A Study of the Effect of Sintering Conditions of Mg0.95Ni0.05Ti3 on Its Physical and Dielectric Properties
Abstract
:1. Introduction
- High permittivity (εr): the εr is inversely related to the wavelength in dielectrics (), so the component size can be effectively reduced.
- High-quality factor (Q factor, Qf): Q factors are inversely related to the dielectric loss (Q = 1/tanδ), so high Q factors can improve frequency selectivity and stability in MW components.
- A temperature coefficient (τf) close to zero: A τf value close to zero ensures that component performance is not affected by external temperatures.
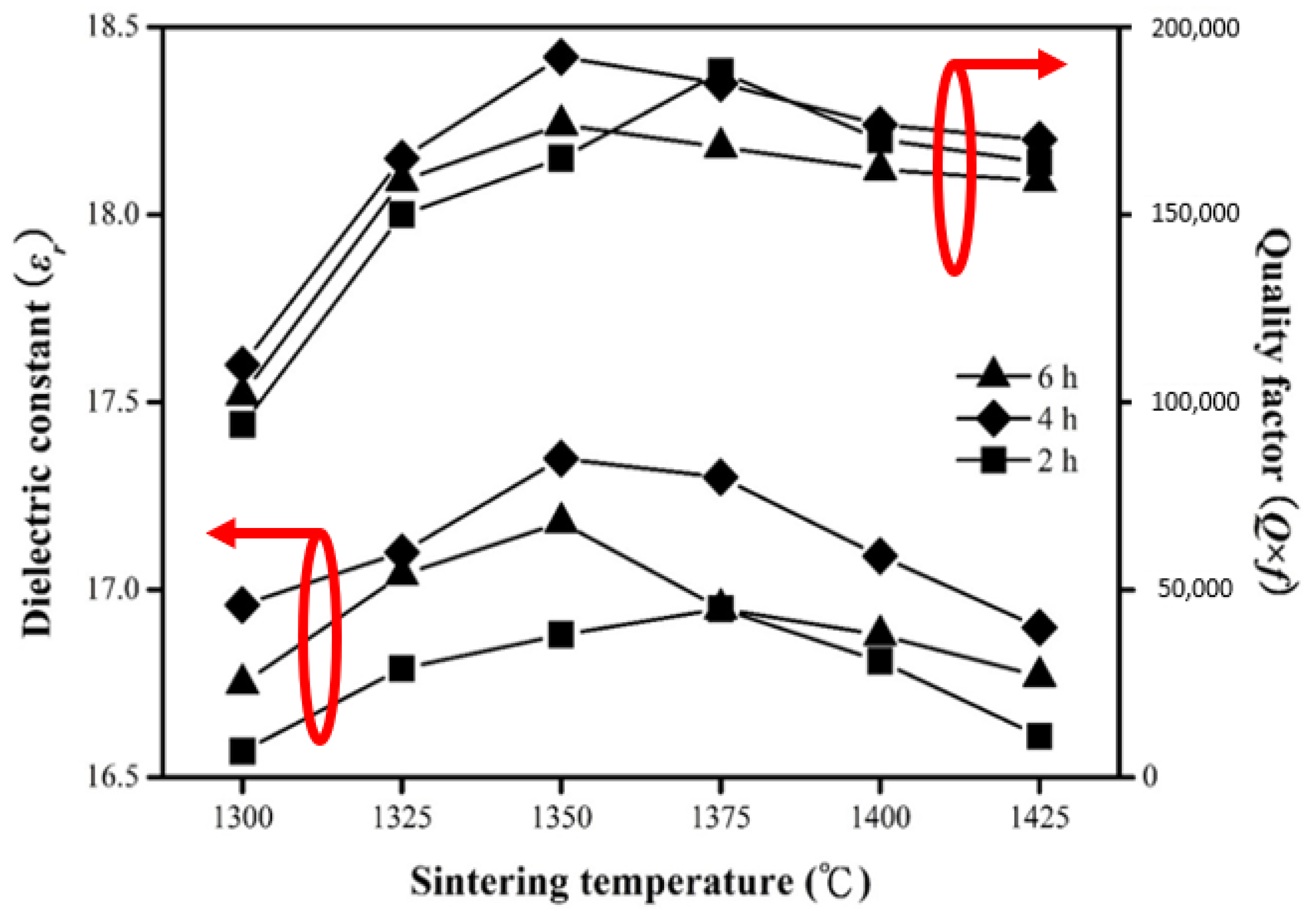
2. Results and Discussion
3. Experimental Procedure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, S.H.; Chen, Y.B. Structure and characterization of B2O3 modified yNd(Mg1/2Ti1/2)O3-(1-y)Ca0.8Sr0.2TiO3 ceramics with a near-zero temperature coefficient at microwave frequency. Ceram. Int. 2017, 43, 2368–2371. [Google Scholar] [CrossRef]
- Nakagoshi, Y.; Sato, J.; Morimoto, M.; Suzuki, Y. Near-zero volume-shrinkage in reactive sintering of porous MgTi2O5 with pseudobrookite-type structure. Ceram. Int. 2016, 42, 9139–9144. [Google Scholar] [CrossRef] [Green Version]
- Bor, B.; Heilmann, L.; Domènech, B.; Kampferbeck, M.; Vossmeyer, T.; Weller, H.; Schneider, G.A.; Giuntini, D. Mapping the Mechanical Properties of Hierarchical Supercrystalline Ceramic-Organic Nanocomposites. Molecules 2020, 25, 4790. [Google Scholar] [CrossRef]
- Palmero, P. Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods. Nanomaterials 2015, 5, 656–696. [Google Scholar] [CrossRef]
- Freitas, A.E.; Manhabosco, T.M.; Batista, R.J.C.; Segundo, A.K.R.; Araújo, H.X.; Araújo, F.G.S.; Costa, A.R. Development and Characterization of Titanium Dioxide Ceramic Substrates with High Dielectric Permittivities. Materials 2020, 13, 386. [Google Scholar] [CrossRef] [Green Version]
- Itaalit, B.; Mouyane, M.; Bernard, J.; Womes, M.; Houivet, D. Effect of Post-Annealing on the Microstructure and Microwave Dielectric Properties of Ba(Co0.7Zn0.3)1/3Nb2/3O3 Ceramics. Appl. Sci. 2016, 6, 2. [Google Scholar] [CrossRef]
- Aljaafari, A.; Sedky, A. Influence of Fine Crystal Percentage on the Electrical Properties of ZnO Ceramic-Based Varistors. Crystals 2020, 10, 681. [Google Scholar] [CrossRef]
- Chen, Y.B.; Tseng, Z.L.; Chen, L.C.; Lin, C.C.; Miao, H.Y.; Li, J.H.; Lin, S.H. Crystal structure and microwave dielectric properties of [(Mg0.6Zn0.4)0.95Co0.05]2TiO4-modified Ca0.6La0.8/3TiO3 cordierite ceramics with a near-zero temperature coefficient. J. Mater. Sci. Mater. Electron. 2018, 13, 10709–10714. [Google Scholar] [CrossRef]
- Somiya, S. Handbook of Advanced Ceramics, 2nd ed.; Elsevier: Tokyo, Japan, 2013. [Google Scholar]
- Sohn, J.H.; Inaguma, Y.; Yoon, S.O. Microwave Dielectric Characteristics of Ilmenite-Type Titanates with High Q Values. Jpn. J. Appl. Phys. 1994, 33, 5466. [Google Scholar] [CrossRef]
- Chen, Y.C.; Su, C.F.; Weng, M.Z.; You, H.M.; Chang, K.C. Improvement microwave dielectric properties of Zn2SnO4 ceramics by substituting Sn4+ with Si4+. J. Mater. Sci. Mater. Electron. 2014, 25, 2120. [Google Scholar] [CrossRef]
- Shen, C.H.; Pan, C.L.; Lin, S.H. Structure, dielectric properties, and applications of (Na0.5Sm0.5)TiO3-modified (Mg0.95Ni0.05)TiO3 ceramics at microwave frequency. Mater. Res. Bull. 2015, 169, 65. [Google Scholar] [CrossRef]
- Huang, C.L.; Wang, J.J. Dielectric Properties of Low Loss (1–x)(Mg0.95Zn0.05)TiO3–xSrTiO3 Ceramic System at Microwave Frequency. J. Am. Ceram. Soc. 2007, 90, 858–862. [Google Scholar] [CrossRef]
- Wang, H.P.; Yang, Q.; Li, D.; Huang, L.; Zhao, S.; Xu, S.D. Sintering Behavior and Microwave Dielectric Properties of MgTiO3 Ceramics Doped with B2O3 by Sol-Gel Method. J. Mater. Sci. Technol. 2012, 28, 751. [Google Scholar] [CrossRef]
- Wang, H.P.; Xu, S.Q.; Zhai, S.Y.; Deng, D.G.; Ju, H.D. Effect of B2O3 Additives on the Sintering and Dielectric Behaviors of CaMgSi2O6 Ceramics. J. Mater. Sci. Technol. 2010, 26, 351. [Google Scholar] [CrossRef]
- Gakkai, D.T.; Gakkai, S.; Gakkai, T. 1976 Joint Convention Record of Four Institutes of Electrical Engineers; Denki Shigakkai Jōchi Rengō Taikai Kikaku Iinkai: Tokyo, Japan, 1976. [Google Scholar]
- Ferreira, V.M.; Azough, F.; Baptista, J.L.; Freer, R. DiC12: Magnesium titanate microwave dielectric ceramics. Ferroelectrics 1992, 133, 127. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Azough, F.; Freer, R. The effect of Cr and La on MgTiO3 and MgTiO3-CaTiO3 microwave dielectric ceramics. J. Mater. Res. 1997, 12, 3293. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Baptista, J.L.; Kamba, S. Dielectric spectroscopy of MgTiO3-based ceramics in the 109–1014Hz region. J. Mater. Sci. 1993, 28, 5894–5900. [Google Scholar] [CrossRef]
- Huang, C.L.; Chen, Y.B. New dielectric material system of Mg0.95Co0.05TiO3 −Zn0.975Ca0.025TiO3 at microwave frequencies. J. Alloys Compd. 2009, 477, 712–715. [Google Scholar] [CrossRef]
- Shen, C.H.; Huang, C.L. Microwave Dielectric Properties of (Mg0.95Ni0.05)TiO3-SrTiO3 Ceramics with a Near-Zero Temperature Coefficient of Resonant Frequency. Int. J. Appl. Ceram. Technol. 2010, 7, 207–216. [Google Scholar]
- Shen, C.H.; Huang, C.L. Microwave Dielectric Properties of (1−x)(Mg0.95Ni0.05)TiO3–x(Ca0.8Sr0.2)TiO3 Ceramic System With Near-Zero Temperature Coefficient. Int. J. Appl. Ceram. Technol. 2012, 9, 447–453. [Google Scholar] [CrossRef]
- Liao, J.; Senna, M. Crystallization of titania and magnesium titanate from mechanically activated Mg(OH)2 and TiO2 gel mixture. Mater. Res. Bull. 1995, 30, 385. [Google Scholar] [CrossRef]
- Huang, C.L.; Pan, C.L. Low-Temperature Sintering and Microwave Dielectric Properties of (1−x)MgTiO3–xCaTiO3 Ceramics Using Bismuth Addition. J. Appl. Phys. 2002, 41, 707. [Google Scholar] [CrossRef]
- Shen, C.H.; Huang, C.L. Phase Evolution and Dielectric Properties of (Mg0.95M0.052+) Ti2O5 (M2+ = Co, Ni, and Zn) Ceramics at Microwave Frequencies. J. Am. Ceram. Soc. 2009, 92, 384–388. [Google Scholar]
- Belous, A.; Ovchar, O.; Durilin, D. High-Q Microwave Dielectric Materials Based on the Spinel Mg2TiO4. J. Am. Ceram. Soc. 2006, 89, 3441–3445. [Google Scholar] [CrossRef]
- Wang, J.J. Microwave dielectric properties of (1−x)(Mg0.95Zn0.05)TiO3–x(Na0.5La0.5)TiO3 ceramic system. J. Alloys Compd. 2009, 486, 423. [Google Scholar] [CrossRef]
- Silverman, B.D. Microwave Absorption in Cubic Strontium Titanate. Phys. Rev. 1962, 125, 1921. [Google Scholar] [CrossRef]
- Hakki, B.W.; Coleman, P.D. A Dielectric Resonator Method of Measuring Inductive Capacities in the Millimeter Range. IEEE Trans. Microwave Theory Tech. 1960, 8, 402. [Google Scholar] [CrossRef]
- Courtney, W.E. Analysis and Evaluation of a Method of Measuring the Complex Permittivity and Permeability Microwave Insulators. IEEE Trans. Microwave Theory Tech. 1970, 18, 476. [Google Scholar] [CrossRef]
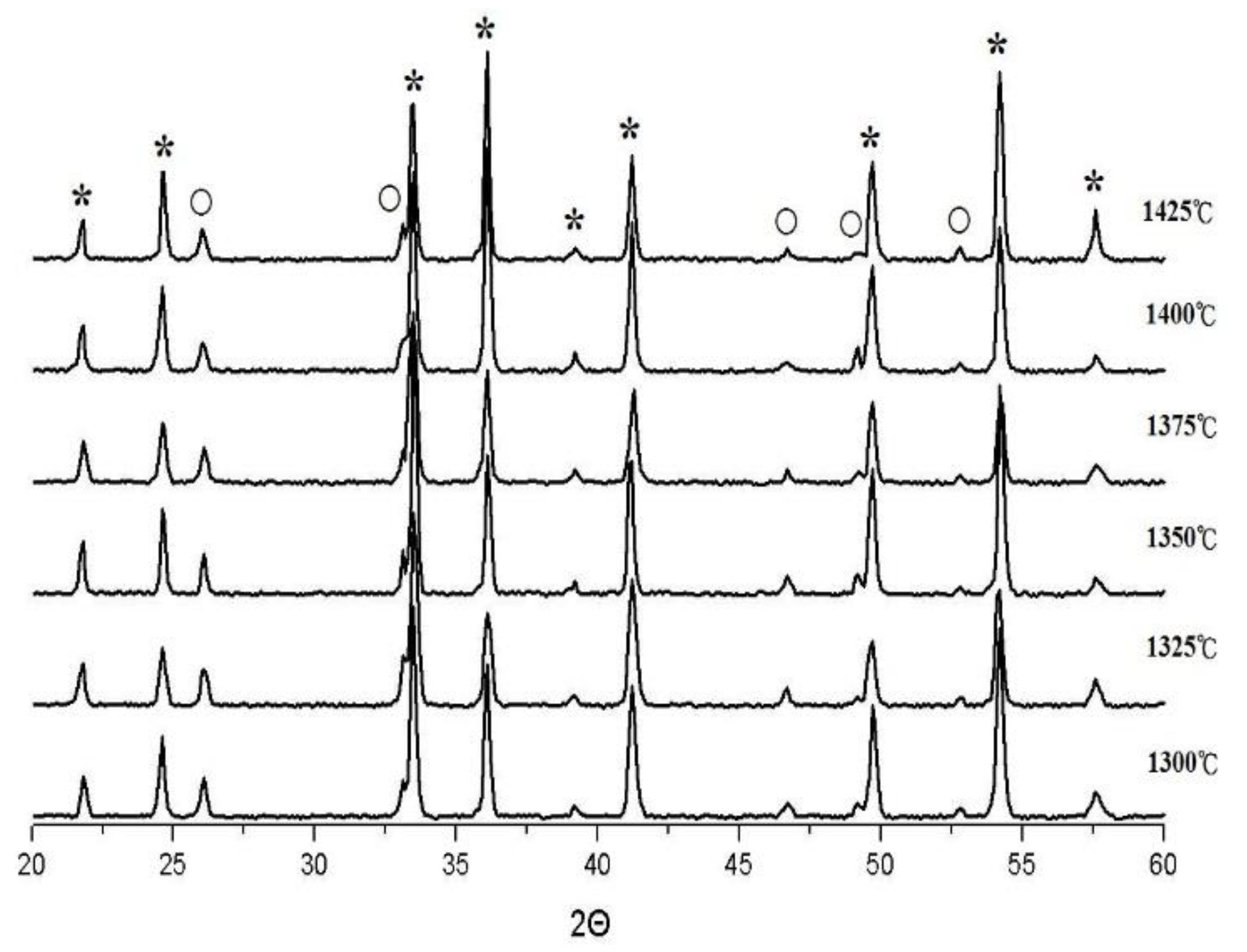
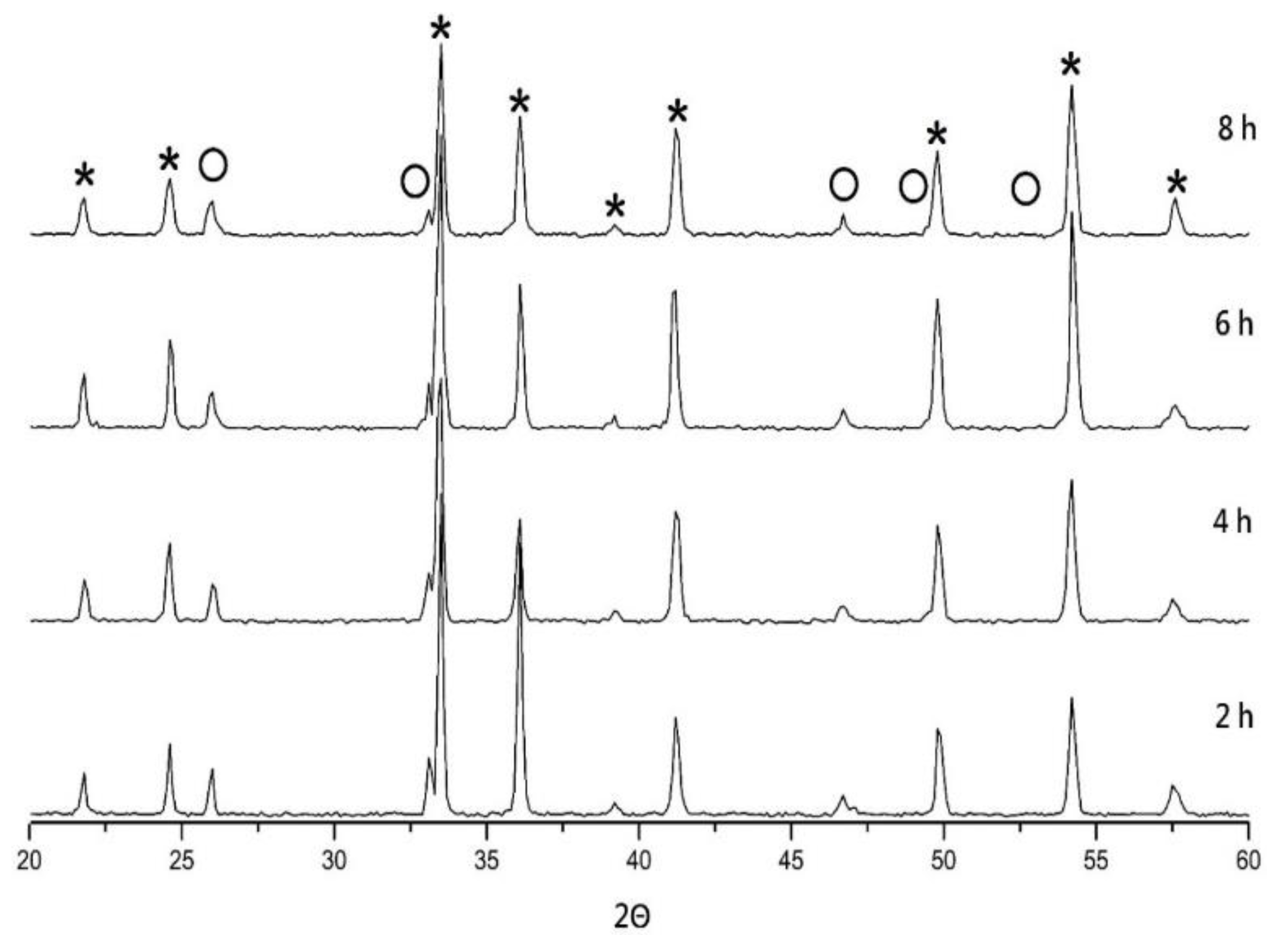

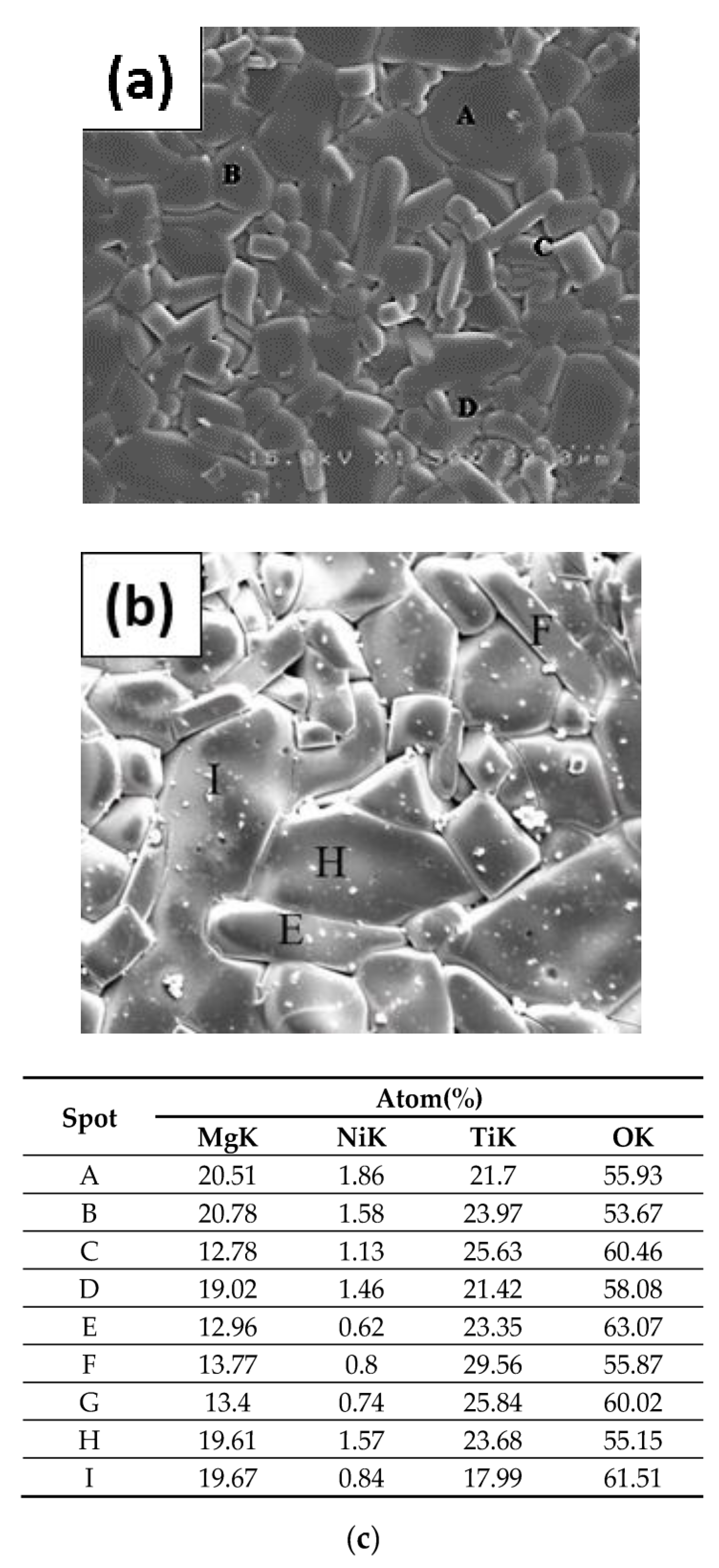
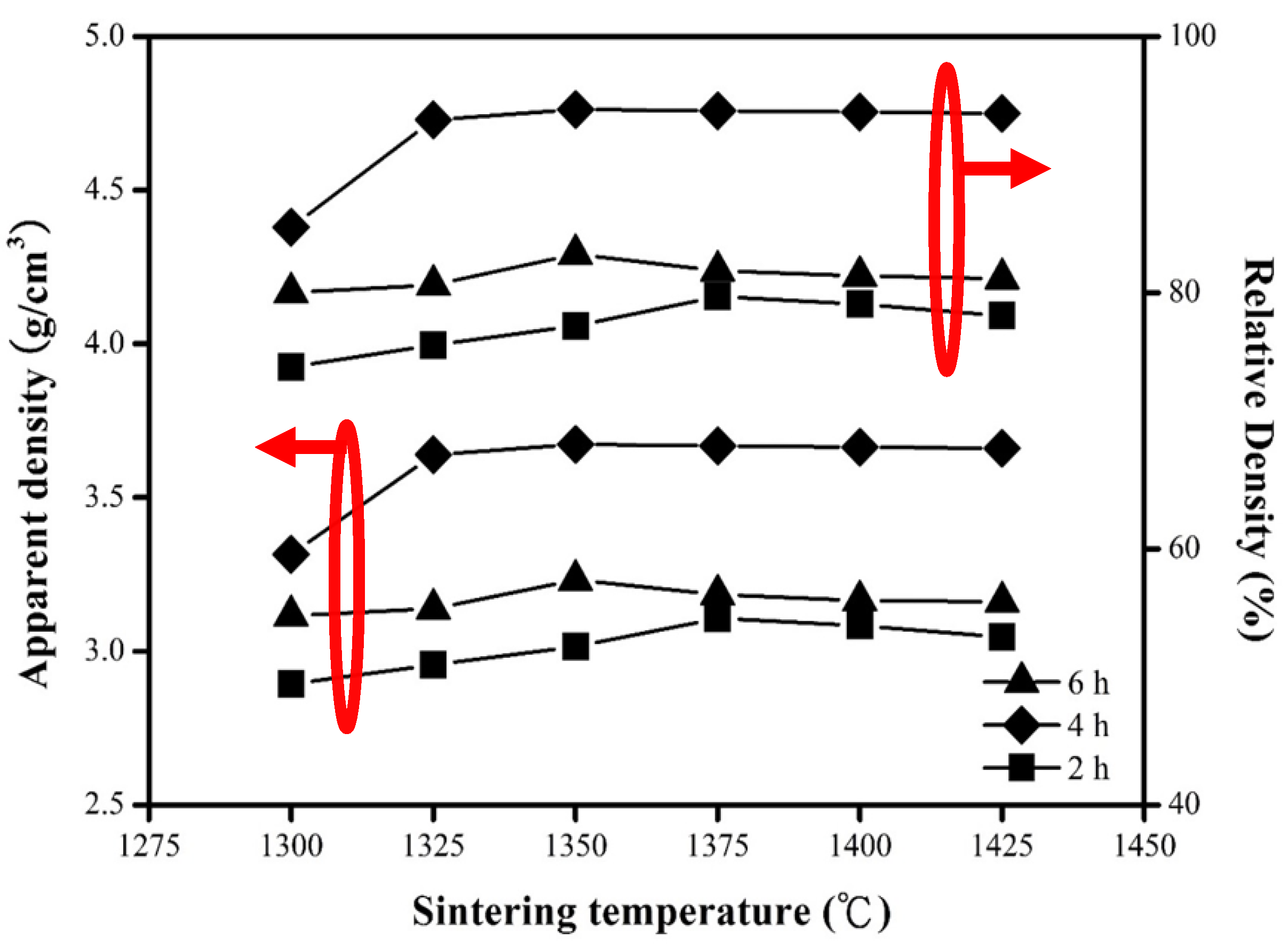

| S.T. | Holding Time | a (nm) | c (nm) |
|---|---|---|---|
| 1325 °C | 2 h | 0.5024 ± 0.00197 | 1.38518 ± 0.00398 |
| 4 h | 0.50395 ± 0.00100 | 1.38357 ± 0.00200 | |
| 6 h | 0.50429 ± 0.00125 | 1.38971 ± 0.00254 | |
| 1350 °C | 2 h | 0.50439 ± 0.00135 | 1.38850 ± 0.00273 |
| 4 h | 0.50459 ± 0.00126 | 1.39054 ± 0.00256 | |
| 6 h | 0.50611 ± 0.00070 | 1.38949 ± 0.00141 | |
| 1375 °C | 2 h | 0.50288 ± 0.00163 | 1.39250 ± 0.00335 |
| 4 h | 0.50386 ± 0.00080 | 1.38478 ± 0.00161 | |
| 6 h | 0.50401 ± 0.00116 | 1.38811 ± 0.00235 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-H.; Pan, C.-L.; Lin, S.-H. A Study of the Effect of Sintering Conditions of Mg0.95Ni0.05Ti3 on Its Physical and Dielectric Properties. Molecules 2020, 25, 5988. https://doi.org/10.3390/molecules25245988
Shen C-H, Pan C-L, Lin S-H. A Study of the Effect of Sintering Conditions of Mg0.95Ni0.05Ti3 on Its Physical and Dielectric Properties. Molecules. 2020; 25(24):5988. https://doi.org/10.3390/molecules25245988
Chicago/Turabian StyleShen, Chun-Hsu, Chung-Long Pan, and Shih-Hung Lin. 2020. "A Study of the Effect of Sintering Conditions of Mg0.95Ni0.05Ti3 on Its Physical and Dielectric Properties" Molecules 25, no. 24: 5988. https://doi.org/10.3390/molecules25245988






