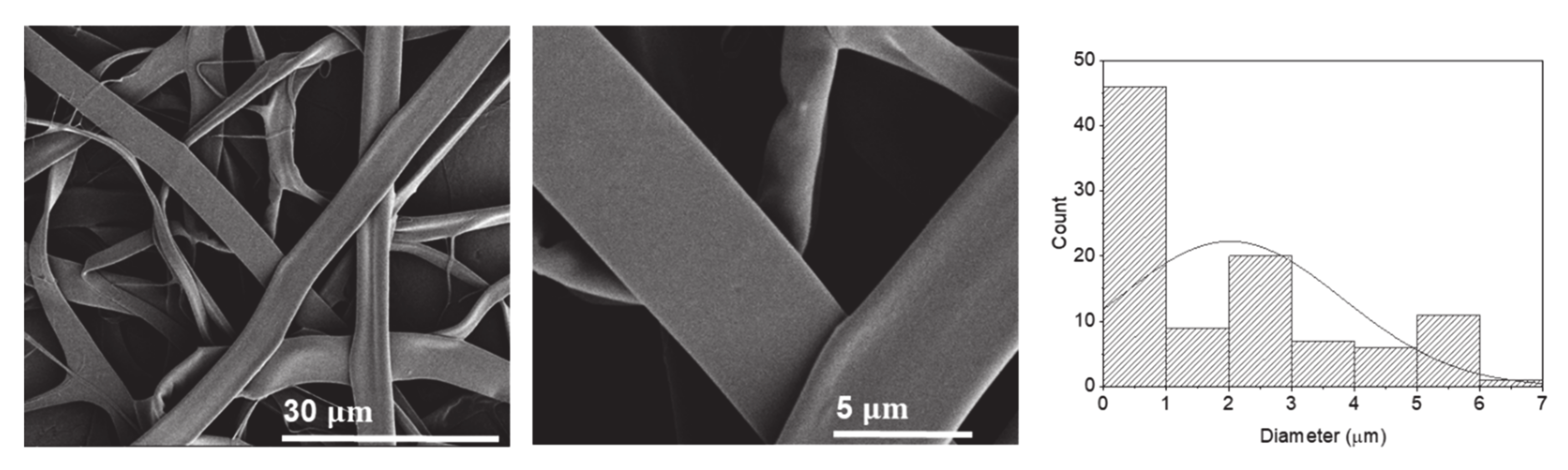

| Sample Designation | Electrical Conductivity (µS/cm) | Average Diameter (µm) |
|---|---|---|
| P1 | 7705.5 ± 146.4 | 0.36 ± 0.34 (particles) |
| P2 | 7705.5 ± 146.4 | 0.68 ± 0.60 (particles) |
| P3 | 7651.5 ± 61.4 | 0.42 ± 0.71 (particles) |
| P4 | 7651.5 ± 61.4 | 1.35 ± 0.93 (particles) |
| P5 | 1263.7 ± 35.8 | 0.39 ± 0.64 (particles) |
| P6 | 1779.2 ± 1.4 | 0.53 ± 0.52 (particles) |
| P7 | 20.3 ± 0.2 | 0.98 ± 0.92 (particles) |
| P8 | 20.6 ± 0.7 | 0.17 ± 0.13 (fibers) |
| P9 | 23.9 ± 1.2 | 1.72 ± 1.11 (fibers) |
| P10 | 2958.1 ± 6.8 | 0.53 ± 0.56 (particles) |
| P11 | 24.5 ± 0.9 | 2.01 ± 1.79 (fibers) |
| Sample Designation | Concentration PK15 (% w/v) | Solvent (% v/v) | Flow Rate (mL/min) | Needle (G) | Distance * (cm) | Voltage (kV) |
|---|---|---|---|---|---|---|
| P1 | 20 | H2O | 0.02 | 24 | 15 | 25 |
| P2 | 20 | H2O | 0.02 | 24 | 15 | 30 |
| P3 | 40 | H2O | 0.02 | 19 | 15 | 25 |
| P4 | 40 | H2O | 0.02 | 19 | 15 | 30 |
| P5 | 10 | H2O:ethanol (9:1) | 0.02 | 24 | 15 | 25 |
| P6 | 20 | H2O:ethanol (9:1) | 0.02 | 24 | 15 | 25 |
| P7 | 5 | HFIP | 0.02 | 24 | 15 | 25 |
| P8 | 10 | HFIP | 0.02 | 24 | 15 | 25 |
| P9 | 20 | HFIP | 0.02 | 24 | 15 | 25 |
| P10 | 40 | H2O: glycerol (9:1) | 0.02 | 19 | 15 | 25 |
| P11 ** | 20 | HFIP | 0.02 | 24 | 15 | 25 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, A.C.; Saldarini, E.; Chronakis, I.S. Electrohydrodynamic Processing of Potato Protein into Particles and Fibers. Molecules 2020, 25, 5968. https://doi.org/10.3390/molecules25245968
Mendes AC, Saldarini E, Chronakis IS. Electrohydrodynamic Processing of Potato Protein into Particles and Fibers. Molecules. 2020; 25(24):5968. https://doi.org/10.3390/molecules25245968
Chicago/Turabian StyleMendes, Ana C., Elena Saldarini, and Ioannis S. Chronakis. 2020. "Electrohydrodynamic Processing of Potato Protein into Particles and Fibers" Molecules 25, no. 24: 5968. https://doi.org/10.3390/molecules25245968







